Abstract
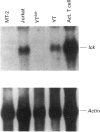
The haematopoietic protein, p95vav, has been shown to be a tyrosine kinase substrate and to have tyrosine kinase-modulated guanine-nucleotide-releasing-factor activity. This implies a function in the control of ras or ras-like proteins. Because ras activation has been shown to be a downstream event following stimulation of the interleukin-2 (IL-2) receptor, we investigated the possibility that vav was involved in IL-2 signal transduction pathways, using human T cells as a model. We found rapid tyrosine phosphorylation of vav in response to IL-2 within 1 min, with maximum increase of phosphorylation of 5-fold occurring by 5 min after treatment in normal human T cells. IL-2 stimulation of the human T-cell line YT and a subclone of the YT cell line (YTlck-) that does not express message for the src-family kinase p56lck also results in a rapid rate of tyrosine phosphorylation of vav of more than 5-fold by 5 min. These results suggest that vav may play an important role in IL-2-stimulated signal transduction and that there is not a strict requirement for the tyrosine kinase p56lck.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Houston H., Allen J., Lints T., Harvey R. The hematopoietically expressed vav proto-oncogene shares homology with the dbl GDP-GTP exchange factor, the bcr gene and a yeast gene (CDC24) involved in cytoskeletal organization. Oncogene. 1992 Apr;7(4):611–618. [PubMed] [Google Scholar]
- Alai M., Mui A. L., Cutler R. L., Bustelo X. R., Barbacid M., Krystal G. Steel factor stimulates the tyrosine phosphorylation of the proto-oncogene product, p95vav, in human hemopoietic cells. J Biol Chem. 1992 Sep 5;267(25):18021–18025. [PubMed] [Google Scholar]
- Bazan J. F. A novel family of growth factor receptors: a common binding domain in the growth hormone, prolactin, erythropoietin and IL-6 receptors, and the p75 IL-2 receptor beta-chain. Biochem Biophys Res Commun. 1989 Oct 31;164(2):788–795. doi: 10.1016/0006-291x(89)91528-3. [DOI] [PubMed] [Google Scholar]
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustelo X. R., Barbacid M. Tyrosine phosphorylation of the vav proto-oncogene product in activated B cells. Science. 1992 May 22;256(5060):1196–1199. doi: 10.1126/science.256.5060.1196. [DOI] [PubMed] [Google Scholar]
- Bustelo X. R., Ledbetter J. A., Barbacid M. Product of vav proto-oncogene defines a new class of tyrosine protein kinase substrates. Nature. 1992 Mar 5;356(6364):68–71. doi: 10.1038/356068a0. [DOI] [PubMed] [Google Scholar]
- Cen H., Papageorge A. G., Zippel R., Lowy D. R., Zhang K. Isolation of multiple mouse cDNAs with coding homology to Saccharomyces cerevisiae CDC25: identification of a region related to Bcr, Vav, Dbl and CDC24. EMBO J. 1992 Nov;11(11):4007–4015. doi: 10.1002/j.1460-2075.1992.tb05494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Duronio V., Welham M. J., Abraham S., Dryden P., Schrader J. W. p21ras activation via hemopoietin receptors and c-kit requires tyrosine kinase activity but not tyrosine phosphorylation of p21ras GTPase-activating protein. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1587–1591. doi: 10.1073/pnas.89.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. A., Wahl L. M., Farrar W. L. Interleukin-2-dependent phosphorylation of the retinoblastoma-susceptibility-gene product p110-115RB in human T-cells. Biochem J. 1992 Mar 15;282(Pt 3):759–764. doi: 10.1042/bj2820759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris D. K., Willette-Brown J., Ortaldo J. R., Farrar W. L. IL-2 regulation of tyrosine kinase activity is mediated through the p70-75 beta-subunit of the IL-2 receptor. J Immunol. 1989 Aug 1;143(3):870–876. [PubMed] [Google Scholar]
