Abstract
Background
Immune checkpoint inhibitors (ICIs) have been a major advance in cancer management. However, we still lack prospective real-world data regarding their usage in people with HIV infection (PWH).
Methods
The ANRS CO24 OncoVIHAC study (NCT03354936) is an ongoing prospective observational cohort study in France of PWH with cancer treated with ICI. We assessed the incidence of grade ≥3 immune-related adverse events (irAEs). All grade ≥3 irAEs were reviewed by an event review.
Results
Between January 17, 2018, and December 05, 2023, 150 participants were recruited from 33 sites and 140 were included in this analysis. At the data cut-off date of December 05, 2023, the median follow-up was 9.2 months (IQR: 3.9–18.3), with a total of 126.2 person-years.
Median age was 59 years (IQR: 54–64) and 111 (79.3%) were men. Median time since HIV diagnosis was 25 years (12–31), the median duration on antiretroviral (ARV) was 19.5 years (7.7–25.4), and the CD4 nadir was 117/µL (51–240). ICI regimens comprised anti-programmed cell death protein-1 (PD-1) for 111 (79.3%) participants, anti-programmed death-ligand 1 for 25 (17.9%), a combination of anti-PD-1 and anti-cytotoxic T-lymphocyte associated protein 4 for 3 (2.1%), and anti-PD-1 along with anti-vascular endothelial growth factor receptor for 1 (0.7%). The most frequent cancers were lung (n=65), head/neck (n=15), melanoma (n=12), liver (n=11) and Hodgkin’s lymphoma (n=9).
During follow-up, a total of 34 grade ≥3 irAEs occurred in 20 participants, leading to an incidence rate of 26.9 per 100 person-years. The Kaplan-Meier estimates of the proportion of participants with at least one episode of grade ≥3 irAEs were 13.8% at 6 months, 15.0% at 12 months and 18.7% at 18 months. One treatment-related death due to myocarditis was reported (0.7%). Multivariable analysis of cumulative incidence showed that participants with time since HIV diagnosis >17 years (incidence rate ratio (IRR)=4.66, p=0.002), with CD4<200 cells/µL (IRR=4.39, p<0.0001), with positive cytomegalovirus (CMV) serology (IRR=2.76, p=0.034), with history of cancer surgery (IRR=3.44, p=0.001) had a higher risk of incidence of grade ≥3 irAEs.
Conclusion
This study showed that the incidence of a first episode of grade ≥3 irAE was 15.0% (95% CI: 9.6% to 22.9%) at 1 year and the cumulative incidence of all severe irAE episodes was 26.9 per 100 person-years. Low CD4 count, positive CMV serology, history of cancer surgery and a longer time since HIV diagnosis were associated with the occurrence of severe irAEs.
Keywords: Immune Checkpoint Inhibitors, Immunocompromised, Immunotherapy, Immune related adverse event - irAE, Infection
WHAT IS ALREADY KNOWN ON THIS TOPIC.
WHAT THIS STUDY ADDS
The study showed that a low CD4 count, a longer time since HIV diagnosis, a history of cancer surgery and positive cytomegalovirus (CMV) serology at the start of ICI were risk factors for the development of severe immune-related adverse events.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our results show the importance of CD4 counts in the management of cancer PWH and highlight the survival benefit for melanoma and Hodgkin’s disease PWH after ICI treatment.
Introduction
Over the past two decades, considerable advances in antiretroviral therapy (ART) have brought HIV replication and its deadly consequences under sustained control. As a result, people with HIV infection (PWH) are now living much longer1 and, as they age, face new cancer challenges, the incidence of which is higher in PWH than in HIV-uninfected individuals.2 Immune checkpoint inhibitors (ICIs), including monoclonal antibodies that block cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed death 1 (PD-1), or its ligand (PD-L1), work to restore T-cell mediated immune responses against various cancer types.3 Indeed, ICIs are highly effective in restoring potent antitumor immunity and do not appear to interact with antiretrovirals.4 Their use has led to a substantial improvement in the survival of individuals with cancer, and they are now commonly administered across a spectrum of malignancies.5 Nonetheless, ICIs may also disrupt immune tolerance, heightening the risk of immune-related adverse events (irAEs), which may include autoimmune reactions.6
PWH were initially excluded from all oncology trials evaluating the efficacy and the safety profile of ICIs.7 Despite the high efficiency of ARTs, PWH remain more exposed to infections, cancers and inflammatory manifestations.8,11 Thus, a higher rate of irAEs, a lower antitumoral efficacy and/or the induction of inflammatory syndromes with the use of ICI in PWH were feared.12,14
More recently, reassuring data on their utilization in this population with a distinct immunological profile have emerged. A review of 176 PWH treated with ICI, primarily from retrospective studies, reported a 12.1% rate of severe adverse events (AEs) (Common Terminology Criteria for Adverse Events (CTCAE) grade >2), which is comparable to the general population.3 This finding was corroborated by three phase I/II trials that investigated the use of durvalumab, nivolumab, and pembrolizumab in virally controlled PWH with cancer, revealing rates of severe AEs at 0%, 6.3%, and 23.3%, respectively.15,17 It is worth noting that one participant, treated with pembrolizumab for Kaposi sarcoma-associated herpesvirus infection (KSHV), developed a lethal polyclonal KSHV-associated B-cell-lymphoproliferation, though this remains the sole reported case to date.17 When it comes to the effect of ICIs on HIV viral markers, such as plasma viral load (VL) and circulating CD4 T levels, the data were similarly reassuring. In the systematic review, 92% of participants exhibited a stable VL (within±50 copies/mL), while 61% of participants undergoing treatment demonstrated stable circulating CD4 T levels (within±100 CD4/mm3).3
Regarding the antitumor efficacy of ICI in PWH, data remains limited. Although the three phase I/II trials reported comparable efficacy to the general population, they included only a small number of participants.15,17 Moreover, a recent large control-matched retrospective study found no difference in terms of progression-free survival (PFS) and overall survival (OS) in PWH with metastatic non-small cell lung cancer (NSCLC) compared with immunocompetent participants with metastatic NSCLC.18
In contrast to these potential side effects, there are arguments in favor of a potentially beneficial effect of immunotherapy on HIV infection itself. Indeed, as PD-1 is overexpressed on HIV-specific CD4 and CD8 blocking the PD-1/PD-L1 axis would increase HIV-specific CD4 and CD8 T cells functions19 20 with potentially an effect on HIV reservoirs.
Despite these recent reassuring findings, prospective real-world data on their utilization in this specific population are still lacking. To extend our knowledge in terms of the management of ICI in the context of cancer and HIV infection, we built through the French national ONCOVIH network, a network that brings together all the expertize required for optimal management of the various cancers affecting PWH, the ANRS CO24 OncoVIHAC study, a prospective, real-world cohort study designed to assess the tolerability of these immunotherapies in PWH with cancer treated with ICIs in France, and to assess the impact of ICIs on the virological and immunological status of PWH.
