Abstract
Background.
In recent years, pragmatic metformin use in pregnancy has stretched to include prediabetes, type 2 diabetes, gestational diabetes and (most recently) pre-eclampsia. With its expanded use, however, concerns of unintended harm have been raised.
Objective.
We developed an experimental primate model and applied triple-quadruple pole LC mass spectrometry (UHPLC-QQQ) for direct quantitation of maternal and fetal tissue metformin levels with detailed fetal biometry and histopathology.
Study design.
Within 30 days of confirmed conception (defined as early pregnancy), n=13 time-bred (TMB) Rhesus dams with gestations designated for fetal necropsy were initiated on twice daily human dose-equivalent 10 mg/kg metformin or vehicle control. Pregnant dams were maintained as pairs and fed either a control chow or 36% fat Western-style diet (WSD). Metformin or placebo vehicle control were delivered in a variety of treats while animals were separated via a slide. A Cesarean was performed at G145, and amniotic fluid and blood were collected and the fetus and placenta were delivered. The fetus was immediately necropsied by trained primate center personnel. All fetal organs were dissected, measured, sectioned, and processed per clinical standards. Fluid and tissue metformin levels were assayed using validated UHPLC-QQQ in SRM against standard curves.
Results.
Among the n=13 G145 pregnancies with fetal necropsy, n=1 dam and its fetal tissues had detectable metformin levels despite being allocated to the vehicle control group (>1 μM metformin/kg maternal weight or fetal/placental tissue), while a second fetus allocated to the vehicle control group had severe fetal growth restriction (birthweight 248.32 g, <1%) and was suspected of having a fetal congenital condition. After excluding these two fetal gestations from further analyses, 11 fetuses from dams initiated on either vehicle control (n=4, 3 female, 1 male fetuses) or 10 mg/kg metformin (n=7, 5 female, 2 male fetuses) were available for analyses. Among dams initiated on metformin by G30 (regardless of maternal diet), we observed significant bioaccumulation within the fetal kidney (0.78–6.06 μmol/kg, mean 2.48 μmol/kg) , liver (0.16–0.73 μmol/kg, mean 0.38 μmol/kg), fetal gut (0.28–1.22 μmol/kg, mean 0.70 μmol/kg), amniotic fluid (0.43–3.33 μmol/L, mean 1.88 μmol/L), placenta (0.16–1.0 μmol/kg , mean 0.50 μmol/kg) and fetal serum (0 –0.66 μmol/L , mean 0.23 μmol/L ), and fetal urine (4.1–174.1 μmol/L mean 38.5 μmol/L ), with fetal levels near biomolar equivalent to maternal levels (maternal serum 0.18–0.86 μmol/L , mean 0.46 μmol/L; maternal urine 42.6–254.0 μmol/L , mean 149.3 μmol/L). WSD feeding neither accelerated nor reduced metformin bioaccumulations in maternal or fetal serum, urine, amniotic fluid, placenta nor fetal tissues. In these 11 animals, fetal bioaccumulation of metformin was associated with less fetal skeletal muscle (57% lower cross-sectional area of gastrocnemius) and decreased liver, heart, and retroperitoneal fat masses (p<0.05), collectively driving lower delivery weight (p<0.0001) without changing the crown-rump length. Sagittal sections of fetal kidneys demonstrated delayed maturation, with disorganized glomerular generations and increased cortical thickness; this renal dysmorphology was not accompanied by structural nor functional changes indicative of renal insufficiency.
Conclusions.
We demonstrate fetal bioaccumulation of metformin with associated fetal growth restriction and renal dysmorphology following maternal initiation of the drug within 30 days of conception in primates. Given these results and the prevalence of metformin use during pregnancy, additional investigation of any potential immediate and enduring effects of prenatal metformin use is warranted.
