Abstract
Seeking a new drug has become a significant milestone in drug discovery. However, it might not be immediately used in urgent situations or during a pandemic. Acute Respiratory Distress Syndrome (ARDS) contributes to mild-to-severe symptoms in patients due to cytokine storms, leading to morbidity and mortality. Hypertension is recognized as an independent risk factor for the severity of ARDS regarding to both ACE Inhibitors (ACEIs) and Angiotensin Receptor Blockers (ARBs) treatment, although the precise mechanism remains unclear. In this study, murine macrophage cell lines (RAW264.7) and alveolar epithelial type II-like cell lines (A549) were utilized to investigate the effect of Losartan (LOS). LOS attenuated nitric oxide production in a dose-dependent manner and collectively reduced intracellular reactive oxygen species (ROS) compared to Diclofenac under LPS-stimulation conditions. For ADRS-mimicking conditions, LPS-induced inflammatory A549 cells were performed to monitor the effect of LOS. The results showed that LOS exhibited a protective effect by increasing cell viability and decreasing intracellular ROS levels. Notably, a high dose of LOS increased intracellular ROS levels. Moreover, LOS treatment downregulated NF-kappaB activation and AT1R at the protein level. Correspondingly, proinflammatory mediator cytokines (TNF-alpha and IL-8) were downregulated, but not IL-6, during LOS treatment. Hence, LOS may provide substantial benefits to ARDS patients by modulating proinflammatory cytokine production through AT1R downregulation and NF-kappaB inactivation. The mechanistic insight into LOS’s anti-inflammatory effect holds promise for reducing mortality rates among ARDS patients.
Key Words: Losartan, lung, inflammation, LPS, hypertension
Introduction
Currently, toxic environments, biological hazards, and chemical exposures have a worldwide impact on human health, leading to the pathogenesis of chronic inflammation. Acute Respiratory Distress Syndrome (ARDS) and cytokine storms primarily exhibit a range of severity from mild to severe, contributing significantly to morbidity and mortality (1). Angiotensin Receptor Blockers (ARBs) are oral drugs commonly used in hypertensive patients due to their safety profile, especially Losartan (LOS). By targeting the angiotensin II receptor, a G-protein coupled receptor known as angiotensin I receptor (AT1R), these drugs primarily attenuate blood pressure and regulate fluid balance by inhibiting Angiotensin II (AngII)-induced AT1R activation (2). A hypertensive patient treated with LOS who tested positive for SAR-Cov-2 showed a mortality rate five times lower than those who were not treated with LOS (3). LOS upregulated both ACE2 mRNA level and ACE2 activity leading to Ang (1-7) elevation with reducing tissue damage (4). LOS inhibited AngII-AT1R activation via NF-kappaB inactivation and JAK2/STAT attenuation under seawater inhalation-induced lung injury in rat (5). The expression of ACE/Ang-II is significantly elevated in individuals with ARDS and those with sepsis, which is the primary cause of ARDS (6). BEAS-2B cells exposed to LPS showed increased expression of renin, Ang II, ACE, and AT1R (7). Following losartan treatment, the decrease in AT1R expression and increase in AT2R expression in the spleen and synovium of adjuvant-induced arthritis rats were positively associated with a reduction in the polyarthritis index (8). Collectively, LOS has been extensively investigated based on repurposing drug strategies. It has demonstrated various therapeutic effects beyond hypertension and heart diseases (9-11).
Interestingly, ARBs seemingly exerted anti-inflammatory effect by regulating cytokine producing and modulating redox imbalance in ADRS. LOS have modulatory effect on macrophage in an independent-AT1R activity manner (12). Downregulation of COX-2 were observed in bleomycin-induced pulmonary fibrosis during LOS treatment (13). In the presence an AT1R antagonist, NFkappaB activation was abolished, resulting in reduction in proinflammatory cytokines at mRNA level in LPS-induced acute lung injury (14). LPS stimulation increased inflammatory factor (TNF-alpha, IL-6, IL-1beta), inflammatory-related protein (COX-2 and p-NFkappaB), and oxidative stress marker (MDA, SOD, and GSH-Px) in LPS-induced A549 inflammation (15). Oxidative stress and proinflammatory cytokine production are hallmark in pathogenesis of ADRS (16). However, LOS attenuated proinflammatory cytokines via PPAR-γ activation but not NFkappB in LPS-treated THP-1(17). LOS improved methotrexate-induced hepatic and pulmonary injuries by enhancing liver enzyme function, restoring normal tissue histology, increasing PPAR-γ expression, decreasing TGF-β/SMAD3 signaling, activating Nrf2-mediated antioxidants, and reducing malondialdehyde levels (18). A reasonable expectation would be whether LOS attenuates inflammation through in a cell-specific manner or cellular microenvironment context by modulating redox state and the production of proinflammatory cytokines (19). Nonetheless, anti-inflammatory mechanism of LOS remains controversial.
Herein, we investigated the anti-inflammatory effects of LOS in an in vitro model mimicking acute respiratory distress syndrome. To simulate inflammation, we used LPS-stimulated macrophages (RAW264.7) and alveolar type 2 progenitor cells (A549). Excessive reactive oxygen species (ROS) exacerbate lung damage by reducing lung viability and increasing the production of pro-inflammatory cytokines. We monitored ROS production and the expression of genes related to pro-inflammatory cytokines under LOS treatment to understand its impact on ARDS-like pathogenesis. Our findings focused on the inactivation of NF-kappa B and the downregulation of AT1R, both of which contribute to reducing lung inflammation. Overall, these data demonstrate the potential of LOS in mitigating LPS-induced lung inflammation, providing insights into its underlying anti-inflammatory mechanisms.