Methods
Study design and participants
This prospective, real-world, observational cohort study (ANRS CO24 OncoVIHAC) was set-up at 33 sites in France (online supplemental table S1). It enrolled participants ≥18 years of age, with HIV infection and a histologically proven cancer, naïve for ICI therapy who initiated an ICI therapy at baseline or were treated with an ICI for less than 30 days, with any CD4 lymphocytes count or HIV VL. ICI regimens included anti-PD-1 or anti-PD-L1 or anti-CTLA-4 administered either as monotherapy or in combination according to cancer guidelines for HIV uninfected individuals. ICI were provided free of charge through the national health insurance according to marketing authorization, temporary authorization for use or temporary recommendation for use. Indications were as followed: melanoma (ipilimumab, nivolumab and pembrolizumab), lung cancer (nivolumab, pembrolizumab, atezolizumab, durvalumab), kidney cancer (nivolumab), bladder cancer (atezolizumab), Hodgkin’s disease (nivolumab, pembrolizumab), anal cancer (pembrolizumab) and head/neck cancer (pembrolizumab, nivolumab). Nivolumab and durvalumab were prescribed every 2 weeks, pembrolizumab and atezolizumab every 3 weeks, and ipilimumab every 3 weeks, but for a total of four doses. Some of these ICIs are administered as first-line therapy or after the failure of previous therapies, and may be administered in conjunction with chemotherapies.
The protocol was approved by the ethics committee of the CPP Sud-Ouest et de l’Outre-mer IV. All participants gave written informed consent to take part in this study registered under ClinicalTrials.gov (NCT03354936).
Procedures
Study visits were scheduled at month 0 (the first day of ICI administration, baseline), 6 months, 12 months, 18 months and 24 months. At baseline, the following data were collected: socio-demographic and lifestyle data, history of HIV infection, current cancer (type, histology, previous treatments), the ICI received, HIV-1 RNA VL, CD4 and CD8 T lymphocytes count, presence of co-infections using serology determinations for hepatitis B virus (HBV), hepatitis C virus (HCV), epstein barr virus (EBV), cytomegalovirus (CMV), history of AIDS-defining events, antiretroviral (ARV) treatments, personal and family history of autoimmune diseases, corticosteroid therapy in the last 6 months, personal and family history of cancer.
At follow-up visits the data collected were current ARV treatment, concomitant therapies, the ICI received (type of treatment, dose, start date, end date and, time and reason for the interruption of ICI if any), clinical and biological AEs, immuno-virological data (HIV-1 RNA VL, CD4 and CD8), evolution of cancer disease and vital status.
Outcomes
The primary outcome was the incidence of the first occurrence of grade ≥3 irAEs during the study period. Grade 3–5 AEs were reviewed by an event review committee composed of infectiologists, immunologists, pharmacologists and oncologists to validate the reported AEs and classify them as irAEs or not, on a consensus basis. All AEs were graded according to the CTCAE V.4.0 (available at https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm).
Secondary outcomes included cumulative incidence of grade ≥3 irAEs, baseline factors associated with a cumulative incidence of irAEs, OS, PFS, the evolution of plasma HIV RNA VL, CD4 count, CD8 count and CD4/CD8 ratio, and changes in ART.
Statistical analysis
Primary and secondary analyses were assessed in participants enrolled in the study who started ICI and who were enrolled at least 6 months prior to the cut-off date. Baseline characteristics were described for the overall cohort, and by cancer types for the most frequent ones.
We used the Kaplan-Meier method to estimate the proportion of participants with grade ≥3 irAEs for the entire cohort and by subgroups, including ICI type, cancer type, period of inclusion, CD4 nadir (<100 vs ≥100 cells/µL), baseline CD4 count (<200 vs ≥200 cells/µL) and baseline CD4:CD8 ratio (<0.4 vs ≥0.4). Log-rank test was used to compare the risk of grade ≥3 irAEs between groups. In this analysis, subjects’ follow-up was right-censored at the time of the first occurrence of grade ≥3 irAEs.
Cumulative incidence rates per 100 person-years of grade ≥3 irAEs were calculated by the total number of grade ≥3 irAEs divided by the total number of person-years of observation. Participants who experienced multiple grade ≥3 irAEs on a given day were considered to have experienced one grade ≥3 irAE on that day. The person-years of observation are the sum of the number of years that each subject of the study population has been under observation. In this analysis, the entire follow-up period was considered.
Univariable and multivariable analyses were performed to identify baseline factors associated with the cumulative incidence of grade ≥3 irAEs, using Poisson regression models with a log link and person-years as the offset. The following baseline variables were tested: age, gender, geographic origin, professional activity, smoking, drug use, alcohol use, corticosteroid therapy in the last 6 months, HIV transmission group, pre-ART VL, CD4 nadir, CD4 count, CD8 count, CD4:CD8 ratio, VL, integrase strand transfer inhibitor-containing regimen, non-nucleoside reverse transcriptase inhibitor-containing regimen, protease inhibitor-containing regimen, positive hepatitis B surface (HBs) antigen, positive HCV antibody, positive EBV antibody, positive CMV antibody, cancer stage at ICI initiation, ICI type, history of chemotherapy, cancer surgery, radio-chemotherapy, radiotherapy, cancer type for the most common ones (lung cancer, head/neck cancer, melanoma, liver cancer, Hodgkin’s disease). Continuous variables were modeled as categorical variables (categorization into terciles) and we grouped the closest category to obtain two categories for certain variables based on the incidence rate ratio of the corresponding univariable model. Variables with a p value<0.20 in the univariable analysis were retained for the multivariable analysis. The backward elimination technique (p<0.05) was applied to identify variables independently associated with the incidence of irAEs. Because some of the variables for the multivariable analysis had missing values, and because it is better to impute missing data than to ignore them, multiple imputations using the chained equations approach were used to fill in missing data from the participant’s other covariables. Five imputations were chosen. Analyses were run on each of the five data sets and the results were combined with Rubin’s rules.
The Kaplan-Meier method was used to estimate overall and PFS rates for the overall cohort and by subgroups. For PFS, the event was death or disease progression. The log-rank test was used to compare survival rates between groups.
The evolution of plasma VL was assessed separately for participants with a plasma VL<50 copies/mL at baseline and those with a VL ≥50 copies/mL at baseline. For participants whose VL was ≥50 copies/mL at baseline, we estimated, among those who had VL measurements during follow-up, the proportion of participants who had an HIV VL<50 copies/mL at the last assessment. For participants whose VL was <50 copies/mL at baseline, we estimated, among those who had VL measurements during follow-up, the proportion of participants who had all VL measurements <50 copies/mL. Changes in CD4 count, CD8 count and the CD4/CD8 ratio between baseline and the participant’s last assessment were compared using the Wilcoxon paired test. Change in antiretroviral treatment was estimated by the proportion of participants who changed their baseline regimen during the follow-up period.
Analyses were performed using SAS (V.9.4, SAS Institute) and Stata/SE (V.13.0, StataCorp, USA) software. All p values and CIs are two-tailed, with a significance level set at 0.05.