Keywords: Metformin use, fetal bioaccumulation, insulin resistance, obesity, fetal programming
Tweetable statement:
In a primate model, maternal metformin intake from early pregnancy results in metformin bioaccumulation in fetal kidney, gut & liver tissues with associated decrease in fetal weight & abnormal renal maturation [hyperlink] identified by @norsketexsci and colleagues @SRWesolowski, @BTGarciaMED, @MaximSeferovic
Introduction
For over three decades, studies aimed at characterizing the Developmental Origins of Health and Disease (DOHaD) have performed experiments in mice, primates and humans and consistently demonstrated a durable effect of maternal-driven disruptions in fetal growth (either growth restriction or macrosomia) on later childhood and adult burden of metabolic, behavioral and immune-related disease.1–20 Two of the most prevalent maternal conditions that lead to accelerated fetal growth and macrosomia are obesity and diabetes, and poorly controlled diabetes in pregnancy (with or without maternal obesity) is significantly linked to a higher incidence and earlier onset of childhood obesity, insulin resistance, and type 2 diabetes (T2D).21–25
Not surprising, following these and other studies describing such a significant link between maternal hyperglycemia and offspring outcomes, several interventions aimed at treating maternal diabetes and predicant insulin resistance have gained clinical traction. Current recommendations for the medical management of either maternal T2D or gestational diabetes not controlled by diet alone (e.g., GDMA2) include frequent monitoring of maternal blood glucose combined with dietary management and insulin therapy to achieve maternal euglycemia.26–32 The American College of Obstetrics and Gynecology31 and the American Diabetes Association32 both recommend that insulin be considered the first line pharmacotherapy for overt T2D in pregnancy, with metformin (dimethylbiguanide) reserved for those who cannot use insulin or decline to do so. Others have reported challenges to 4 to 6 times daily injections of insulin therapy, potentially leading to prevalent use of metformin in lieu of insulin.28–30 Additionally, over the past decade, pragmatic use of metformin has expanded beyond treatment of T2D to now include use both prior to and during pregnancy for obesity, prediabetes, polycystic ovary syndrome (PCOS), gestational diabetes (GDMA1 and GDMA2), and even preeclampsia.33–36
With increased prevalence of metformin use during pregnancy, based on its pregnancy pharmacology and experimental data (e.g., secondary analyses of human clinical trials and limited experimental studies in mice), we and others have raised concerns that maternal metformin use in pregnancy may be accompanied by a potential risk of longer term harm to her offspring.37–50 The biology of these potential risks has been described. First, metformin does not undergo first-pass metabolism in the maternal liver and is instead transported across the placenta via binding to drug transporters on the basal and apical membranes of syncytiotrophoblasts and through paracellular diffusion.51–54 Therefore, a mechanism for risk of fetal bioaccumulation is present. Second, fetal mice, sheep, and Rhesus macaque fetal tissues express metformin transporters.51–60 Thus, tissue-specific effects of metformin may impact fetal growth potential via its effects on AMP-associated kinase (AMPK) activation and 1-carbon metabolism in somatic cells, both of which we and others have shown to be pivotal in fetal programming of later metabolic disease.2–12 Third, metformin acts to inhibit the mammalian target of rapamycin (mTOR) pathway, which is a key regulator of cell proliferation and anabolic processes.60 Fourth, metformin is also known to significantly alter the functional ecology of the adult gut microbiome61–63. In addition, some studies propose that metformin may have anti-folate activity and can alter microbial folate metabolism, leading to relative folate deficiency.64 As a result, metformin exposure in a fetus may mimic the “methyl folate trap” which has been linked to paradoxical obesity and insulin resistance in formerly growth restricted neonates.2–12
To experimentally address raised concerns for fetal metformin bioaccumulation following maternal initiation and continued use in pregnancy, we designed a model in the Rhesus macaque. In the current manuscript, we describe our experimental model, and the findings of our initial fetal cohort at the time of necropsy on gestational day (G)145.
Methods
IACUC approval and compliance of reporting with ARRIVE guidelines 2.0.
All animal care and procedures for these studies were performed according to the Institutional Animal Care and Use Committee (IACUC) at the ONPRC at Oregon Health and Science University and comply with the ethical standards with the NIH guide on the care and use of laboratory animals from the Animal Welfare Act. Separate IACUC approval for the handling of tissues and specimen was obtained at Baylor College of Medicine (AN-8851). Social and environmental enrichment activities were provided to all animals in the study. All elements of ARRIVE (Animal Research: Reporting of In Vivo Experiments) Essential 10 guidelines have been met. All fetal tissues have been banked and stored for these and other future mechanistic studies.
Timed-mated breeding.
In these experimental animal studies, we utilized Rhesus macaques from the Time-Mated Breeding Resource (TMB) at Oregon National Primate Research Center (ONPRC, Beaverton, OR). The TMB program is designed to optimize use of primates for studies on periconception and prenatal exposures by enabling precise timing of gestational exposure while reducing the number of animals required for research studies. We assured the precise initiation of maternal metformin (or vehicle control) in each dam during a uniform early gestational window of fetal development and organogenesis (i.e., by G30), and not during the preconception period. A term Rhesus gestation approximates a mean of 167 days (range 160–172 days), and a measurable crown-rump length (CRL) is detected by G18–24 on ultrasound (for reference, a human term gestation is 280 days and a CRL is sonographically detected by G30–45). In accordance with ONPRC well-established and validated gestational age biometry normograms65–67, we validated our conception dating (as described below) with crown-rump length biometry by greatest of three lengths from 21 to 30 days, and femur and biparietal diameter at G90, G115 and at G145 in our Rhesus macaques.
