Abstract
Treatment failure after intravesical instillation of Bacillus Calmette-Guerin immunotherapy (BCG) for non-muscle-invasive bladder cancer (BCa) occurs frequently. The exact effects of BCG on cellular redox status and gene expression remain unclear. We assessed oxidative stress biomarkers and changes in miR-155-5p expression in response to BCG. Twenty-seven patients with BCa were recruited for measuring tissue and serum malondialdehyde (MDA) and total antioxidant capacity (TAC) levels, and tissue expression of miR-155-5p at two-time points: pre and 6 weeks post BCG. Recurrence of BCa was observed after 20 months. R statistical software was used for paired comparisons of biomarkers, as well as the correlation between variables. Significant increases in TAC were observed after BCG (P= <0.001). Tissue MDA levels were significantly reduced (P= 0.003). miR-155-5p was slightly overexpressed after BCG (median fold change=1.3, P=0.25). At the 20-month follow-up, it was observed that improved MDA and TAC changes were significant only in patients without recurrence of BCa. In patients with recurrence, the pre-treatment expression ratio of miR-155-p5 was positively correlated with TAC (R=0.63, P= 0.032) and negatively correlated with MDA (R=-0.72, P=0.037). In patients with recurrence of BCa pre-treatment miR-155-5p showed negative correlation with its expression changes after BCG (R=-0.78, P=0.004). Conclusions: Treatment with BCG has some beneficial effects on the oxidative stress status, which is probably modulated by miR-155-5p. A well-controlled oxidative balance may enhance overall survival of BCa. Considering its high recurrence rate, our pilot experiment can open a window toward better management of patients with BCa.
Key Words: Bacillus Calmette-Guerin, bladder cancer, malondialdehyde, miR-155-5p, total antioxidant capacity
Introduction
Urothelial carcinoma of the bladder (BCa), one of the most common malignancies worldwide, is divided into two general categories: non-muscle-invasive bladder cancer (NMIBC) and muscle invasive bladder cancer (MIBC) (1). NMIBC which accounts for the most newly diagnosed bladder cancer patients is limited to the epithelium or the subepithelial connective tissue of the bladder and includes stages (Ta, Tis and T1), whereas MIBC includes stages T2, T3 and T4 (1, 2). The progression of NMIBC to MIBC is known to result in worse prognosis.
Transurethral resection along with intravesical instillations of Bacillus Calmette-Guerin immune-therapy (BCG) for six weeks are recommended as the main strategies for the treatment of high-risk NMIBC patients (2). BCG seems to act by activating the innate and adaptive immune systems (3, 4). Cellular redox status, gene expression, signaling pathways and tumor biology may be affected by BCG. BCG internalization causes the recruitment of immune cells such as T-cytotoxic and phagocyted into the bladder. The release of chemokines and cytokines occurs as a result of immune cell activation (5). Despite the high effectiveness rate, failure to respond to BCG therapy, recurrence and progression of the disease are inevitable in many cases (6).
Redox imbalance potentially is a well-known risk factor for the development and exacerbation of malignancies. Oxidative stress imbalance, lipid peroxidation and nitric oxide formation in bladder cancer cells following BCG have been reported previously (7, 8). Nevertheless, it is safe to mention that the efficient use of consequences such as the creation of oxidative stress can be considered an effective therapeutic approach in destroying cancer cells. Shah et al. reported the intracellular production of stress-induced free radicals and changes in cellular redox status by the generation of H2O2 followed by BCG instillations as a requirement for increasing BCG efficacy (9).
The advent of genomic and epigenomic studies, especially in the field of up and downregulated expression of genes involved in metabolism-related pathways, can open a window toward better understanding the pathogenesis of diseases. Although the whole genome can be transcribed to RNAs, only a small portion of the transcriptome encodes proteins, most of which are non-coding RNAs (ncRNAs). Accumulating evidence suggests that the aberrant expression of ncRNAs is useful for identifying oncogenic or tumor suppressing pathways and can serve as diagnostic, prognostic or therapeutic target biomarkers. Kim et al., using microarray gene expression profiling of BCa patients who were treated with BCG, demonstrated that lots of genes were significantly different between BCa patients who responded to treatment and those with BCa recurrence. Further analysis by Kim et al. revealed the annotations of most genes involved in cellular immune response, growth, proliferation and inflammatory response (10).