Results
Baseline characteristics of study population
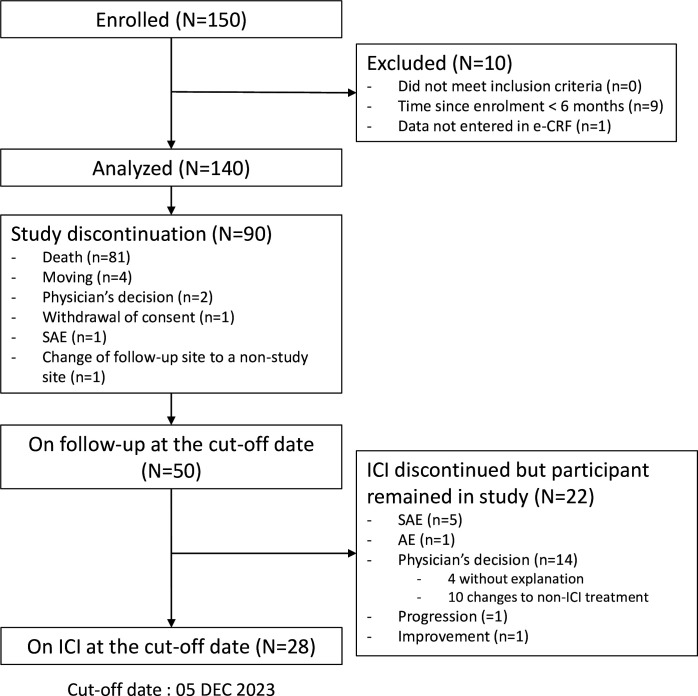
Between January 17, 2018, to December 05, 2023, 150 participants had been enrolled from 33 sites, 9 of whom enrolled less than 6 months ago and 1 had no data reported (online supplemental table S1), leaving a total of 140 participants analyzed. At the data cut-off date of December 05, 2023, the median follow-up of participants was 9.2 months (IQR: 3.9–18.3), with a total of 126.2 person-years, including a median duration under ICI of 5.4 months (IQR: 2.1–12.7). During the follow-up period, 81 (58%) participants died, and 9 (6%) prematurely discontinued study follow-up. From the remaining 50 participants, 28 were on ICI at the cut-off date, 22 stopped ICI due to cancer progression (n=1), AE/serious adverse event (SAE) (n=6), improvement (n=1) and physician decision (n=14 including 4 without explanation and 10 switched to a non-ICI treatment), (figure 1).
Figure 1. Flowchart of the study. AE, adverse event; e-CRF, electronic case report form; SAE, serious adverse event.
Baseline characteristics are shown in table 1. Participants were mostly men (79.3%), the median age was 59 years (IQR: 54–64), and 111 (79.3%) were current or former smokers. Median time since HIV diagnosis was 25 years (12–31), with a median CD4 nadir of 117 /µL (51–240). They have been on ART for a median of 19.5 years (7.7–25.4) and four had never received antiretroviral treatment. At study entry, the median CD4 count was 336/µL (210–598), CD4:CD8 ratio 0.7 (0.3–1.0) and HIV plasma VL was <50 copies/mL for 107/127 (84.3%) participants. In the 20 participants with viremia at baseline, the median HIV RNA VL was 460 copies/mL (IQR: 106–39,550).
Table 1. Baseline characteristics of the study participants at ICI initiation for the overall cohort and by the most frequent cancer.
| Overall | Most frequent cancer (≥9 cases) | |||||
| Median (IQR) or n (%) | N=140 | LungN=65 | Head/neckN=15 | Cutaneous melanomaN=12 | Liver cancerN=11 | Hodgkin’s diseaseN=9 |
| Age, years | N=139 | N=65 | N=15 | N=12 | N=11 | N=8 |
| 59 (54–64) | 59 (55–63) | 55 (51–64) | 59 (53–72) | 67 (60–73) | 53 (48–54) | |
| Sex at birth | ||||||
|
111 (79.3) | 48 (73.9) | 11 (73.3) | 11 (91.7) | 11 (100.0) | 8 (88.9) |
|
29 (20.7) | 16 (26.1) | 4 (26.7) | 1 (8.3) | 0 (0.0) | 1 (11.1) |
| Geographical origin | ||||||
|
110 (78.6) | 55 (84.6) | 10 (66.7) | 10 (83.4) | 8 (72.7) | 7 (77.8) |
|
13 (9.3) | 2 (3.1) | 2 (13.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
17 (12.1) | 8 (12.3) | 3 (20.0) | 2 (16.7) | 3 (27.3) | 2 (22.2) |
| Professional activity | 70 (50.0) | 30 (46.2) | 8 (53.3) | 7 (58.3) | 3 (27.3) | 6 (66.7) |
| Current or former smokers | 111 (79.3) | 65 (100) | 10 (66.7) | 4 (33.3) | 8 (72.8) | 7 (77.8) |
| Current or former alcohol users | 90 (64.3) | 47 (72.3) | 11 (73.3) | 8 (66.7) | 6 (54.6) | 5 (55.6) |
| Current or former drug users | 49 (35.0) | 32 (49.3) | 3 (20.0) | 1 (8.3) | 4 (36.4) | 5 (55.6) |
| Year of inclusion in the study | ||||||
|
26 (18.6) | 12 (18.5) | 3 (20.0) | 4 (33.3) | 0 (0.0) | 2 (22.2) |
|
22 (15.7) | 7 (10.8) | 3 (20.0) | 3 (25.0) | 0 (0.0) | 2 (22.2) |
|
29 (20.7) | 20 (30.8) | 3 (20.0) | 1 (8.3) | 1 (9.1) | 1 (11.1) |
|
35 (25.0) | 13 (20.0) | 2 (13.3) | 4 (33.3) | 4 (36.4) | 3 (33.3) |
|
21 (15.0) | 9 (13.9) | 3 (20.0) | 0 (0.0) | 4 (36.4) | 1 (11.1) |
|
7 (5.0) | 4 (6.2) | 1 (6.7) | 0 (0.0) | 2 (18.2) | 0 (0.0) |
| HIV risk group | ||||||
|
41 (29.3) | 20 (30.8) | 2 (13.3) | 6 (50.0) | 5 (45.5) | 5 (55.6) |
|
40 (28.6) | 15 (23.1) | 5 (33.3) | 3 (25.0) | 0 (0.0) | 3 (33.3) |
|
24 (17.1) | 16 (24.6) | 0 (0.0) | 1 (8.3) | 2 (18.2) | 1 (11.1) |
|
35 (25.0) | 14 (21.5) | 8 (53.3) | 2 (16.7) | 4 (36.4) | 0 (0.0) |
| Previous AIDS-defining event | 38 (27.1) | 18 (27.7) | 3 (20.0) | 2 (16.7) | 2 (18.2) | 2 (22.2) |
| Time since HIV diagnosis, years | N=140 | N=65 | N=15 | N=12 | N=11 | N=9 |
| 25 (12–31) | 25 (13–31) | 25 (6–29) | 18 (8–30) | 33 (27–35) | 14 (8–26) | |
| Duration of antiretroviral therapy, years | N=119 | N=53 | N=12 | N=10 | N=9 | N=9 |
| 19.5 (7.7–25.4) | 18.1 (10.1–24.4) | 20.7 (8.6–24.6) | 14.2 (5.8–23.6) | 22.2 (17.2–26.9) | 10.3 (7.4–16.7) | |
| Pre-ART HIV pVL log10 (copies/mL) | N=74 | N=35 | N=7 | N=5 | N=2 | N=7 |
| 4.7 (3.8–5.3) | 4.5 (3.8–5.3) | 4.9 (4.1–5.4) | 5.1 (3.0–5.3) | 5.5 (1.5–5.8) | 4.5 (1.3–5.2) | |
| CD4 count nadir (cells/μL) | 117 (51–240) | 114 (55–286) | 94 (69–180) | 276 (212–388) | 120 (56–239) | 67 (41–160) |
|
50 (45.1) | 24 (46.2) | 6 (54.6) | 0 (0.0) | 4 (50.0) | 4 (57.1) |