Materials and methods
Cell culture
A549 and RAW264.7 cells were routinely maintained in Dulbecco’s Modified Eagle medium (DMEM) (Gibco,MA, USA) supplemented with 10% Fetal Bovine Serum (FBS) (Gibco, MA, USA) and 1% penicillin/streptomycin solution (Gibco,MA, USA) in a humidified 5% CO2 incubator. The cells were replenished every 3 days. It should be noted that RAW264.7 cells beyond the 18th passage were excluded from the study. Morphology was routinely observed during passaging.
Cytotoxicity assessment
A549 cells (5x103) were seeded into a 96-well plate prior to the assay setup. After cell attachment, the medium was replaced with fresh DMEM containing various concentrations of LPS (1.5-100 µg/mL) or LOS (0.3-100 mM). The cells were then incubated for an additional 72 h. Cell viability was assessed using the MTT assay. Briefly, 10 µL of MTT solution (5 mg/mL) was added to each well, followed by a further incubation of 3 hours. The formed formazan crystals were dissolved in dimethyl sulfoxide (DMSO), and the absorbance of the solution was measured at a wavelength of 570 nm.
Nitric oxide production
RAW 264.7 cells (1X105 cells/well) were used to determine NO production under LPS stimulation. Following cell attachment, the cells were pre-treated with various concentrations of Losartan for 1 hour. The pre-treated cells were then stimulated with LPS for an additional 24 hours. For post-treatment, cells were first stimulated with a desired concentration of LPS for 24 hours, followed by treatment with various concentrations of Losartan. Diclofenac (75 µg/mL final concentraion) was used as a reference drug control. The conditioned medium was collected to determine the released NO, following the instructions for Griess's reagent (Promega, WI, USA). The viability of the remaining cells was assessed using the MTT assay, as described above.
Reactive oxygen species measurement
Intracellular reactive oxygen species production was determined using H2DCFDA staining. Briefly, A549 cells (5x103 cells/well) were seeded into a 96-well flat-black, clear-bottom plate. After cell attachment, the cells were stimulated with LPS stimulation medium (fresh medium containing 1% FBS or 10% and 10 µg/mL LPS) for 24 hours. The LPS-stimulated cells were treated with various concentrations of LOS for another 24h. At the indicated time, the treated cells were extensively washed with 1X PBS. Fresh medium containing 10 µM H2DCFDA (Thermofiser, MA, USA) was then replaced, and the cells were incubated for another 24 h. H2O2 was used as a positive control. At the indicated time, the fluorescence intensity of the stained cells was measured at an excitation of 485 nm and an emission of 528 nm using a microplate reader. Cell viability in the remaining cells was determined using the MTT assay.
Western blotting analysis
After the described treatment, the treated cells were washed twice with ice-cold 1X PBS. The treated wells were lysed with RIPA buffer supplemented with 100 µM Na3VO4 on ice for 15 minutes. The cell lysates were clarified by centrifugation, and the total protein concentration was determined using the BCA assay. SDS-PAGE was then performed, followed by blotting onto a nitrocellulose membrane. The membranes were incubated in a blocking solution (3% BSA in PBS-0.1% Tween 20) for 1 hour. The blocked membranes were incubated with the primary antibody at 4 °C for 18 hours. Primary antibody list was shown in Table 1. At the indicated time, the membranes were probed with HRP-conjugated secondary antibody at room temperature for 30 minutes. Luminescence signals from the probed membranes were detected using an ECL substrate.
Table 1.
Primary and secondary antibody list.
| Antibodies | Company | Dilution |
|---|---|---|
| Anti-phospho NF-kappaB (#3033T) | Cell signaling Technology | 1:1000 |
| Anti-AT1R (ab124734) | 1:1000 | |
| Anti-GAPDH (#5174) | Cell signaling Technology | 1:1000 |
| Anti-mouse, HRP-linked (#7076) | Cell signaling Technology | 1:1000 |
| Anti-Rabbit, HRP-linked (#7074) | Cell signaling Technology | 1:5000 |
Quantitative PCR
The treated cells were harvested at the indicated times. Total RNA was extracted following the instructions of the easy-spin™ Total RNA Extraction Kit (iNtRON, Korea). For cDNA synthesis, 1 µg of total RNA was used as the template, following the ReverseAid First Strand cDNA Synthesis Kit instructions with Oligo(dT)16 primer. The newly synthesized cDNA was then used as the template for qPCR. The Maxima SYBR Green/ROX qPCR Master Mix (2X) (Thermofiser, MA, USA) was used for the PCR reaction, performed on a CFX Connect Real-Time PCR Detection System (Bio-rad, CA, USA). GAPDH was used as an internal control. Primer list was shown in Table 2. All primers were design using Primer 3. Relative gene expression was calculated using the delta-delta Ct method.