- Fung M. R., Scearce R. M., Hoffman J. A., Peffer N. J., Hammes S. R., Hosking J. B., Schmandt R., Kuziel W. A., Haynes B. F., Mills G. B. A tyrosine kinase physically associates with the beta-subunit of the human IL-2 receptor. J Immunol. 1991 Aug 15;147(4):1253–1260. [PubMed] [Google Scholar]
- Galland F., Katzav S., Birnbaum D. The products of the mcf-2 and vav proto-oncogenes and of the yeast gene cdc-24 share sequence similarities. Oncogene. 1992 Mar;7(3):585–587. [PubMed] [Google Scholar]
- Garcia G. G., Evans G. A., Michiel D. F., Farrar W. L. Characterization of a tyrosine kinase activity associated with the high-affinity interleukin 2 receptor complex. Biochem J. 1992 Aug 1;285(Pt 3):851–856. doi: 10.1042/bj2850851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves J. D., Downward J., Izquierdo-Pastor M., Rayter S., Warne P. H., Cantrell D. A. The growth factor IL-2 activates p21ras proteins in normal human T lymphocytes. J Immunol. 1992 Apr 15;148(8):2417–2422. [PubMed] [Google Scholar]
- Gulbins E., Coggeshall K. M., Baier G., Katzav S., Burn P., Altman A. Tyrosine kinase-stimulated guanine nucleotide exchange activity of Vav in T cell activation. Science. 1993 May 7;260(5109):822–825. doi: 10.1126/science.8484124. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Kono T., Kobayashi N., Kawahara A., Levin S. D., Perlmutter R. M., Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991 Jun 14;252(5012):1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- Heidaran M. A., Molloy C. J., Pangelinan M., Choudhury G. G., Wang L. M., Fleming T. P., Sakaguchi A. Y., Pierce J. H. Activation of the colony-stimulating factor 1 receptor leads to the rapid tyrosine phosphorylation of GTPase-activating protein and activation of cellular p21ras. Oncogene. 1992 Jan;7(1):147–152. [PubMed] [Google Scholar]
- Horak I. D., Gress R. E., Lucas P. J., Horak E. M., Waldmann T. A., Bolen J. B. T-lymphocyte interleukin 2-dependent tyrosine protein kinase signal transduction involves the activation of p56lck. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1996–2000. doi: 10.1073/pnas.88.5.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P., Margolis B., Schlessinger J. Vav: a potential link between tyrosine kinases and ras-like GTPases in hematopoietic cell signaling. Bioessays. 1993 Mar;15(3):179–183. doi: 10.1002/bies.950150306. [DOI] [PubMed] [Google Scholar]
- Katzav S., Martin-Zanca D., Barbacid M. vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. EMBO J. 1989 Aug;8(8):2283–2290. doi: 10.1002/j.1460-2075.1989.tb08354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N., Kono T., Hatakeyama M., Minami Y., Miyazaki T., Perlmutter R. M., Taniguchi T. Functional coupling of the src-family protein tyrosine kinases p59fyn and p53/56lyn with the interleukin 2 receptor: implications for redundancy and pleiotropism in cytokine signal transduction. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4201–4205. doi: 10.1073/pnas.90.9.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linnekin D., Evans G. A., D'Andrea A., Farrar W. L. Association of the erythropoietin receptor with protein tyrosine kinase activity. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6237–6241. doi: 10.1073/pnas.89.14.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B., Hu P., Katzav S., Li W., Oliver J. M., Ullrich A., Weiss A., Schlessinger J. Tyrosine phosphorylation of vav proto-oncogene product containing SH2 domain and transcription factor motifs. Nature. 1992 Mar 5;356(6364):71–74. doi: 10.1038/356071a0. [DOI] [PubMed] [Google Scholar]
- Michiel D. F., Garcia G. G., Evans G. A., Farrar W. L. Regulation of the interleukin 2 receptor complex tyrosine kinase activity in vitro. Cytokine. 1991 Sep;3(5):428–438. doi: 10.1016/1043-4666(91)90047-h. [DOI] [PubMed] [Google Scholar]