|
61 (55.0) | 28 (53.9) | 5 (45.5) | 9 (100.0) | 4 (50.0) | 3 (42.9) |
|
29 | 13 | 4 | 3 | 3 | 2 |
| CD4 count (cells per μL) | 336 (210–598) | 374 (251–608) | 254 (136–268) | 630 (370–704) | 363 (281–616) | 230 (145–364) |
|
29 (24.0) | 12 (21.1) | 5 (38.5) | 1 (8.3) | 0 (0.0) | 2 (33.3) |
|
92 (76.0) | 45 (78.9) | 8 (61.5) | 11 (91.7) | 9 (100.0) | 4 (66.7) |
|
19 | 8 | 2 | 0 | 2 | 3 |
| CD4:CD8 ratio | 0.7 (0.3–1.0) | 0.7 (0.4–1.0) | 0.5 (0.2–0.8) | 0.7 (0.6–1.1) | 1.1 (0.9–1.4) | 0.2 (0.1–0.4) |
|
37 (31.6) | 13 (23.6) | 7 (53.8) | 1 (8.3) | 2 (22.2) | 5 (83.3) |
|
80 (68.4) | 42 (76.4) | 6 (46.2) | 11 (91.7) | 7 (77.8) | 1 (16.7) |
|
23 | 10 | 2 | 0 | 2 | 3 |
| HIV RNA viral load, copies/mL | ||||||
|
107 (84.3) | 51 (86.4) | 10 (83.3) | 11 (91.7) | 9 (100.0) | 9 (100.0) |
|
20 (15.7) | 8 (13.6) | 2 (16.7) | 1 (8.3) | 0 (0.0) | 0 (0.0) |
|
13 | 6 | 3 | 0 | 2 | 0 |
| Antiretroviral regimen | ||||||
|
23 (16.4) | 11 (16.9) | 1 (6.7) | 6 (50.0) | 2 (18.2) | 1 (11.1) |
|
61 (43.6) | 29 (44.6) | 5 (33.3) | 6 (50.0) | 5 (45.5) | 5 (55.6) |
|
6 (4.3) | 4 (6.2) | 1 (6.7) | 0 (0.0) | 1 (9.1) | 0 (0.0) |
|
10 (7.1) | 4 (6.2) | 1 (6.7) | 0 (0.0) | 1 (9.1) | 0 (0.0) |
|
32 (22.9) | 13 (20.0) | 4 (26.7) | 0 (0.0) | 2 (18.2) | 3 (33.3) |
|
8 (5.7) | 4 (2.9) | 3 (2.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Serology status | ||||||
|
12/118 (10.2) | 3/54 (5.6) | 2/12 (16.7) | 1/10 (10.0) | 4/9 (44.4) | 0 (0.0) |
|
29/132 (22.0) | 16/63 (25.4) | 1/13 (7.7) | 0 (0.0) | 4/10 (40.0) | 0 (0.0) |
|
67/130 (51.5) | 32/64 (50.0) | 4/12 (33.3) | 4/11 (36.4) | 5/10 (50.0) | 4/7 (57.1) |
|
73/130 (56.2) | 37/63 (58.7) | 6/12 (50.0) | 4 (33.3) | 5/10 (50.0) | 4/7 (57.1) |
| Personal history of autoimmune/inflammatory diseases† | 9 (6.4) | 4 (6.2) | 2 (13.3) | 1 (8.3) | 0 (0.0) | 2 (22.2) |
| Cancer stage at ICI initiation | ||||||
|
84 (60.0) | 49 (75.4) | 4 (26.7) | 7 (58.3) | 7 (63.6) | 0 (0.0) |
|
56 (40.0) | 16 (24.6) | 11 (73.3) | 5 (41.7) | 4 (36.4) | 9 (100.0) |
| Latest cancer therapy | ||||||
| Chemotherapy | 40/140 (28.6) | 18/65 (27.7) | 4/15 (26.7) | 0 (0.0) | 2/11 (18.2) | 6/9 (66.7) |
| Surgery | 16/140 (11.4) | 7/65 (10.8) | 1/15 (6.7) | 4/12 (33.3) | 1/11 (9.1) | 0 (0.0) |
| Radiochemotherapy | 16/140 (11.43) | 9/65 (13.9) | 3/15 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Radiotherapy | 11/140 (7.9) | 3/65 (4.6) | 3/15 (20.0) | 0 (0.0) | 2/11 (18.2) | 0 (0.0) |
| Targeted therapy | 4/140 (2.9) | 0 (0.0) | 0 (0.0) | 2/12 (16.7) | 0 (0.0) | 0 (0.0) |
| Other | 15/140 (10.7) | 7/65 (10.8) | 2/15 (13.3) | 1/8 (8.3) | 2/11 (18.2) | 3/9 (33.3) |
| Missing | 38 | 21 | 2 | 5 | 4 | 0 |
| Time since cancer diagnosis, years | 0.5 (0.1–1.5) | 0.2 (0.1–0.7) | 0.7 (0.5–2.0) | 0.3 (0.1–1.5) | 0.4 (0.1–1.1) | 3.9 (1.9–7.8) |
| Corticosteroid therapy within the last 6 months | 29 (20.7) | 19 (29.2) | 1 (6.7) | 2 (16.7) | 0 (0.0) | 3 (33.3) |
Continuous variables were described by medians and 25th percentile and 75th percentile and categorical variables by numbers and percentages. have sex with men; User; ; reverse transcriptase inhibitor; -nucleoside reverse
Other ARV treatment: Monotherapy (n=3), quadritherapy (n=1), naïve(n=4).
Autoimmune hemolytic anemia (n=1), Crohn's disease (n=1), myositis (n=1), rheumatoid polyarthritis (n=1), psoriasis (n=1), immunological thrombocytopenic purpura (n=2), ankylosing spondylitis (n=1), antiphospholipid antibodies (n=1).
ARTantiretroviral therapyARVantiretroviralCMVcytomegalovirusEBVepstein barr virusHBshepatitis B surfaceHBVhepatitis B virusHCVhepatitis C virusICIimmune checkpoint inhibitorIDUintravenous drug userINSTIintegrase strand transfer inhibitorMSMmen who have sex with menNNRTInon-nucleoside reverse transcriptase inhibitorNRTInucleoside reverse transcriptase inhibitorPIprotease inhibitorpVLplasma viral load
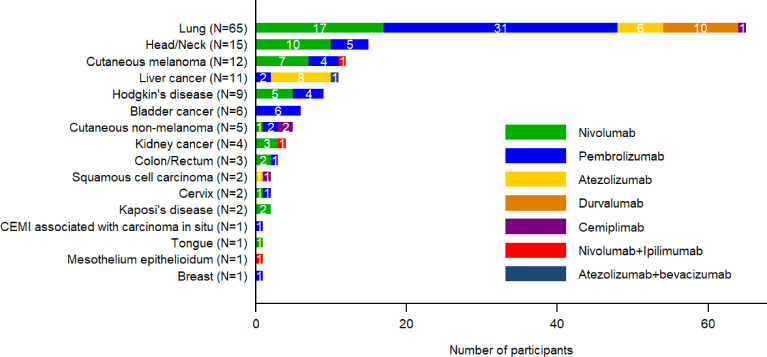
The types of cancer and the ICIs prescribed for these cancers are reported in figure 2. The most frequent cancers were lung (n=65, 46%), head/neck (n=15, 11%), melanoma (n=12, 9%), liver (n=11, 8%) and Hodgkin’s lymphoma (n=9, 6%). ICI regimens comprised anti-PD-1 for 111 (79%) participants (pembrolizumab (n=58, 41%), nivolumab (n=49, 35%), cemiplimab (n=4, 3%)), anti-PD-L1 for 25 (18%) participants (atezolizumab (n=15, 11%) and durvalumab (n=10, 7%)), a combination of anti-PD-1 and anti-CTLA4 for 3 (2%) (nivolumab+ipilimumab), and anti-PD-L1 along with anti-vascular endothelial growth factor receptor (VEGFR) for 1 (<1%) (atezolizumab+bevacizumab).