Table 2.
qPCR primer list.
| Primers | FW | RW |
|---|---|---|
| TNF-alpha | GCTGCACTTTGGAGTGATCG | GCTTGAGGGTTTGCTACAACA |
| IL-6 | GTAGCCGCCCCACACAG | CATGTCTCCTTTCTCAGGGCTG |
| IL-8 | GCACTCCATAAGGCACAAACTT | CTTGGCAAAACTGCACCTTCAC |
| GAPDH | GACATCATACTTGGCAG | CTCGTGGAGTCTACTGGT |
Statistical analysis
Each experiment was carried out three times independently, and the data were presented as mean ± standard deviation. The IC50 value was determined using non-linear regression analysis with the log (inhibitor) vs. response - Variable slope (four parameters) model. ANOVA was employed to identify statistically significant data, followed by post-hoc analysis as needed. Graphs and statistical analyses were generated using Prism 9 software.
Results
LPS-induced macrophage activation was attenuated NO production by LOS
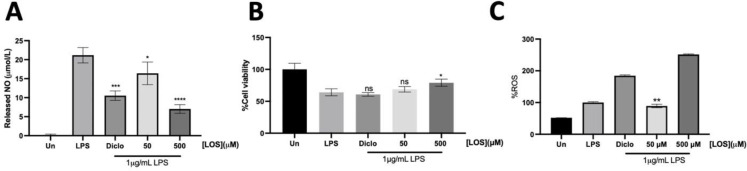
To investigate the anti-inflammatory effects of LOS, we utilized an in vitro murine macrophage system to simulate LPS-induced inflammation. RAW264.7 cells exhibit macrophage-like properties, suitable for studying macrophage functions. They are also more cost-effective and accessible than human cell lines, simplifying experimental setups. The results revealed that LOS significantly attenuated nitric oxide production under LPS stimulation in a concentration-dependent manner without inducing cytotoxicity (Figure 1A-B). Notably, LOS demonstrated a more pronounced anti-inflammatory effect compared to the well-known cyclooxygenase inhibitor, Diclofenac. Therefore, the anti-inflammatory efficacy of LOS is primarily confirmed by its ability to attenuate NO production.
Fig.1.
Anti-inflammation assessment of Losartan on murine macrophage RAW264.7 cell lines under LPS-induced inflammation, (A) Released nitric oxide (NO) was determined by Griess’s reagent. (B) Cell viability was assessed by MTT assay. (C) Intracellular ROS determination was done by H2DCFDA staining. Asterisk* was used to indicate significant data (p<0.05).LOS; Losartan, Diclo; Diclofenac, Un; untreated, ns; no significant
LPS-induced intracellular ROS production was attenuated by low-dose LOS in RAW264.7 cells
During LPS stimulation, NO was generated by inducible Nitric Oxide Synthase (iNOS) through LPS/TRL-4/NFκB activation. The proinflammatory state was further exacerbated by an increase in proinflammatory factors and ROS. The elevation of ROS led to adverse inflammatory effects by activating the mitogen-activated protein kinase (MAPK) signaling pathway (20, 21). At 50µM LOS, intracellular ROS levels significantly decreased (p<0.05) (Fig. 1C). As expected, LPS-stimulated RAW264.7 cells showed an increased intracellular ROS level compared to the untreated condition, as indicated by H2DCF-DA staining. Notably, intracellular ROS levels dramatically increased in the presence of Diclofenac and high-dose LOS (500 µM). Markedly, LOS significantly improved cell viability of RAW264.7 cells at 500µM LOS under LPS stimulation (Fig.1C). Therefore, LOS demonstrated both anti-inflammatory and antioxidant activities by reducing NO production and decreasing intracellular ROS levels in a concentration-dependent manner.
LPS-induced A549 inflammation was attenuated by NF-kappaB inactivation
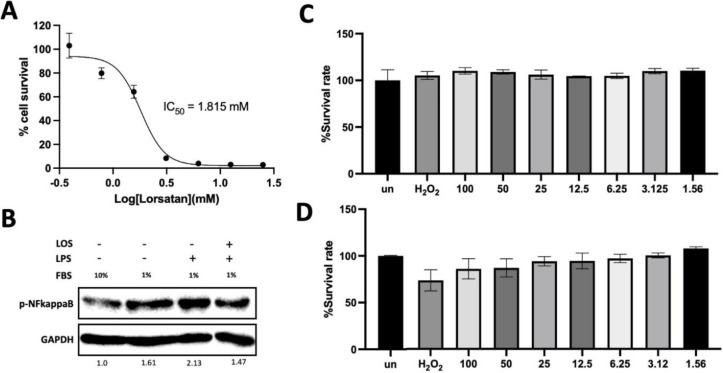
The optimal concentrations of LPS and LOS were initially optimized before setting up the experiment. LOS demonstrated mild toxicity on A549 cells in a concentration-dependent manner, with an IC50 of approximately 1.81 mM (Figure 2A). LPS was used as an exogenous stimulus to simulate acute distress respiratory syndrome (ADRS). Gradually increasing concentrations of LPS had a noticeable impact on A549 cell viability. Noted, 1%FBS impaired A549 cell viability rather than 10% FBS did (Figure 2C-D). Thus, 6.25 µg/mL LPS was a promising concentration that could not exert any effect on cell survival rate of A549. LOS modulated LPS-induced inflammation by decreasing NF-kappaB activation (Figure 2B). Thus, LOS exert anti-inflammatory activity by suppressing NF-kappaB activation. For the sake of practicality in calculations and to demonstrate a comparable effect to 6.25 µg/mL, we opted to use 5 µg/mL for the further experiment.
Fig.2.