Micro-RNAs (miRNAs) are small non-coding RNAs which modulate gene expression at the post-transcriptional level. Their dysregulations have frequently been reported as important diagnostics, prognostics or therapeutic target biomarkers. miR-155-5p, encoded by MIR155 host gene (MIR155HG), is known as a proinflammatory and oncogenic miRNA which its upregulated expression has been reported in lymphocytes and also macrophages (11). Diagnostic and prognostic value of miR-155 has been investigated in various types of cancers including leukemia, and pancreatic, breast and colorectal cancer (12). Aberrant expression of miR-155 has been reported to be associated with oxidative stress in cardiac and pulmonary diseases (13).
Overexpression of miR-155 results in cellular proliferation is through activating Wnt/β-catenin signaling (14). In addition, antiapoptotic properties are attributed to miR-155 since it can block Caspase 3 mediated apoptosis (15). The production of miR-155 induced by Kirsten rat sarcoma 2 viral oncogene homolog (K-Ras) activation is another proposed mechanism which results in the reduced production of antioxidant enzymes, accumulation of free reactive radicals and oxidative imbalance (16).
As mentioned above, BCG causes altering the redox status of the host cells. Increased expression of stress induced genes and activation of sensitive related signaling pathways are expected outcomes of such events. Nuclear factor erythroid 2 (NF-E2)- related factor 2 (Nrf2) is a cytoplasmic transcription factor which induces the expression of antioxidant related genes. In the physiological status, cytoplasmic Nrf2 is suppressed by binding to Kelch-like ECH-associated protein 1 (Keap1), an Nrf2 inhibitor. Oxidative stress induced by BCG results in segregation of Nrf2-Keap1 and the translocation of Nrf2 to the nucleus to induce the expression of antioxidant-related genes. On the other hand, overexpression of miR-155 contributes to oxidative damage by suppressing Nrf2 (17, 18).
To the best of our knowledge, there are no previous studies which focused on evaluating the oxidative stress status and miR-155-5p changes in BCa patients treated with BCG. Despite the high recurrence rate of BCa in patients treated with BCG, however, it remains still a curative strategy in patients with NMIBC. Highlighting the importance of adopting good management, the present pilot study aimed to investigate the possible effects of BCG immunotherapy on expression levels of miR-155-5p as well as lipid peroxidation and total antioxidant capacity.
Materials and methods
Study design
This study was approved by the ethics committee of our university (Ethic number: IR.MUBABOL. REC.1399.147). A total of 27 hospitalized patients with BCa signed a written informed consent form and participated in the study. Bladder cancer diagnosing and staging were performed by a trained pathologist according to cystoscopy and histopathological findings based on the World Health Organization guideline (19). Following the cystoscopy procedure, transurethral resection of the bladder tumor at initiation and 2-6 weeks apart is recommended for patients with Ta-high graded, T1 patients and those who have partially cancerous tissue after surgery (1).
The study participants included BCa patients with NMIBC (grade Ta-high grade and T1) whose BCG instillation was prescribed according to the European Organization for Research and Treatment of Cancer (EORTIC) scoring system for 6 weeks consecutive (20). Patients with a previous history of BCG immunotherapy, muscle invasive bladder cancer patients and those with Ta-low grade BCa were excluded. We also obtained BCa recurrence data in a 20-month follow-up.
Sampling and storage
Tissue specimens from participants were obtained surgically (Pre and 6 week post BCG immunotherapy) and transferred to the laboratory within nuclease-free tubes containing RNA later reagent (Yekta Tajhiz Azma, Iran). Blood samplings of patients with BCa were also performed before and after the 6th week of treatment with BCG (Pastocys, Pasteur Institute of Iran, Iran). Whole bloods were collected within gel-free clot activating tubes and were centrifuged (2500 g, 15 minutes, 4°C) and the resulting serums were isolated and stored at -80°C until analyses.
RNA extraction, cDNA synthesis and real time PCR
After total RNA extraction, real-time quantitative relative PCR (RT-qPCR) was performed to evaluate tissue miR-155-5p expression levels versus U6 small-nuclear RNA 1 (RNU6-1) as an internal control before and after BCG.