Figure 2. Number of immune checkpoint inhibitors (ICI) received by cancer-type. CEMI, Squamous cell carcinoma.
Incidence of grade ≥3 irAEs
Overall, a total of 34 grades ≥3 irAEs occurred in 20 participants, leading to an incidence rate of 26.9 per 100 person-years (95% CI: 18.7 to 37.7). The most frequent grade ≥3 irAEs were liver enzymes elevation (n=3), cytopenia (n=3), anemia (n=3), pneumonia (n=3), lipase elevation (n=2), myocarditis (n=2), acute hepatitis (n=1) (online supplemental table S2). One death, due to myocarditis and considered to be treatment-related, was reported (0.7%). The majority of events were reversible after systemic glucocorticoid use. Indeed, during the study, 41/140 (29.3%) participants received glucocorticoid for a median duration of 12.1 months (IQR: 5.1–45.9). Most of them received glucocorticoid in the context of irAE. Of the 20 participants with grade ≥3 irAEs, 15 discontinued ICI due to these grade ≥3 irAEs.
With regards to the dynamics of grade ≥3 irAEs, using Kaplan-Meier estimates, the proportion of participants with at least one grade ≥3 irAEs was 13.8% (95% CI: 8.8% to 21.4%) at 6 months, 15.0% (95% CI: 9.6% to 22.9%) at 12 months and 18.7% (95% CI: 12.1% to 28.3%) at 18 months. Subgroup analyses did not evidence any statistically significant difference between subgroups (table 2).
Table 2. First occurrence and cumulative incidence of grade ≥3 immune-related adverse events (irAEs) overall and by ICI and cancer types, CD4 nadir, baseline CD4 count, baseline CD4/CD8 ratio, and year of inclusion in the study.
| Kaplan-Meier estimate of the first occurrence of grade ≥3 immune-related AEs | Cumulative incidence of grade ≥3 immune-related AEs | ||||||||
| Number of events/N | Month 6% (95% CI) | Month 12% (95% CI) | Month 18% (95% CI) | P value | Person-years | Total number of events | Incidence rate per 100 person-years(95% CI) | P value | |
| Overall | 20/140 | 13.8 (8.8 to 21.4) | 15.0 (9.6 to 22.9) | 18.7 (12.1 to 28.3) | 126.2 | 34 | 26.9 (18.7 to 37.6) | ||
| Cancer type | 0.37 | 0.0021 | |||||||
| Lung | 10/65 | 16.6 (8.9 to 29.7) | 16.6 (8.9 to 29.7) | 21.0 (11.2 to 37.2) | 55.8 | 21 | 37.6 (23.3 to 57.5) | ||
| Head/neck | 0/15 | 0.0 (0.0 to 21.8) | 0.0 (0.0 to 21.8) | 25.0 (3.9 to 87.2) | 9.7 | 0 | 0.0 (0.0 to 38.0) | ||
| Melanoma | 2/12 | 17.5 (4.7 to 53.9) | 17.5 (4.7 to 53.9) | 17.5 (4.7 to 53.9) | 15.2 | 2 | 13.2 (1.6 to 47.5) | ||
| Liver | 1/11 | 0 | 20.0 (3.1 to 79.6) | 20.0 (3.1 to 79.6) | 9.6 | 1 | 10.4 (0.2 to 58.4) | ||
| Hodgkin’s disease | 0/9 | 0.0 (0.0 to 33.6) | 0.0 (0.0 to 33.6) | 0.0 (0.0 to 33.6) | 13.9 | 0 | 0.0 (0.0 to 26.5) | ||
| ICI type | 0.69 | 0.24 | |||||||
| Nivolumab | 5/49 | 11.0 (4.2 to 27.4) | 14.9 (6.3 to 33.0) | 14.9 (6.3 to 33.0) | 41.5 | 6 | 14.5 (5.3 to 31.5) | ||
| Pembrolizumab | 8/58 | 15.2 (7.8 to 28.3) | 15.2 (7.8 to 28.3) | 15.2 (7.8 to 28.3) | 51.4 | 18 | 35.0 (20.8 to 55.3) | ||
| Atezolizumab | 3/15 | 13.3 (3.5 to 43.6) | 13.3 (3.5 to 43.6) | 25.7 (8.4 to 63.4) | 15.7 | 5 | 31.8 (10.3 to 74.3) | ||
| Other* | 4/18 | 17.6 (6.1 to 45.3) | 17.6 (6.1 to 45.3) | 31.4 (11.6 to 68.3) | 17.6 | 5 | 28.4 (9.2 to 66.3) | ||
| CD4 nadir, cells/µL | 0.71 | 0.13 | |||||||
| <100 | 7/50 | 13.6 (6.3 to 27.9) | 13.6 (6.3 to 27.9) | 20.3 (9.1 to 41.7) | 38.3 | 16 | 41.8 (23.9 to 67.8) | ||
| ≥100 | 11/61 | 18.1 (10.0 to 31.3) | 20.4 (11.7 to 34.0) | 20.4 (11.7 to 34.0) | 65.0 | 16 | 24.6 (14.1 to 40.0) | ||
| Baseline CD4 count, cells/µL | 0.070 | 0.0004 | |||||||
| <200 | 7/29 | 28.6 (14.6 to 51.3) | 28.6 (14.6 to 51.3) | 28.6 (14.6 to 51.3) | 24.7 | 16 | 64.8 (37.0 to 105.2) | ||
| ≥200 | 11/92 | 10.8 (5.7 to 19.8) | 12.5 (6.8 to 22.1) | 15.0 (8.3 to 26.3) | 85.7 | 16 | 18.7 (10.7 to 30.3) | ||
| Baseline CD4:CD8 ratio | 0.19 | 0.007 | |||||||
| <0.4 | 7/37 | 27.2 (13.5 to 50.1) | 27.2 (13.5 to 50.1) | 27.2 (13.5 to 50.1) | 28.1 | 15 | 53.4 (29.9 to 88.0) | ||
| ≥0.4 | 10/80 | 10.4 (5.3 to 19.7) | 12.1 (6.5 to 22.1) | 15.1 (8.1 to 27.1) | 79.0 | 16 | 20.3 (11.6 to 32.9) | ||
| Year of inclusion | 0.77 | 0.35 | |||||||
| 2018 | 4/26 | 15.9 (6.3 to 37.1) | 15.9 (6.3 to 37.1) | 15.9 (6.3 to 37.1) | 29.7 | 6 | 20.2 (7.4 to 44.0) | ||
| 2019 | 4/22 | 15.6 (5.3 to 40.9) | 23.2 (9.2 to 51.7) | 23.2 (9.2 to 51.7) | 16.0 | 5 | 31.3 (10.1 to 72.9) | ||
| 2020 | 5/29 | 22.9 (9.9 to 47.8) | 22.9 (9.9 to 47.8) | 22.9 (9.9 to 47.8) | 24.9 | 9 | 36.1 (16.5 to 68.6) | ||
| 2021 | 3/35 | 6.5 (1.7 to 23.9) | 6.5 (1.7 to 23.9) | 12.8 (4.0 to 36.7) | 32.5 | 5 | 15.4 (5.0 to 35.9) | ||
| 2022–2023 | 4/28 | 11.8 (3.9 to 32.5) | 11.8 (3.9 to 32.5) | 22.8 (7.9 to 56.0) | 23.1 | 9 | 39.0 (17.8 to 74.0) | ||
irAE incidences rate (IR) were calculated as the total number of irAEs over person-years of observation and compared between groups using a Poisson distribution.