Determination of the optimal LPS-induced inflammatory condition on A549, (A) dose-dependent effect of LOS on survival rate of A549. (B) NF-Kappa B activation was done under LPS-induced inflammation in the presence of LOS. (C) and (D) Survival rate was determined under the various concentration of LPS in the presence of 10% FBS or 1% FBS. H2O2 was used as a ROS inducer.
LOS improved cell survival rate under LPS stimulation by downregulating AT1R and NF-kappaB inactivation
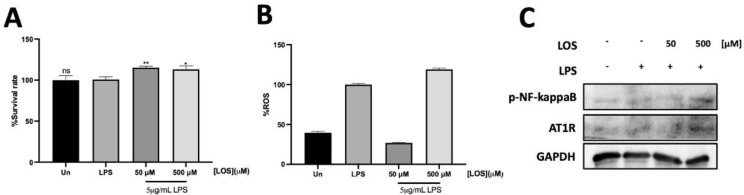
Interestingly, pharmacokinetic parameters of LOS demonstrated Cmax~200-250ng/mL (~0.5µM) when administrated dose~50-80 mg by single oral administration (22, 23). 50 and 500 µM concentrations of LOS were chosen for further investigation due to their non-cytotoxic effects. Upon LPS stimulation, LOS demonstrated a dose-dependent increase in cell survival rates under LPS-induced inflammation conditions (Figure 3A). Additionally, LOS decreased intracellular ROS levels compared to the inflammation induced by LPS alone. Interestingly, there was a significant increase in intracellular ROS with higher concentration of LOS (Fig.3B). To further investigate inflammatory-related protein regulators, at a concentration of 50 µM LOS, phosphorylated NF-kappaB and intracellular ROS decreased. However, at 500 µM LOS, phosphorylated NF-kappaB dramatically increased, correlating with intracellular ROS production (Fig. 3B and 3C). Our investigation demonstrated that 50 µM and 500 µM concentrations of LOS are non-cytotoxic and significantly affect cell survival and inflammation. These findings highlight the potential of LOS as an anti-inflammatory agent, with its efficacy and safety depending on the concentration used.
Fig.3.
Anti-inflammation assessment of Losartan on murine macrophage A549 cell lines under LPS-induced inflammation, (A) Protective effect of LOS was assessed on LPS-induced inflammatory A549 cell lines. (B) Intracellular ROS of LOS treatment was determined under LPS-induced inflammation using H2DCFDA staining. (C) Lung inflammatory related proteins were analyzed using western blotting.
LOS downregulated Lung inflammatory cytokines under LPS stimulation
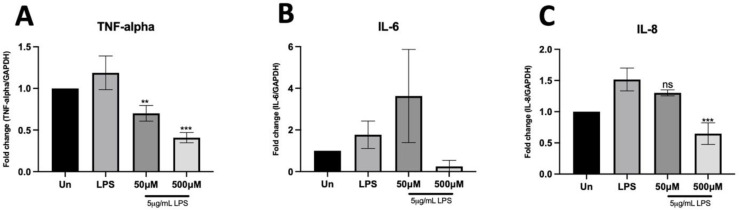
LOS demonstrated a significant modulatory effect on LPS-induced inflammation through the NF-kappaB pathway, leading to a reduction in proinflammatory cytokine production. This attenuation of NF-kappaB had a transcriptional effect on cytokine production, mitigating the cytokine storm associated with ARDS. Specifically, TNF-alpha, IL-6, and IL-8 were downregulated in a dose-dependent manner with LOS treatment compared to the LPS-stimulated condition (Figure 4A-C). Notably, at 500 µM LOS, TNF-alpha and IL-8 levels were significantly reduced to basal levels, even without associated NF-kappaB activation. Therefore, LOS modulating-proinflammatory cytokine production via the NF-kappaB pathway may play a critical role in the inflammatory response and tissue regeneration in ARDS.
Fig.4.
Inflammatory cytokine gene expression was analyzed using quantitiative PCR, (A), (B), and (C) demonstrsted inflmmatory cytokine mRNA expression (TNF-alpha, IL-6, and IL-8, respectively) in the presence of LOS treatment under LPS-induced inflammation.
Discussion
Inflammation in both acute and chronic conditions significantly impacts human health worldwide. Foreign particles such as PM2.5 or bacterial/viral infections systematically aggravate our organs, especially the lungs. Acute Respiratory Distress Syndrome (ARDS) contributes to mild-to-severe symptoms in patients due to cytokines strom, leading to morbidity and mortality. Losartan (LOS), the angiotensin receptor blockers exhibits pleiotropic properties that offer multifaceted benefits by reducing injury mechanisms, dampening inflammation, and safeguarding various vital functions, thereby presenting a promising therapeutic approach for treating a wide range of inflammatory (13, 14, 24). Herein, Losartan (LOS), an Angiotensin Receptor Blocker (ARB), has been re-purposed as an alternative strategy in ARDS treatment. Our study reveals that Losartan (LOS) exhibits significant potential in modulating inflammation related to Acute Respiratory Distress Syndrome (ARDS) through its impact on the NF-kappaB pathway and cytokine production. A549 cells were chosen to represent Alveolar Type II pneumocytes, specifically AT2 cells that play a crucial role in lung epithelium, including acting as self-renewing progenitors during lung regeneration (25). Pharmacokinetic data indicate that LOS reaches a Cmax of approximately 200-250 ng/mL (~0.5 µM) with a single oral dose of 50-80 mg. In our experiments, LOS at concentrations of 50 µM and 500 µM showed non-cytotoxic effects and a dose-dependent increase in cell survival under LPS-induced inflammation.