Total RNAs extraction was based on phenol/chloroform method. Briefly, approximately 50 mg of tissue specimens were homogenized in nucleases free water and applied to Reboex® reagent (Genall, Korea) and RNA extraction was performed according to the manufacturer’s instructions. The quality and quantity of the extracted RNA were verified using NanoDrop™ UV-Vis. spectrophotometer (Thermo Fisher Scientific Inc.).
Complementary DNA for miR-155-5p and RNU6-1 were synthetized specifically using 0.2 microgram RNA, unique stem-loop primers and MMLV-reverse transcriptase (Yekta Tajhiz, Tehran, Iran) commercial kit. RT-qPCR was subsequently performed by specific primers and Real-Q Plus 2x Master Mix Green (Ampliqon, Denmark) in a thermal cycler Rotor-Gene Q MDx thermocycler (QIAGEN, Hilden, Germany) system. Primer designing for the RT-qPCR, was performed using Oligo7 software (Molecular Biology Insights, Inc., Cascade, CO, USA) and Primer-BLAST (NCBI). Specific stem-loops and primer sequences are provided in Table 1.
Table 1.
Gene annotation and primer sequences for cDNA synthesis and qRT-PCR.
| hsa- miR-155-5p |
miRbase Accession
number: MIMAT0000646 |
Chromosome: chr21 |
|---|---|---|
| hsa-miR-155-5p Forward primer | CGGCGCTTAATGCTAATCGTGATAG | |
| hsa-miR-155-5p Stem-loop | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACCCCT | |
| U6 small nuclear 1 (RNU6-1) | Ensembl ID: ENSG00000206625 | Chromosome: chr15 |
| RNU6 Forward primer | GCTCGCTTCGGCAGCACATATAC | |
| RNU6-1 Stem-Loop | GTCGTTACCAGAGCAGGGTCCGAGGTATTCGCTCTGGTAACGACCGTGTCATCCTTG | |
| Universal reverse primer | GTGCAGGGTCCGAGGT | |
All experiments were performed in duplicate. The efficiency and exponential threshold of amplifications were determined for each sample individually using LinRegPCR software (Version 2021.2, MB, Amsterdam UMC, Netherland). Relative quantification of miR-155-5p expression levels was calculated using REST software.
Serum and tissue TAC assessment
Total antioxidant capacity in serum and tissue samples was measured by Ferric reducing antioxidant power (FRAP) assay according to the standard protocol (21). Basically, in the FRAP assay, the intensity of the colored product represents the amount of reduced Fe3+ which can be measured using spectrophotometer at 593 nm. The FRAP’s working solution contains dissolved 2, 4, 6 tripyridyl-s-triazine (TPTZ, Merck, Germany) in HCl 40 mM (Merk, India), FeCl3 20 mM (Merck, Darmstadt, Germany) and acetate buffer 0.3 M (containing Acetic acid and sodium acetate, Merck, Germany). Different concentrations of FeSO4 (Merck, Darmstadt, Germany) were used as standard solutions. Serum and homogenized tissue samples were mixed with a working solution and heated up to 37° C for 5 minutes to produce colored complex. Total antioxidant capacities of the samples were obtained by plotting a standard curve and comparing the absorbance of the samples with the standards.
Serum and tissue lipid peroxidation measurement
Cellular membrane is enriched with phospholipid molecules. Oxidative stress causes cellular damage and as a result, Malondialdehyde (MDA), by product of lipid peroxidation process, can be released into extracellular matrix and body fluids. MDA was measured spectrophotometry according to the Thiobarbituric acid reactive substance (TBARS, Sigma Aldrich, India) method (22). TBARS is based on the precipitation reaction between MDA, Trichloroacetic acid (Merck, Germany) and thiobarbituric acid (TBA), which produces a pink color and can be measured at 535 nm wavelength. An equal amount of homogenized tissue in solvent was mixed with TBA solution and boiled at water bath (90-100 °C) for 15 min. After incubation time, the tubes were chilled on ice, centrifuged at 1500 rpm for 10 min and the supernatants were separated for measuring the absorbances. An MDA standard solution was purchased and the MDA concentration of samples were calculated according to the standard curve.