Other includes: Durvalumab (n=10), Nnivolumab + Iipilimumab (n=3), Ccemiplimab (n=4), and Aatezolizumab+bevacizumab (n=1).ICI=
ICIimmune checkpoint inhibitor
Multivariable analysis of cumulative incidence of grade ≥3 irAEs showed that participants with time since HIV diagnosis >17 years (incidence rate ratio (IRR)=4.66, p=0.002), with CD4<200 cells/µL (IRR=4.39, p<0.0001), with positive CMV serology (IRR=2.76, p=0.034), with history of cancer surgery (IRR=3.44, p=0.001) had a higher risk of incidence of grade ≥3 irAEs than others (online supplemental table S3). In contrast, participants receiving nivolumab experienced a 62% reduction in the risk of incidence of grade ≥3 irAEs.
Overall survival and progression-free survival
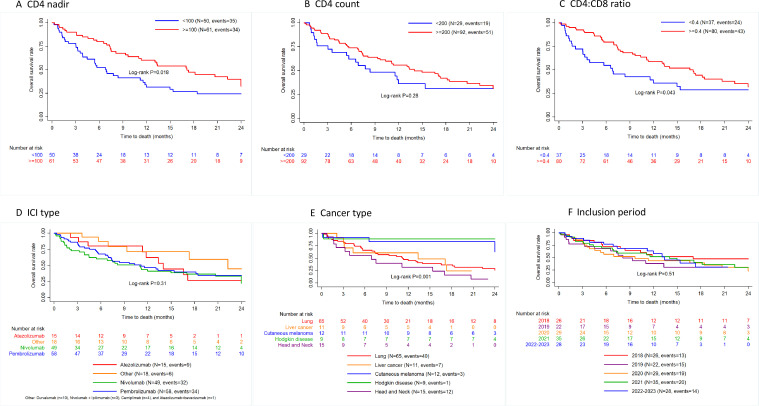
During follow-up, 81 participants died. The OS rates as estimated using Kaplan-Maier estimates were 68.6% (95% CI: 60.0% to 75.8%) at 6 months, 49.2% (95% CI: 40.1% to 57.6%) at 12 months and 39.3% (95% CI: 30.3% to 48.1%) at 18 months (online supplemental table S4). The OS rates differed according to cancer type, CD4 nadir and CD4:CD8 ratio levels. Participants with lung cancer (36.4%), liver cancer (24.2%) and head/neck cancer (15.9%) had a lower 18-month survival rates than those with melanoma (83.3%) and Hodgkin’s (88.9%). Participants with CD4 nadir ≥100 cells/µL (47.4%) and CD4:CD8 ratio ≥0.4 (44.3%) had higher survival rates as compared with those with CD4 nadir <100 cells/µL (26.8%) and CD4:CD8 ratio <0.4 (28.8%), respectively (figure 3 and online supplemental table S4). By contrast, no differences in survival rate was evidenced according to the inclusion period, the baseline CD4 count and the ICI type. Moreover, glucocorticoid use was not associated with survival.
Figure 3. Kaplan-Meier estimates of the probability of survival according to CD4 nadir (A); CD4 count at baseline (B); CD4:CD8 ratio at baseline (C); immune checkpoint inhibitor (ICI) type (D); cancer type (E) and inclusion period (F).
With regard to PFS rates, Kaplan-Meier estimates were 48.8% (95% CI: 40.1% to 57.0%) at 6 months, 32.3% (95% CI: 24.3% to 40.5%) at 12 months and 25.3% (95% CI: 17.8% to 33.5%) at 18 months (online supplemental table S4). PFS rates at month 18 were higher in participants with Hodgkin’s disease (98.5%) than in those with head/neck cancer (38.4%), melanoma (31.4%), liver (29.5%) and lung cancer (25.5%), p=0.002 and in those with a CD4 nadir ≥100 cells/µL (29.8%) versus 16.7% in those CD4 nadir <100 cells/µL, p=0.007. Inclusion period, baseline CD4 count, baseline CD4:CD8 ratio and ICI received were not associated with a significant difference in PFS (online supplemental appendix figure S1 and table S4).
Evolution of plasma viral load, CD4, CD8 and CD4:CD8 ratio
A median of 3 HIV RNA VLs assessments per participant (IQR: 1–5) were collected from 81 participants over the study follow-up. In participants with baseline HIV RNA<50 copies/mL, HIV viremia remains suppressed in 57/67 (85.1%) throughout follow-up and 62/67 (92.5%) had their last VL<50 copies/mL (online supplemental table S5 and figure S2A). Of the 14 participants with baseline HIV RNA≥50 copies/mL, 11/14 (78.6%) achieved VL<50 copies/mL during the follow-up and 9/14 (64.3%) had the last VL<50 copies/mL (online supplemental table S5 and figure S2B). There was no significant change in CD4 and CD8 T cells counts and CD4:CD8 ratio over time, respectively, −20 cells/µL, p=0.062 and −44 cells/µL, p=0.17, and −0.003, p=0.33 (online supplemental table S6).
Changes in antiretroviral therapy
During follow-up, 9 of the 140 participants (6.4%) discontinued their baseline ART and switched to another ARV regimen in the context of viral suppression. Changes were proposed during multidisciplinary meetings mainly to prevent potential toxicity of cancer therapy, minimize drug interaction and improve participants’ quality of life (online supplemental table S7).
Discussion
As HIV can be considered a chronic disease when HIV viral replication is durably suppressed, prolonged life together with persistent damages in the immune system, high prevalence of persistent oncogenic viruses and increased exposure hazards have moved cancer as a leading cause of death in PWH and a new challenge for clinical management of both entities, cancer and HIV. The ONCOVIH network, which gathers all types of expertize required for optimal management of the different cancers in PWH, has set-up this national prospective OncoVIHAC cohort to evaluate in real life and on a large scale the safety of the use of ICI in this double context of HIV infection and cancer.
The ANRS CO24 OncoVIHAC cohort study conducted in France has encouraged the deployment through this prospective, real-world national cohort to assess the safety of PWH receiving ICI for different types of cancer in PWH within thanks to close collaboration set-up through the ONCOVIH network between oncologists, infectiologists and pharmacologists, within the framework of regular national or regional interdisciplinary meetings. Our results over a 2-year follow-up showed a proportion of PWH experiencing a severe irAEs between 13.8% (95% CI: 8.8% to 21.4%) at 6 months, 15.0% (95% CI: 9.6% to 22.9%) at 12 months and 18.7% (95% CI: 12.1% to 28.3%) at 18 months. This is in agreement with the results of irAEs in HIV-uninfected populations demonstrating that such therapies can be used in PWH who did not experience excessive treatment-related immune toxicities, as the majority of events were reversible after systemic glucocorticoid use, and then safely managed. Indeed, grade ≥3 irAEs occur in approximately 5–30% of patients in the general population.21 Furthermore, in a systematic review and meta-analysis of data from 36 phase II and III randomized controlled trials (n=15 370) involving HIV-uninfected people with cancer,22 grade ≥3 irAEs were 15.1% for atezolizumab, 14.1% for nivolumab, 19.8% for pembrolizumab and 28.6% for ipilimumab, as observed in the present analysis. In contrast, the study by El Zarif et al23 in PWH reported a relatively lower incidence rate of grade ≥3 irAEs of 7.7% at 6 months compared with our results. This difference could be explained by the retrospective nature of the first study, a source of a selection bias and result in the omission of some events. The incidence of fatal-ICI-associated AEs in our study was rare (0.7%), close to that reported in HIV-uninfected individuals, ranging from 0.3% to 1.3%.24
Our study suggests that the incidence of irAEs was lower with nivolumab than with other ICI, as shown in another study.25 In addition, viral infections, such as CMV could play a role in remodeling the tumor’s immune microenvironment, altering the host immune response and thus favoring the development of irAEs.26 Surgery can also produce immunogenic lesions on non-malignant host cells, and is therefore likely to induce irAEs. Lower CD4 cell count and longer duration since HIV diagnosis played a role in the incidence of serious treatment-related toxicity.