LOS, known for its anti-inflammatory properties through AT1R blockade, demonstrated significant attenuation of LPS-induced macrophage activation and nitric oxide production in vitro. It outperformed Diclofenac in reducing proinflammatory cytokines. In RAW264.7 cells, LOS at low doses decreased intracellular ROS levels, but higher doses led to elevated ROS. Fimasartan, AT1R blocker reduced NO production by downregulating iNOS expression via NK-kappaB inactivation (26). LOS administration in diabetic rats suppressed Vascular Endothelial Growth Factor (VEGF) expression and excess NO in the pancreas (27). Inhibition of AT1R by LOS decreased NO-mediated hypertension by modulating NADPH oxidase, oxidative stress and proinflammatory mediator production in L-NAME-induced hypertension rat (28). Therefore, LOS would be confirmed its anti-inflammatory effect by attenuating NO production via AT1R inactivation. LOS downregulated NF-kappaB activation induced by LPS, potentially offering protection against acute respiratory distress syndrome (ARDS). LOS has been extensively documented for its anti-inflammatory activity, achieved through the blockade of the G-protein-coupled receptor, AT1R, leading to the modulation of cytokines and chemokines (29).
LPS is a well-known activator of the inflammasome via TRL-4 receptor that is commonly expressed on A549 cell surface (30). LPS modulated surfactant protein level in AT2 cells that could affect on pulmonary surfactant (31, 32). LPS upregulated AT1R expression leading to increase Maximal binding (Bmax) of AngII/AT1R in ARDS (33). During LPS stimulation, LPS induced metabolic reprogramming of macrophages by generating ROS production (34). ATP through oxidative phosphorylation during LPS stimulation shifted to glycolysis, leading to an elevation of succinate accumulation, which drive mitochondrial ROS production (35). Diclofenac; a nonsteroidal anti-inflammation drug a nonsteroidal anti-inflammatory drug, elevated ROS production by triggering mitochondrial dysfunction and impairing antioxidant mechanisms, notably attenuating Superoxide Dismutase 2 (SOD2) in the neuroblastoma cell line SH-SY-5Y (36). Diclofenac-induced oxidative stress also increased the risk of cardiovascular diseases by depolarizing mitochondrial potential (37). LOS exerted anti-inflammatory activity by reducing NO production. However, increasing LOS concentration slightly impaired SOD activity and increased malondialdehyde (MDA) levels compared to Low- and Mid-dose LOS in diabetes-induced hepatic damage (38).
Noted, LOS, formulated as a potassium salt, exhibits efficient oral absorption, with a systemic bioavailability of approximately 33%. High concentration of LOS would increase potassium level in culture medium during treatment. Lung preservation solutions containing high potassium (K+) concentrations can lead to membrane depolarization, activate NADPH oxidase, and result in an increase in ROS production in the pulmonary artery (39). Collectively, the documented side effects of LOS include the potential to cause hyperkalemia, which is characterized by the high levels of potassium in the blood and can be harmful to renal function. (40). LPS upregulated d AT1R expression level that led homeostasis unbalance leading to tissue damage. AT1R underwent upregulation in human pulmonary microvascular endothelial cells (HPMECs), reaching its peak level within 8 h under LPS-induced inflammation (33). Interestingly, LOS downregulated AT1R expression at protein level under LPS-induced inflammation. ARBs exhibited a response in human Type II diabetic nephropathy, wherein it downregulated the AT1R receptor while concurrently upregulating AT2R which contributed to tissue regeneration (41). LOS led to a significant reduction in the vascular expression of AT1R, accompanied by a notable increase in AT2R expression (42). Cross-talk between inflammation and AngII/AT1R promoted proinflammatory cytokine production via activated mitogen-acticated protein kinase (MAPKs), c-Jun and NF-kappaB (43). Interestingly, LOS exhibited both anti-inflammatory and antioxidant activities by reducing NO production and intracellular ROS levels in a dose-dependent manner in A549. However, caution is warranted with high LOS concentrations due to potential hyperkalemia risks.
NF-kappaB plays a key role in cellular response to stimuli. LPS is recognized by TRL-4 that is responsible for pathogen pattern recognition receptor resulting in cellular response. LPS-induced inflammation mechanism has been reported to drive proinflammatory cytokines via NF-kappaB activation (44-46). Phosphorylation of Ser536 increased upon LPS stimulation. It was noted that phosphorylated NF-kappaB was also detected under the 1% FBS condition, whereas it was downregulated under the 10% FBS condition (Figure 2B). Ser536 plays a pivotal role in the response noxious stimuli (47). Nonetheless, serum starvation induced cellular stress, leading to the activation of autophagy via NF-kappaB activation (48, 49). Enalapril and LOS inhibited NF-κB activation by modulating p65 phosphorylation (24). LOS has a protective effect on sepsis-induced cardiomyopathy by modulating macrophage polarization from M1 to M2 via NF-kappaB activation and MAPK pathway (50). Consequently, LOS would attenuated LPS-induced inflammation by downregulating AT1R leading to NF-kappaB inactivation.