Statistical analysis
Statistical analyses were performed using the R software (R Core Team 2023; https://www.R-project.org/). Descriptive statistical analyses were performed as frequencies and median values along with Q1 and Q3 quartiles (Q1-Q3). miR-155-5 p expression ratio versus RNU6-1 was determined based on the Pfaffl method using REST software (Qiagen, Hilden, Germany; Downloaded from http://www. REST. de.com) (23). The differences in tissue and serum MDA and TAC between pre and post BCG as well as recurrent BCa versus non-recurrent BCa were assessed using the Wilcoxon rank test and Mann-Whitney U test respectively. Participants were categorized into high and low miR-155-5p expression groups based on miR-155-5p expression ratio before treatment. Two-sided Fisher exact test and χ2 test were applied to compare the differences between clinical features between groups. Spearman correlation coefficients were calculated to determine the possible correlation between miR-155-5p expression ratio and other parameters. P-values less than 0.05 were considered statistically significant.
Results
Clinical characteristics and demographic information of participants
Our study participants included 27 patients with bladder cancer who received BCG immunotherapy. The clinical characteristics and demographic information of the study participants are summarized in Table 2. Among the participants, nine diabetic patients (33.3 %), 13 patients with hypertension (48 %), 8 patients with cardiovascular disease (29 %), 5 patients with hyperlipidemia (18.5 %) and 13 smoker (48 %) were observed. Further follow-up of participants within 20 months apart indicated that 12 patients had recurrence of BCa while 12 patients had no recurrence (the follow-up data of 3 patients were not accessible).
Table 2.
Association between miR-155-5p expression and clinical features of participants.
| Variable | Total Number | overexpressed | under expressed | P-value* |
|---|---|---|---|---|
| Gender | - | |||
| Female | 5 | 3 | 2 | |
| Male | 22 | 14 | 8 | |
| Recurrence | 1.00 | |||
| NO | 12 | 5 | 7 | |
| Yes | 12 | 4 | 8 | |
| Diabetes | 0.011 | |||
| NO | 18 | 4 | 14 | |
| Yes | 9 | 7 | 2 | |
| Hypertension | 0.252 | |||
| NO | 14 | 10 | 4 | |
| Yes | 13 | 6 | 7 | |
| Cardiovascular diseases | 0.4 | |||
| NO | 19 | 9 | 10 | |
| Yes | 8 | 2 | 6 | |
| Hyperlipidemia | 0.37 | |||
| NO | 22 | 8 | 14 | |
| Yes | 5 | 3 | 2 | |
| Smoking | 0.704 | |||
| NO | 14 | 5 | 9 | |
| Yes | 13 | 6 | 7 | |
| Number of Tumors | 1.00 | |||
| singular | 15 | 6 | 9 | |
| Multiple | 12 | 7 | 5 | |
| Grade | 0.618 | |||
| Low grade | 5 | 1 | 4 | |
| High grade | 22 | 10 | 12 | |
| Stage | 0.692 | |||
| T1 | 9 | 3 | 6 | |
| Ta | 18 | 8 | 10 |
Two-sided fisher exact test and Χ2 test were applied to compare the frequencies of participants with under/overexpression of miR-155-5p with different clinical features.
The expression levels of miR-155-5p before treatment were considered for dividing expression differences at cut-off=1.00 into two overexpression and under expression groups. A significant higher expression of miR-155-5p was observed in diabetic person (P=0.011). There were no significant differences in other clinicopathological features.
Tissue miR-155-5p expression levels before and after BCG immunotherapy
Differences in the expression levels of miR-155-5p were compared using the REST software. A slight but not significant over expression of miR-155-5p was observed with a median fold change of 1.3 (Q1-Q3: 0.57-2.08; and P=0.259).