Data on the effect of CD4+T cell count on irAEs are conflicting. Some studies suggest that low CD4+T cell counts in participants receiving ICI therapy may be associated with a higher risk of irAEs27 whereas other suggest that a higher CD4+T cell counts prior to receiving ICI therapy may be associated with a higher risk of irAEs.23 In this prospective cohort study, we showed that participants with CD4 T cell count <200/µL at ICI initiation have a higher risk of irAEs, which is in line with the first hypothesis.
The onset of immune-mediated toxicities was followed by discontinuation of immunotherapy in most cases in the study, in line with the literature, which recommends discontinuation of systemic therapy in the event of grade 3–4 toxicity, as well as moderate-dose to high-dose corticosteroid therapy.28 29
Over the course of the study, 81 participants died, leading to a survival rate of 68.6% at 6 months and 49.2% at 12 months. Individuals with cutaneous melanoma, Hodgkin’s, CD4 nadir ≥100 cells/µL and CD4:CD8 ratio ≥0.4, appeared to have a higher survival rate than others. These results are in line with those reported by El Zarif et al, among whom participants with melanoma had a higher survival rate than participants with other tumor types. Our results confirm the importance of CD4 count and CD4:CD8 ratio in the prognosis of PWH with cancer, as ICI treatment did not reduce the high risk of death in PWH with cancer whose CD4 count and CD4:CD8 ratio were low. This reinforces the absolute need to treat HIV-infected individuals before massive damage to the immune system occurs.
Our study is entirely reassuring, confirming that ICI treatment has no deleterious impact on HIV virological control and immunological parameters in this population.1516 30,32 HIV remained suppressed during ICI treatment. Indeed, 85.1% of participants maintained undetectable HIV-RNA during ICI treatment. In addition, CD4 and CD8 counts, as well as the CD4:CD8 ratio, remained stable. Indeed, as CD4 cell count and HIV VL depend strongly on HIV treatment, these results are expected as the ARV treatment also remained stable over time with only nine participants changing their baseline ARV treatment.
Our study has several limitations: First, our cohort does not include a population of HIV-uninfected participants with cancers to estimate whether HIV infection plays a role in the incidence of immune-related toxicity in PWH with cancer. Second, the presence of missing data for certain important variables, including CD4 nadir, CD4 count, CD4:CD8 ratio and duration of ART, can have an impact on the multivariate analysis, even if the missing data have been imputed. Third, women and individuals PWH from sub-Saharan origin were poorly represented, possibly due to a younger age and thus less at risk of age-related cancers, which may limit the generalization of our results. However, one key strength of this ongoing cohort is that it is prospective and real-world, that it has enabled a prospective evaluation of the safety of these treatments, and that it has strengthened close collaboration between infectiologists, oncologists and pharmacologists to optimize the management of PWH with cancer.
Fourth, the study follow-up was too short (9.2 months) to cover all the potential long-term side effects of ICIs. However, of the 34 grade ≥3 irAEs, 29 occurred within the first 6 months of follow-up and the remaining 5 irAEs occurred after 10 months of follow-up. This suggests that severe irAEs occur early in ICI treatment and that the potential long-term side effect of ICI appears to be limited. Furthermore, when we assessed the relationship between ICI exposure and irAE, we found that the risk of developing severe irAE decreased by 63% after 10 months of ICI treatment compared with less than 10 months (IRR 0.37, 95% CI: 0.14 to 0.95).
In conclusion, this prospective real-life cohort study has added to current knowledge concerning the incidence and risk factors for severe irAE, as well as survival according to cancer type in PWH receiving ICI. The study showed that a low CD4 count, a longer time since HIV diagnosis, a history of cancer surgery and positive CMV serology at the start of ICI were risk factors for the development of severe irAEs. These data also suggest that ICI treatment has no impact on HIV control, with no disruption in control of viral replication and no decrease in CD4 T-cell count. The survival benefit for PWH with melanoma and Hodgkin’s disease after ICI is encouraging and reinforces the value of these treatments in this population.
supplementary material
Acknowledgements
We thank the participants of this study for their time and dedication to this research for the benefit of their community. We thank our event review committee for continuous support. We also thank the data management and statistical analysis staff who made this study possible through continuous interactions with study staff at study sites and between visits.
Footnotes
Funding: This study was funded by the ANRS/MIE Maladies Infectieuses Emergentes (National Agency for Research on AIDS and Emerging Infectious Diseases).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: The protocol was approved by the ethics committee of the CPP Sud-Ouest et de l’Outre-mer IV. All participants gave written informed consent to take part in this study registered under ClinicalTrials.gov (NCT03354936). Participants gave informed consent to participate in the study before taking part.
Data availability free text: Data requests can be submitted to the scientific committee of the ANRS CO24 ONCOVIHAC Study (Corresponding author; lambert.assoumou@inserm.fr) and need to be approved by the French data protection authority (la Commission Nationale de l’Informatique et des Libertes (CNIL)) and ANRS Maladies Infectieuses Emergentes. French law requires that those who wish to access cohort or clinical study data require permission from CNIL (for more on CNIL data permissions see https://www.cnil.fr/) by completing a form that can be provided by the coauthor LA (lambert.assoumou@iplesp.upmc.fr). The scientific committee will evaluate each proposal for compatibility with general objectives, ethical approval, and informed consent forms of the ANRS CO24 OncoVIHAC project, and for possible overlap with ongoing work. De-identified participant data and the study protocol (including informed consent forms) can be made available upon request, 1 year after publication of this manuscript.
Collaborators: on behalf of the ANRS CO24 OncoVIHAC Study Group.
Contributor Information
Lambert Assoumou, Email: lambert.assoumou@iplesp.upmc.fr.
Raghiatou Baldé, Email: raghiatou.balde@iplesp.upmc.fr.
Christine Katlama, Email: christine.katlama@aphp.fr.
Baptiste Abbar, Email: baptiste.abbar@aphp.fr.
Pierre Delobel, Email: delobel.p@chu-toulouse.fr.
Thierry Allegre, Email: tallegre@ch-aix.fr.
Armelle Lavole, Email: armelle.lavole@aphp.fr.
Alain Makinson, Email: a-makinson@chu-montpellier.fr.
Olivia Zaegel-Faucher, Email: Olivia.ZAEGEL@ap-hm.fr.
Laurent Greillier, Email: laurent.greillier@ap-hm.fr.
Cathia Soulie, Email: cathia.soulie-ext@aphp.fr.
Marianne Veyri, Email: marianne.veyri@aphp.fr.
Mathilde Bertheau, Email: mathilde.bertheau@inserm.fr.
Michèle Algarte Genin, Email: michele.genin@inserm.fr.
Séverine Gibowski, Email: severine.gibowski@inserm.fr.
Anne-Geneviève Marcelin, Email: anne-genevieve.marcelin@aphp.fr.
Kevin Bihan, Email: kevin.bihan@aphp.fr.
Marine Baron, Email: marine.baron@aphp.fr.