The NF-κB pathway can exhibit both anti-oxidant and pro-oxidant roles in the context of oxidative stress (48). Inhibition of NF-κB activation can lead to an increase in TNFα-induced ROS production, lipid peroxidation, and protein oxidation (51). On the contrary, prolonged activation of NF-κB has been demonstrated to mitigate hyperoxia-induced mortality in adults and enhance survival while preserving lung development in neonatal mice (52). It also maintains alveolarization by inhibiting the anti-angiogenic cytokine macrophage inflammatory protein 2 in neonates exposed to LPS (53).
Moreover, LOS downregulated inflammatory cytokines TNF-alpha, IL-6, and IL-8 under LPS stimulation, indicating its potential in mitigating the cytokine storm associated with ARDS. These proinflammatory cytokines were detected in SAR-CoV2 infected patients who got severe pulmonary inflammation and extensive lung damage (54). This study underscores the intricate role of the NF-kappaB pathway in inflammation and tissue protection, highlighting LOS as a promising therapeutic candidate for ARDS management.
In conclusion, the intricate interplay between LOS and the NF-kappaB pathway underscores its potential in inflammation modulation and tissue protection. LOS demonstrated significant attenuation of LPS-induced inflammation, primarily through its impact on the NF-kappaB pathway and cytokine production. LOS downregulated proinflammatory cytokines TNF-alpha, IL-6, and IL-8, suggesting its potential in mitigating the cytokine storm associated with severe inflammatory conditions like ARDS. However, caution is warranted regarding LOS concentrations to avoid potential side effects such as hyperkalemia. Overall, LOS represents an innovative approach to managing inflammatory, offering hope for improved ARDS patient outcomes.
Acknowledgment
The authors thank the Health System Research Institute for the financial support from the under-contact number 63-053 and Department of Medical Services, Minister of Public Health, Nonthaburi, Thailand.
References
- 1.Nejat R, Sadr AS. Are losartan and imatinib effective against SARS-CoV2 pathogenesis? A pathophysiologic-based in silico study. In Silico Pharmacol. 2020;9:1. doi: 10.1007/s40203-020-00058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore GJ, Ridgway H, Kelaidonis K, et al. Actions of Novel Angiotensin Receptor Blocking Drugs, Bisartans, Relevant for COVID-19 Therapy: Biased Agonism at Angiotensin Receptors and the Beneficial Effects of Neprilysin in the Renin Angiotensin System. Molecules. 2022:27. doi: 10.3390/molecules27154854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirjalili M, Soodejani MT, Raadabadi M, et al. Does Losartan reduce the severity of COVID-19 in hypertensive patients? BMC Cardiovasc Disord. 2022;22:116. doi: 10.1186/s12872-022-02548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel VB, Zhong JC, Grant MB, et al. Role of the ACE2/Angiotensin 1-7 Axis of the Renin-Angiotensin System in Heart Failure. Circ Res. 2016;118:1313–26. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li C, Bo L, Li P, et al. Losartan, a selective antagonist of AT1 receptor, attenuates seawater inhalation induced lung injury via modulating JAK2/STATs and apoptosis in rat. Pulm Pharmacol Ther. 2017;45:69–79. doi: 10.1016/j.pupt.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Doerschug KC, Delsing AS, Schmidt GA, et al. Renin-angiotensin system activation correlates with microvascular dysfunction in a prospective cohort study of clinical sepsis. Crit Care. 2010;14:R24. doi: 10.1186/cc8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye R, Liu Z. ACE2 exhibits protective effects against LPS-induced acute lung injury in mice by inhibiting the LPS-TLR4 pathway. Exp Mol Pathol. 2020;113:104350. doi: 10.1016/j.yexmp.2019.104350. [DOI] [PubMed] [Google Scholar]
- 8.Wang D, Hu S, Zhu J, et al. Angiotensin II type 2 receptor correlates with therapeutic effects of losartan in rats with adjuvant-induced arthritis. J Cell Mol Med. 2013;17:1577–87. doi: 10.1111/jcmm.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashemzehi M, Rahmani F, Khoshakhlagh M, et al. Angiotensin receptor blocker Losartan inhibits tumor growth of colorectal cancer. EXCLI J. 2021;20:506–21. doi: 10.17179/excli2020-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan B, Gleim G, Panish J. Use of losartan in diabetic patients in the primary care setting: review of the results in LIFE and RENAAL. Curr Med Res Opin. 2004;20:1909–17. doi: 10.1185/030079904X13040. [DOI] [PubMed] [Google Scholar]