Tissue and serum oxidative stress biomarkers before and after BCG immunotherapy
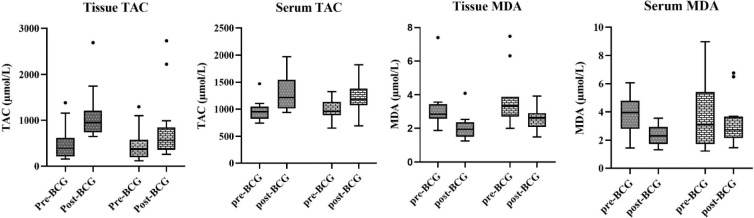
The differences between serum and tissue TAC and MDA levels were compared using Wilcoxon signed rank test (Figure 1). Both serum and tissue TAC levels were significantly higher after BCG (P = 0.003 and P < 0.001 respectively). A significant decrease in the tissue MDA levels was observed (P=0.003). Although the serum MDA levels was slightly lower after treatment, it was not significant (P=0.072). However, in a 20-month follow- up, such changes were not seen in patients with recurred BCa except tissue TAC (P-value=0.002). Based on BCa recurrence, the changes of MDA and TAC are presented in Table 3.
Fig. 1.
The comparison of TAC and MDA before and after BCG immunotherapy were assessed using Wilcoxon rank test.
Table 3.
Differences in tumor recurrence MDA and TAC before and after BCG immunotherapy.
| No Recurrent BCa | Recurrent BCa | P-value | ||
|---|---|---|---|---|
| Tissue TAC | Pre BCG | 389 (226.0-519.0) | 252 (168.5-460.5) | 0.360 |
| (µmol/L) Median | Post BCG | 949 (760-2691) | 604 (337.8-842.8) | 0.067 |
| (Q1-Q3) | P-value | 0.013 | 0.002 | |
| Tissue MDA | Pre BCG | 2.84 (2.62-3.32) | 3.34 (2.69-3.78) | 0.386 |
| (µmol/L) Median | Post BCG | 1.94 (1.53-2.18) | 2.68 (2.4-2.91) | 0.027 |
| (Q1-Q3) | P-value | 0.05 | 0.080 | |
| Serum TAC | Pre BCG | 955 (874.5-1059.5) | 915 (830.5-1009) | 0.489 |
| (µmol/L) Median | Post BCG | 1214 (1065-1535) | 1201 (1182-1424) | 0.964 |
| (Q1-Q3) | P-value | 0.008 | 0.086 | |
| Serum MDA | Pre BCG | 3.95 (3.12-4.57) | 4.70 (2.75-6.32) | 0.356 |
| (µmol/L) Median | Post BCG | 2.29 (1.73-2.76) | 2.87 (2.35-3.69) | 0.076 |
| (Q1-Q3) | P-value | 0.02 | 0.67 | |
The differences in tissue and serum MDA and TAC between pre and post BCG as well as recurred BCa versus no recurrence BCa were assessed using Wilcoxon rank test and Mann-Whitney U test, respectively.
Correlation between miR-155-5p and MDA and TAC
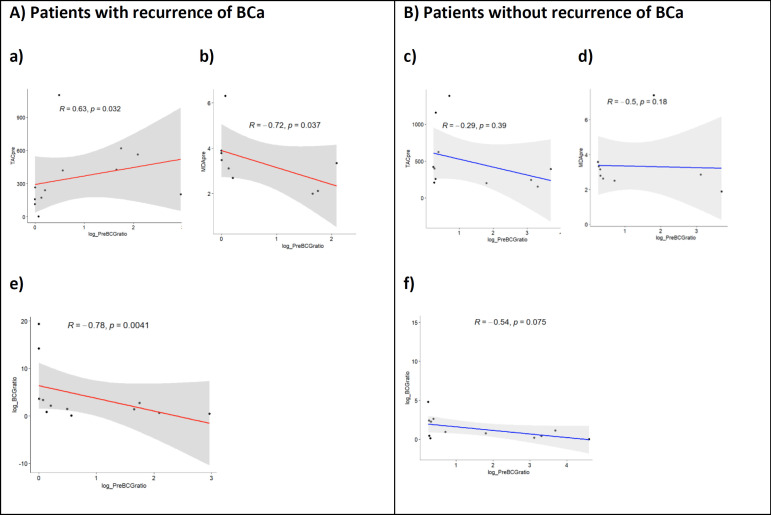
Based on the recurrence of BCA, non-parametric correlations between serum and tissue oxidative-related analytes and miR-155-5p expression levels were evaluated. In patients with recurrent BCA, significant negative and positive correlations were observed between pre-treatment expression levels of miR-155-5p and tissue MDA and TAC levels respectively (Figure 2, a-d). In addition, in patients with recurrent BCA, the expression levels of miR-155-5p were significantly correlated with its expression changes after BCG (P-value=0.004) (Figure 2 e, f).