- Mills G. B., Arima N., May C., Hill M., Schmandt R., Li J., Miyamoto N. G., Greene W. C. Neither the LCK nor the FYN kinases are obligatory for IL-2-mediated signal transduction in HTLV-I-infected human T cells. Int Immunol. 1992 Nov;4(11):1233–1243. doi: 10.1093/intimm/4.11.1233. [DOI] [PubMed] [Google Scholar]
- Otani H., Siegel J. P., Erdos M., Gnarra J. R., Toledano M. B., Sharon M., Mostowski H., Feinberg M. B., Pierce J. H., Leonard W. J. Interleukin (IL)-2 and IL-3 induce distinct but overlapping responses in murine IL-3-dependent 32D cells transduced with human IL-2 receptor beta chain: involvement of tyrosine kinase(s) other than p56lck. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2789–2793. doi: 10.1073/pnas.89.7.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui H., Djeu J. Y., Evans G. A., Kelly P. A., Farrar W. L. Prolactin receptor triggering. Evidence for rapid tyrosine kinase activation. J Biol Chem. 1992 Nov 25;267(33):24076–24081. [PubMed] [Google Scholar]
- Saltzman E. M., Thom R. R., Casnellie J. E. Activation of a tyrosine protein kinase is an early event in the stimulation of T lymphocytes by interleukin-2. J Biol Chem. 1988 May 25;263(15):6956–6959. [PubMed] [Google Scholar]
- Satoh T., Nakafuku M., Miyajima A., Kaziro Y. Involvement of ras p21 protein in signal-transduction pathways from interleukin 2, interleukin 3, and granulocyte/macrophage colony-stimulating factor, but not from interleukin 4. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3314–3318. doi: 10.1073/pnas.88.8.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen P. H., Mui A. L., Murthy S. C., Krystal G. Interleukin-3, GM-CSF, and TPA induce distinct phosphorylation events in an interleukin 3-dependent multipotential cell line. Blood. 1989 Feb;73(2):406–418. [PubMed] [Google Scholar]
- Thoreau E., Petridou B., Kelly P. A., Djiane J., Mornon J. P. Structural symmetry of the extracellular domain of the cytokine/growth hormone/prolactin receptor family and interferon receptors revealed by hydrophobic cluster analysis. FEBS Lett. 1991 Apr 22;282(1):26–31. doi: 10.1016/0014-5793(91)80437-8. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss S. D., Robb R. J., Weil-Hillman G., Hank J. A., Sugamura K., Tsudo M., Sondel P. M. Increased expression of the interleukin 2 (IL-2) receptor beta chain (p70) on CD56+ natural killer cells after in vivo IL-2 therapy: p70 expression does not alone predict the level of intermediate affinity IL-2 binding. J Exp Med. 1990 Oct 1;172(4):1101–1114. doi: 10.1084/jem.172.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl L. M., Katona I. M., Wilder R. L., Winter C. C., Haraoui B., Scher I., Wahl S. M. Isolation of human mononuclear cell subsets by counterflow centrifugal elutriation (CCE). I. Characterization of B-lymphocyte-, T-lymphocyte-, and monocyte-enriched fractions by flow cytometric analysis. Cell Immunol. 1984 May;85(2):373–383. doi: 10.1016/0008-8749(84)90251-x. [DOI] [PubMed] [Google Scholar]
- Whetton A. D., Monk P. N., Consalvey S. D., Huang S. J., Dexter T. M., Downes C. P. Interleukin 3 stimulates proliferation via protein kinase C activation without increasing inositol lipid turnover. Proc Natl Acad Sci U S A. 1988 May;85(10):3284–3288. doi: 10.1073/pnas.85.10.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yodoi J., Teshigawara K., Nikaido T., Fukui K., Noma T., Honjo T., Takigawa M., Sasaki M., Minato N., Tsudo M. TCGF (IL 2)-receptor inducing factor(s). I. Regulation of IL 2 receptor on a natural killer-like cell line (YT cells). J Immunol. 1985 Mar;134(3):1623–1630. [PubMed] [Google Scholar]