- 11.Mainetti LE, Rico MJ, Kaufman CD, et al. Losartan improves the therapeutic effect of metronomic cyclophosphamide in triple negative mammary cancer models. Oncotarget. 2020;11:3048–60. doi: 10.18632/oncotarget.27694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regan DP, Coy JW, Chahal KK, et al. The Angiotensin Receptor Blocker Losartan Suppresses Growth of Pulmonary Metastases via AT1R-Independent Inhibition of CCR2 Signaling and Monocyte Recruitment. J Immunol. 2019;202:3087–102. doi: 10.4049/jimmunol.1800619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molina-Molina M, Serrano-Mollar A, Bulbena O, et al. Losartan attenuates bleomycin induced lung fibrosis by increasing prostaglandin E2 synthesis. Thorax. 2006;61:604–10. doi: 10.1136/thx.2005.051946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F, Xia ZF, Chen XL, et al. Angiotensin II type-1 receptor antagonist attenuates LPS-induced acute lung injury. Cytokine. 2009;48:246–53. doi: 10.1016/j.cyto.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Ge J, Yang H, Zeng Y, et al. Protective effects of wogonin on lipopolysaccharide-induced inflammation and apoptosis of lung epithelial cells and its possible mechanisms. Biomed Eng Online. 2021;20:125. doi: 10.1186/s12938-021-00965-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang JD, McArdle PJ, O'Reilly PJ, et al. Oxidant-antioxidant balance in acute lung injury. Chest. 2002;122:314S–20S. doi: 10.1378/chest.122.6_suppl.314s. [DOI] [PubMed] [Google Scholar]
- 17.An J, Nakajima T, Kuba K, et al. Losartan inhibits LPS-induced inflammatory signaling through a PPARgamma-dependent mechanism in human THP-1 macrophages. Hypertens Res. 2010;33:831–5. doi: 10.1038/hr.2010.79. [DOI] [PubMed] [Google Scholar]
- 18.Abbas NAT, El-Sayed SS, El-Fatah A, et al. Losartan prevents methotrexate-induced liver and lung injury in rats via targeting PPAR-γ/TGF-β1/SMAD3 and Nrf2/redox signaling. Zagazig univ med j. 2023;29:135–48. [Google Scholar]
- 19.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure-lowering on outcome incidence in hypertension: 5 Head-to-head comparisons of various classes of antihypertensive drugs - overview and meta-analyses. J Hypertens. 2015;33:1321–41. doi: 10.1097/HJH.0000000000000614. [DOI] [PubMed] [Google Scholar]
- 20.Baek SH, Park T, Kang MG, et al. Anti-Inflammatory Activity and ROS Regulation Effect of Sinapaldehyde in LPS-Stimulated RAW 264 7 Macrophages. Molecules. 2020:25. doi: 10.3390/molecules25184089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu X, Gao H, Hou Y, et al. Dihydronortanshinone, a natural product, alleviates LPS-induced inflammatory response through NF-kappaB, mitochondrial ROS, and MAPK pathways. Toxicol Appl Pharmacol. 2018;355:1–8. doi: 10.1016/j.taap.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Bae JW, Choi CI, Kim MJ, et al. Frequency of CYP2C9 alleles in Koreans and their effects on losartan pharmacokinetics. Acta Pharmacol Sin. 2011;32:1303–8. doi: 10.1038/aps.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang G, Xiao CQ, Li Z, et al. Effect of soy extract administration on losartan pharmacokinetics in healthy female volunteers. Ann Pharmacother. 2009;43:1045–9. doi: 10.1345/aph.1L690. [DOI] [PubMed] [Google Scholar]
- 24.Kim JM, Heo HS, Choi YJ, et al. Inhibition of NF-kappaB-induced inflammatory responses by angiotensin II antagonists in aged rat kidney. Exp Gerontol. 2011;46:542–8. doi: 10.1016/j.exger.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee DF, Salguero FJ, Grainger D, et al. Isolation and characterisation of alveolar type II pneumocytes from adult bovine lung. Sci Rep. 2018;8:11927. doi: 10.1038/s41598-018-30234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryu S, Shin JS, Cho YW, et al. Fimasartan, anti-hypertension drug, suppressed inducible nitric oxide synthase expressions via nuclear factor-kappa B and activator protein-1 inactivation. Biol Pharm Bull. 2013;36:467–74. doi: 10.1248/bpb.b12-00859. [DOI] [PubMed] [Google Scholar]
- 27.Lee MY, Shim MS, Kim BH, et al. Effects of spironolactone and losartan on diabetic nephropathy in a type 2 diabetic rat model. Diabetes Metab J. 2011;35:130–7. doi: 10.4093/dmj.2011.35.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rincon J, Correia D, Arcaya JL, et al. Role of Angiotensin II type 1 receptor on renal NAD(P)H oxidase, oxidative stress and inflammation in nitric oxide inhibition induced-hypertension. Life Sci. 2015;124:81–90. doi: 10.1016/j.lfs.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Arif G, Khazaal S, Farhat A, et al. Angiotensin II Type I Receptor (AT1R): The Gate towards COVID-19-Associated Diseases. Molecules. 2022:27. doi: 10.3390/molecules27072048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hornef MW, Normark BH, Vandewalle A, et al. Intracellular recognition of lipopolysaccharide by toll-like receptor 4 in intestinal epithelial cells. J Exp Med. 2003;198:1225–35. doi: 10.1084/jem.20022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin J, Tian J, Wang L, et al. Apoptosis and surfactant protein-C expression inhibition induced by lipopolysaccharide in AEC II cell may associate with NF-kappaB pathway. J Toxicol Sci. 2017;42:53–61. doi: 10.2131/jts.42.53. [DOI] [PubMed] [Google Scholar]
- 32.Wang WN, Zhou JH, Wang P, et al. The localization of SP-B and influences of lipopolysaccharide on it. Eur Rev Med Pharmacol Sci. 2016;20:2338–45. [PubMed] [Google Scholar]