Fig. 2.
Non-parametric correlation between serum and tissue oxidative related analytes and miR-155-5p expression levels was calculated using Spearman correlation coefficients test. R: spearman correlation coefficient; P: P-value.
Discussion
Twenty-seven patients diagnosed with bladder cancer (BCa) participated in this study. From each individual tissue and blood samples were prior to and following BCG immunotherapy. The levels of miR-155-5p in the tissue samples and serum MDA and tissue TAC levels were compared between the two-time points. The results showed a significant increase in tissue TAC levels and a decrease in tissue MDA levels after BCG treatment, which probably suggests an improvement in the oxidative stress status. Additionally, based on the results, we observed a slight overexpression of miR-155-5p after BCG treatment. Although, higher tissue and serum levels of TAC and lower MDA levels indicated an improvement in oxidative stress status after treatment; however, considering a 20-month follow-up of participants when TAC and MDA changes were evaluated, such changes could only be seen in patients without recurrence of BCa.
Although BCG immunotherapy is a common treatment strategy for patients with NMIBC, a high rate of BCa recurrence has been reported frequently (6). Some proposed mechanisms of BCG include activating the innate and adaptive immune system and acting through recruitment of macrophages and T- cytotoxic cells into bladder (3-5). These events affect tumor cell homeostasis, cellular signaling pathways and redox status. Redox imbalance which alters the production of factors involved in stress response is a well-known risk factor in the pathogenesis of bladder cancer (8, 9).
Induction of cell death is a proposed mechanism of BCG due to the induction of oxidative damage in urothelial cells. Oxidative imbalance which is induced by BCG is mainly the result of nitric oxide, free O2º and H2O2 production which are involved in urothelial cell damage (9). BCG causes the host’s immune response and activates oxidative stress sensitive signaling pathways, including Nrf2 and NF-κB (24, 25). Activation of Nrf2 results the overexpression of antioxidant related genes (17). As previously stated, higher oxidative damage and depletion of antioxidant repositories are expected in BCG affected cells. Despite the above mentioned mechanism of BCG, higher antioxidant capacity and lower lipid peroxidation were significant in our study which probably indicated the activation of body antioxidant pathways. Compensatory activation of Nrf2 and other antioxidative signaling pathways are probable justifications for the improved oxidative stress status after treatment.
The versatile controversial and intricated function of miR-155 has been reported previously (11). It is mainly expressed in macrophages and lymphocytes and has multifaceted functions as oncomiR, tumor suppressor, and proinflammatory (11, 14, 26, 27).
The oncogenic feature of miR-155 is probably due to its antiapoptotic and cell proliferative effects. Tumor cell growth and proliferation attributed properties of miR-155-5p is mediated through activating canonical Wnt signaling which increases nuclear and cytoplasmic β-catenin and leading tumorigenesis (14). The antiapoptotic effect of miR-155 which is related to blocking Caspase-3 activity is another proposed oncogenic feature of miR-155. Caspase 3 mediated apoptosis occurs through re-expression of tumor protein 53-induced nuclear protein 1 (TP53INP1), a stress-induced p53 target gene. miR-155 by targeting TP53INP1 inhibits apoptosis (15).
In a study by Wang et al. up-regulation of miR-155 was reported due to K-ras signaling activation. K-ras by activating Mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-κB) signaling pathway causes increased expression of miR-155 which potentially inhibits the expression of Forkhead box class O 3a (FOXO3a). Therefore, the production of two main antioxidant enzymes, catalase and superoxide dismutase (SOD), which are dependent to FOXO3a negatively regulates and consequently results in accumulating free reactive oxidant species (16). miR-155 can inhibit the activity of Nrf2 by two mechanisms: First, direct repression of Nrf2 and second, through enhancing the cellular levels of Keap1, the inhibitor of Nrf2 (28). This inhibitory effect represses the expression of genes related to the body’s defense against oxidative stress (18). As inferred, the stress-induced expression of miR-155 can modulate oxidative imbalance by suppressing Nrf2. In our study, expression changes of miR-155-5p, increase of antioxidant capacity and decreased lipid peroxidation changes were not significant in patients with recurred BCa. However, in patients who responded to BCG, a significant decrease in lipid peroxidation status and increased total antioxidant capacity were observed. This may be due to insufficient expression of Nrf2, defect in producing antioxidant enzymes or any underlying personal properties (e.g., genetic susceptibility) that affect response to treatment.