Dominique Costagliola, Email: dominique.costagliola@iplesp.upmc.fr.
Olivier Lambotte, Email: olivier.lambotte@aphp.fr.
Jean-Philippe Spano, Email: jean-philippe.spano@aphp.fr.
Data availability statement
Data are available upon reasonable request.
References
- 1.Trickey A, Sabin CA, Burkholder G, et al. Life expectancy after 2015 of adults with HIV on long-term antiretroviral therapy in Europe and North America: a collaborative analysis of cohort studies. Lancet HIV. 2023;10:e295–307. doi: 10.1016/S2352-3018(23)00028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 3.Abbar B, Baron M, Katlama C, et al. Immune checkpoint inhibitors in people living with HIV: what about anti-HIV effects? AIDS. 2020;34:167–75. doi: 10.1097/QAD.0000000000002397. [DOI] [PubMed] [Google Scholar]
- 4.Guihot A, Cadranel J, Lambotte O, et al. Biological follow-up of patients with HIV treated with anti-PD-1 or anti-PD-L1 for non-small cell bronchial carcinoma: a task group proposal. Rev Mal Respir. 2016;33:419–21. doi: 10.1016/j.rmr.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Makinson A, Tenon J-C, Eymard-Duvernay S, et al. Human immunodeficiency virus infection and non-small cell lung cancer: survival and toxicity of antineoplastic chemotherapy in a cohort study. J Thorac Oncol. 2011;6:1022–9. doi: 10.1097/JTO.0b013e318217b6e0. [DOI] [PubMed] [Google Scholar]
- 6.Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27:559–74. doi: 10.1093/annonc/mdv623. [DOI] [PubMed] [Google Scholar]
- 7.Vora KB, Ricciuti B, Awad MM. Exclusion of patients living with HIV from cancer immune checkpoint inhibitor trials. Sci Rep. 2021;11:6637. doi: 10.1038/s41598-021-86081-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hleyhel M, Belot A, Bouvier AM, et al. Risk of AIDS-defining cancers among HIV-1-infected patients in France between 1992 and 2009: results from the FHDH-ANRS CO4 cohort. Clin Infect Dis. 2013;57:1638–47. doi: 10.1093/cid/cit497. [DOI] [PubMed] [Google Scholar]
- 9.Hleyhel M, Hleyhel M, Bouvier AM, et al. Risk of non-AIDS-defining cancers among HIV-1-infected individuals in France between 1997 and 2009: results from a French cohort. AIDS. 2014;28:2109–18. doi: 10.1097/QAD.0000000000000382. [DOI] [PubMed] [Google Scholar]
- 10.Hunt PW, Lee SA, Siedner MJ. Immunologic Biomarkers, Morbidity, and Mortality in Treated HIV Infection. J Infect Dis. 2016;214 Suppl 2:S44–50. doi: 10.1093/infdis/jiw275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexandrova Y, Costiniuk CT, Jenabian MA. Pulmonary Immune Dysregulation and Viral Persistence During HIV Infection. Front Immunol. 2021;12:808722. doi: 10.3389/fimmu.2021.808722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iordache L, Launay O, Bouchaud O, et al. Autoimmune diseases in HIV-infected patients: 52 cases and literature review. Autoimmun Rev. 2014;13:850–7. doi: 10.1016/j.autrev.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Bourgarit A, Carcelain G, Martinez V, et al. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20:F1–7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 14.Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature New Biol. 2009;458:206–10. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Cao M, Morán T, Dalmau J, et al. Assessment of the Feasibility and Safety of Durvalumab for Treatment of Solid Tumors in Patients With HIV-1 Infection: The Phase 2 DURVAST Study. JAMA Oncol. 2020;6:1063–7. doi: 10.1001/jamaoncol.2020.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavole A, Mazieres J, Schneider S, et al. Assessment of nivolumab in HIV-Infected patients with advanced non-small cell lung cancer after prior chemotherapy. The IFCT-1602 CHIVA2 phase 2 clinical trial. Lung Cancer (Auckl) 2021;158:146–50. doi: 10.1016/j.lungcan.2021.05.031. [DOI] [PubMed] [Google Scholar]
- 17.Uldrick TS, Gonçalves PH, Abdul-Hay M, et al. Assessment of the Safety of Pembrolizumab in Patients With HIV and Advanced Cancer-A Phase 1 Study. JAMA Oncol. 2019;5:1332–9. doi: 10.1001/jamaoncol.2019.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarif TE, Nassar A, Adib E, et al. 437 Safety and efficacy of immune checkpoint inhibitors (ICI) in patients living with HIV (PLWH) and metastatic non-small cell lung cancer (NSCLC): a matched cohort study from the international CATCH-IT consortium. SITC 37th Annual Meeting (SITC 2022) Abstracts; Nov, 2022. [DOI] [Google Scholar]
- 19.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature New Biol. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 20.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 21.Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563–80. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 22.Xu C, Chen Y-P, Du X-J, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ. 2018;363:k4226. doi: 10.1136/bmj.k4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Zarif T, Nassar AH, Adib E, et al. Safety and Activity of Immune Checkpoint Inhibitors in People Living With HIV and Cancer: a Real-World Report From the Cancer Therapy Using Checkpoint Inhibitors in People Living With HIV-International (CATCH-IT) Consortium. J Clin Oncol . 2023;41:3712–23. doi: 10.1200/JCO.22.02459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang DY, Salem J-E, Cohen JV, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4:1721–8. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang P-F, Chen Y, Song S-Y, et al. Immune-Related Adverse Events Associated with Anti-PD-1/PD-L1 Treatment for Malignancies: A Meta-Analysis. Front Pharmacol. 2017;8:730. doi: 10.3389/fphar.2017.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naigeon M, Roulleaux Dugage M, Danlos F-X, et al. Human virome profiling identified CMV as the major viral driver of a high accumulation of senescent CD8+ T cells in patients with advanced NSCLC. Sci Adv. 2023;9:eadh0708. doi: 10.1126/sciadv.adh0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myint PT, Ali FS, Verghese D, et al. The safety and efficacy of immune-checkpoint inhibitor therapy in HIV infected cancer patients. J C O. 2020;38:e15141. doi: 10.1200/JCO.2020.38.15_suppl.e15141. [DOI] [Google Scholar]
- 28.Thompson JA, Schneider BJ, Brahmer J, et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:387–405. doi: 10.6004/jnccn.2022.0020. [DOI] [PubMed] [Google Scholar]
- 29.Thompson JA, Schneider BJ, Brahmer J, et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020. J Natl Compr Canc Netw. 2020;18:230–41. doi: 10.6004/jnccn.2020.0012. [DOI] [PubMed] [Google Scholar]
- 30.Cook MR, Kim C. Safety and Efficacy of Immune Checkpoint Inhibitor Therapy in Patients With HIV Infection and Advanced-Stage Cancer: A Systematic Review. JAMA Oncol. 2019;5:1049–54. doi: 10.1001/jamaoncol.2018.6737. [DOI] [PubMed] [Google Scholar]
- 31.Castelli V, Lombardi A, Palomba E, et al. Immune Checkpoint Inhibitors in People Living with HIV/AIDS: Facts and Controversies. Cells. 2021;10:2227. doi: 10.3390/cells10092227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajdev L, Jackie Wang C-C, Joshi H, et al. Assessment of the safety of nivolumab in people living with HIV with advanced cancer on antiretroviral therapy: the AIDS Malignancy Consortium 095 Study. Cancer. 2024;130:985–94. doi: 10.1002/cncr.35110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.