- 33.Li HP, Qiu HB, Wang HQ. Effect of lipopolysaccharide on angiotensin II type 1 receptor expression and function in human pulmonary microvascular endothelial cells. Mol Med Rep. 2015;12:8289–93. doi: 10.3892/mmr.2015.4481. [DOI] [PubMed] [Google Scholar]
- 34.Mills EL, Kelly B, Logan A, et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell. 2016;167:457–70 e13. doi: 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–42. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cecere F, Iuliano A, Albano F, et al. Diclofenac-induced apoptosis in the neuroblastoma cell line SH-SY5Y: possible involvement of the mitochondrial superoxide dismutase. J Biomed Biotechnol. 2010;2010:801726. doi: 10.1155/2010/801726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thai PN, Ren L, Xu W, et al. Chronic Diclofenac Exposure Increases Mitochondrial Oxidative Stress, Inflammatory Mediators, and Cardiac Dysfunction. Cardiovasc Drugs Ther. 2023;37:25–37. doi: 10.1007/s10557-021-07253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oltulu F, Buhur A, Gurel C, et al. Mid-dose losartan mitigates diabetes-induced hepatic damage by regulating iNOS, eNOS, VEGF, and NF-kappaB expressions. Turk J Med Sci. 2019;49:1582–9. doi: 10.3906/sag-1901-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murar J, Hong ZG, Hong FX, et al. High potassium lung preservation solutions increase production of NADPH oxidase-dependent reactive oxygen species. J Heart Lung Transplant. 2003;22:S101–S2. [Google Scholar]
- 40.Goncalves AR, El Nahas AM. High serum potassium levels after using losartan can reflect more severe renal disease. Diabetologia. 2011;54:2963–4; author reply 5-7. doi: 10.1007/s00125-011-2220-7. [DOI] [PubMed] [Google Scholar]
- 41.Ma YP, Yang Y, Jiang SM, et al. Angiotensin II type 1 receptor blockers favorably affect renal angiotensin II and MAS receptor expression in patients with diabetic nephropathy. J Renin Angiotensin Aldosterone Syst. 2020;21:1470320320919607. doi: 10.1177/1470320320919607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He DH, Lin JX, Zhang LM, et al. Early treatment with losartan effectively ameliorates hypertension and improves vascular remodeling and function in a prehypertensive rat model. Life Sci. 2017;173:20–7. doi: 10.1016/j.lfs.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Khaidakov M, Ding Z, et al. Cross-talk between inflammation and angiotensin II: studies based on direct transfection of cardiomyocytes with AT1R and AT2R cDNA. Exp Biol Med (Maywood) 2012;237:1394–401. doi: 10.1258/ebm.2012.012212. [DOI] [PubMed] [Google Scholar]
- 44.Ngkelo A, Meja K, Yeadon M, et al. LPS induced inflammatory responses in human peripheral blood mononuclear cells is mediated through NOX4 and Gialpha dependent PI-3kinase signalling. J Inflamm (Lond) 2012;9:1. doi: 10.1186/1476-9255-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X, Yin S, Chen Y, et al. LPS‑induced proinflammatory cytokine expression in human airway epithelial cells and macrophages via NF‑kappaB, STAT3 or AP‑1 activation. Mol Med Rep. 2018;17:5484–91. doi: 10.3892/mmr.2018.8542. [DOI] [PubMed] [Google Scholar]
- 46.Ubanako P, Xelwa N, Ntwasa M. LPS induces inflammatory chemokines via TLR-4 signalling and enhances the Warburg Effect in THP-1 cells. PLoS One. 2019;14:e0222614. doi: 10.1371/journal.pone.0222614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109 Suppl:S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 48.Lingappan K. NF-kappaB in Oxidative Stress. Curr Opin Toxicol. 2018;7:81–6. doi: 10.1016/j.cotox.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kohno T, Kubo Y, Yasui K, et al. Serum starvation activates NF-kappaB through G protein beta2 subunit-mediated signal. DNA Cell Biol. 2012;31:1636–44. doi: 10.1089/dna.2012.1666. [DOI] [PubMed] [Google Scholar]
- 50.Chen XS, Wang SH, Liu CY, et al. Losartan attenuates sepsis-induced cardiomyopathy by regulating macrophage polarization via TLR4-mediated NF-kappaB and MAPK signaling. Pharmacol Res. 2022;185:106473. doi: 10.1016/j.phrs.2022.106473. [DOI] [PubMed] [Google Scholar]
- 51.Djavaheri-Mergny M, Javelaud D, Wietzerbin J, et al. NF-kappaB activation prevents apoptotic oxidative stress via an increase of both thioredoxin and MnSOD levels in TNFalpha-treated Ewing sarcoma cells. FEBS Lett. 2004;578:111–5. doi: 10.1016/j.febslet.2004.10.082. [DOI] [PubMed] [Google Scholar]
- 52.Yang G, Abate A, George AG, et al. Maturational differences in lung NF-kappaB activation and their role in tolerance to hyperoxia. J Clin Invest. 2004;114:669–78. doi: 10.1172/JCI19300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hou Y, Liu M, Husted C, et al. Activation of the nuclear factor-kappaB pathway during postnatal lung inflammation preserves alveolarization by suppressing macrophage inflammatory protein-2. Am J Physiol Lung Cell Mol Physiol. 2015;309:L593–604. doi: 10.1152/ajplung.00029.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Darif D, Hammi I, Kihel A, et al. The pro-inflammatory cytokines in COVID-19 pathogenesis: What goes wrong? Microb Pathog. 2021;153:104799. doi: 10.1016/j.micpath.2021.104799. [DOI] [PMC free article] [PubMed] [Google Scholar]