We could not find any previous study that focused on the effect of BCG immunotherapy on the expression levels of miR-155-5p as well as body oxidative status. As discussed above, BCG induces accumulation of immune cells and thus, results in overexpression miR-155-5p. Overexpression on miR-155-5p which modulates oxidative damage of BCG in affected cells and also higher MDA and lower TAC status were expected in our study. No significant correlation was seen between miR-155-5p expression level with MDA and TAC levels. In our pilot study, the possible association between oxidant/antioxidant changes were evaluated only based on MDA and TAC changes. It is possible that other oxidant/antioxidant biomarkers, for example SOD and catalase, nitric oxide or even 8-hydroxyguanosine (8-OHdG) levels show a greater correlation with miR-155-5p levels.
Regarding the established higher expression levels of miR-155 in malignant tissue, we evaluated miR-155-5p expression exclusively in BCa patients and only twice sampling in our pilot study was performed. Our results need to be investigated in another larger-scale sample size and time series analysis. Moreover, using a control group for comparing the miR-155-5p expression level between healthy individuals or adjacent normal tissue may have results with more strength.
In conclusion, it seems the oxidative status of BCa patients is affected by BCG immunotherapy. Increased miR-155-5p expression, higher antioxidant capacity and lower MDA levels were seen as possible biomarkers which can be seen in most of BCa patients whose treatment with BCG was successful. The research implies that BCG therapy may enhance oxidative stress levels, potentially influenced by miR-155-5p expression. A balanced oxidative state can improve the overall survival of bladder cancer patients. The study demonstrates the potential for improved management of NMIBC patients with BCG immunotherapy, given the high recurrence rate of the disease.
Acknowledgements
The authors are thankful to the following; all participants in the study, to Atiyeh Al-e-Ahmad for her assistance in molecular analyses and to the Vice-Chancellery for Research for financially supporting his project. The present research was supported by Babol University of Medical Sciences, Babol, Iran [grant number: 9809119].
References
- 1.Babjuk M, Burger M, Capoun O, et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ) Eur Urol. 2022;81:75–94. doi: 10.1016/j.eururo.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Chang SS, Boorjian SA, Chou R, et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol. 2016;196:1021–9. doi: 10.1016/j.juro.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 3.Lim CJ, Nguyen PHD, Wasser M, et al. Immunological Hallmarks for Clinical Response to BCG in Bladder Cancer. Front Immunol. 2020;11:615091. doi: 10.3389/fimmu.2020.615091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han J, Gu X, Li Y, et al. Mechanisms of BCG in the treatment of bladder cancer-current understanding and the prospect. Biomed Pharmacother. 2020;129:110393. doi: 10.1016/j.biopha.2020.110393. [DOI] [PubMed] [Google Scholar]
- 5.Luo Y, Askeland EJ, Newton MR, et al. Immunotherapy of urinary bladder carcinoma: BCG and beyond. Cancer Treat‐Conventional Innovative Approaches. 2013;361:55283. [Google Scholar]
- 6.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–49. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 7.Rahmat JN, Esuvaranathan K, Mahendran R. Bacillus Calmette-Guerin induces cellular reactive oxygen species and lipid peroxidation in cancer cells. Urology. 2012;79:1411 e15–20. doi: 10.1016/j.urology.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Hosseini A, Koskela LR, Ehren I, et al. Enhanced formation of nitric oxide in bladder carcinoma in situ and in BCG treated bladder cancer. Nitric Oxide. 2006;15:337–43. doi: 10.1016/j.niox.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Shah G, Zielonka J, Chen F, et al. H2O2 generation by bacillus Calmette-Guerin induces the cellular oxidative stress response required for bacillus Calmette-Guerin direct effects on urothelial carcinoma biology. J Urol. 2014;192:1238–48. doi: 10.1016/j.juro.2014.05.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YJ, Ha YS, Kim SK, et al. Gene signatures for the prediction of response to Bacillus Calmette-Guerin immunotherapy in primary pT1 bladder cancers. Clin Cancer Res. 2010;16:2131–7. doi: 10.1158/1078-0432.CCR-09-3323. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Wang Q, Guan Y, et al. Breast cancer cell-derived microRNA-155 suppresses tumor progression via enhancing immune cell recruitment and antitumor function. J Clin Invest. 2022:132. doi: 10.1172/JCI157248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Men CP. Correlation of Increased Expression of MicroRNA-155 in Bladder Cancer and Prognosis. Lab Med. 2015;46:118–22. doi: 10.1309/LMWR9CEA2K2XVSOX. [DOI] [PubMed] [Google Scholar]
- 13.Climent M, Viggiani G, Chen YW, et al. MicroRNA and ROS Crosstalk in Cardiac and Pulmonary Diseases. Int J Mol Sci. 2020:21. doi: 10.3390/ijms21124370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Li M, Zuo K, et al. Upregulated miR-155 in papillary thyroid carcinoma promotes tumor growth by targeting APC and activating Wnt/beta-catenin signaling. J Clin Endocrinol Metab. 2013;98:E1305–13. doi: 10.1210/jc.2012-3602. [DOI] [PubMed] [Google Scholar]
- 15.Gironella M, Seux M, Xie MJ, et al. Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proc Natl Acad Sci U S A. 2007;104:16170–5. doi: 10.1073/pnas.0703942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang P, Zhu CF, Ma MZ, et al. Micro-RNA-155 is induced by K-Ras oncogenic signal and promotes ROS stress in pancreatic cancer. Oncotarget. 2015;6:21148–58. doi: 10.18632/oncotarget.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tebay LE, Robertson H, Durant ST, et al. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic Biol Med. 2015;88:108–46. doi: 10.1016/j.freeradbiomed.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimta AA, Cenariu D, Irimie A, et al. The Role of Nrf2 Activity in Cancer Development and Progression. Cancers (Basel) 2019:11. doi: 10.3390/cancers11111755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babjuk M, Burger M, Comperat EM, et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ) - 2019 Update. Eur Urol. 2019;76:639–57. doi: 10.1016/j.eururo.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Cambier S, Sylvester RJ, Collette L, et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1-3 Years of Maintenance Bacillus Calmette-Guerin. Eur Urol. 2016;69:60–9. doi: 10.1016/j.eururo.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 21.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 22.Rael LT, Thomas GW, Craun ML, et al. Lipid peroxidation and the thiobarbituric acid assay: standardization of the assay when using saturated and unsaturated fatty acids. J Biochem Mol Biol. 2004;37:749–52. doi: 10.5483/bmbrep.2004.37.6.749. [DOI] [PubMed] [Google Scholar]
- 23.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen F, Zhang G, Cao Y, et al. A synthetic polyvalent ligand for alpha5beta1 integrin activates components of the urothelial carcinoma cell response to bacillus Calmette-Guerin. J Urol. 2013;189:1104–9. doi: 10.1016/j.juro.2012.08.218. [DOI] [PubMed] [Google Scholar]
- 25.Shah G, Zhang G, Chen F, et al. iNOS expression and NO production contribute to the direct effects of BCG on urothelial carcinoma cell biology. Urologic Oncology: Seminars and Original Investigations. Elsevier; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong W, He L, Richards EJ, et al. Upregulation of miRNA-155 promotes tumour angiogenesis by targeting VHL and is associated with poor prognosis and triple-negative breast cancer. Oncogene. 2014;33:679–89. doi: 10.1038/onc.2012.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C, Jiang X, Gu S, et al. MicroRNA-155 regulates arsenite-induced malignant transformation by targeting Nrf2-mediated oxidative damage in human bronchial epithelial cells. Toxicol Lett. 2017;278:38–47. doi: 10.1016/j.toxlet.2017.07.215. [DOI] [PubMed] [Google Scholar]