Time-bred dams (TMB Resource) are maintained as socially-housed, female-female pairs prior to being assigned to our studies. TMB maintains a proven reproductive history for prior live offspring births and carefully tracks reproductive hormone levels and menses. Circulating estrogen levels were monitored daily starting 8 days after menses. Once estrogen levels exceeded 100 pg/ml, females were moved into a cage and paired with a proven male breeder for up to 6 days, after which the female was checked for circulating progesterone levels (P4) to verify ovulation. Beginning on day 21 of the mated cycle (or one week after ovulation), blood was collected and assayed for P4. If P4 remained elevated (P4>1 ng/ml), an ultrasound was performed at approximately G27 to confirm pregnancy with early fetal biometry nomograms as detailed above. Once pregnancy was confirmed and gestational age was established as G21-G30, pregnant dams were randomly allocated to the drug group (metformin at 10 mg/kg, twice daily) or vehicle control and assigned to their diet (Western style or chow).
Maternal diet during gestation.
Female Rhesus macaques were maintained on one of two diets during these experiments: either a Western-style, high-fat and caloric dense diet (WSD) with 36% of calories from fat or regular chow breeding diet (CD) with 15% of calories from fat; we have previously published our findings in the Japanese and Rhesus macaque on these diets, inclusive of the rates of obesity over intervals of feeding.2–7,9–15,68–74 Following the experimental design of randomization to metformin or vehicle control, both dietary exposures were initiated at the time of pregnancy confirmation and gestational age establishment and were continuously maintained throughout pregnancy.
Metformin or vehicle control administration and validation of consumption and adherence.
Metformin hydrochloride was administered in the formulation of RioMet, a cherry-flavored suspension for clinical use, which was subsequently provided in a variety of Rhesus macaque preferable treats (Supplemental Figure 1). The goal of administering in individual and varied treats that the pregnant dams preferred was to enable ready consumption by the dam in the metformin allocated group, and prevention of sharing of treats with her partner. The same protocol was followed for vehicle control dams in their allocated cohort. Based on cage-side observations, animals consumed the whole treat within 5–10 minute minutes. If a dam was observed to not take and eat her treat within 5–10 minutes when administered at either of the twice daily “treat times”, the first treat was removed and a different treat (see Supplemental Figure 1) was immediately offered until treat ingestion was observed. This pattern was repeated twice daily throughout gestation and until the time of delivery, which was occurred onG145 in the current study reporting.
Cesarean delivery and fetal necropsy.
Pregnancies were delivered by Cesarean between G143–145. After an overnight fast, dams were sedated with 10–15 mg/kg ketamine with 0.1 ml glycopyrrolate and positioned in dorsal recumbency, followed by sterile prep and draping. The abdomen was entered via 10 cm linear ventral midline laparotomy, followed by delivery and draping of the gravid uterus with moistened laparotomy sponges. The fetus was balloted to the fundic region, and a 5–10 ml sample of amniotic fluid was sterile removed with a 20 gauge needle and syringe. A transverse hysterotomy was thereafter made and the fetus was delivered. 2–5 ml cord blood samples were obtained by the delivering surgeon from both the umbilical artery and vein prior to the cord being clamped with Hartman hemostats and transected. The placenta was separated from the wall of the uterus via gentle blunt dissection and gentle traction to minimize iatrogenic disruption, and the placenta was delivered with the trailing membranes. The hysterotomy incision was closed with two layers of 3–0 coated vicryl in a continuous Cushing pattern, the second imbricating the first and in every case hemostasis was noted. The abdomen was thoroughly irrigated and suctioned clear of all saline and blood. The rectus fascia and subcutaneous tissue was closed with continuous 3–0 coated vicryl, followed by skin apposition with continuous intradermal 4–0 monocryl. All dams survived the surgery without incident. In the current studies, the fetuses were euthanized according to the AVMA guidelines for the Euthanasia of Animals. At the time of surgical necropsy by trained personnel, anthropomorphic measurements and organ weights were obtained prior to tissue processing. The cord blood samples were immediately run using an i-STAT1 blood analyzer (Abbot Laboratories) using the CG4+ and EC8+ cartridges. The following analytes from the umbilical artery, representing the fetal artery, were analyzed for the current studies reported herein: pH, PCO2, PO2, lactate, glucose, blood urea nitrogen (BUN)/urea, hematocrit, sodium, potassium, and chloride.
Quantitation of metformin.
10 μL of NHP serum, urine or amniotic fluid (AF) samples were added into 60 μL of ice-cold methanol containing internal standard (IS, 0.1 μmol/L of agomelatine), vortexed, and centrifuged at 15,000 rcf for 15 min at 4°C. 45 μL of each supernatant was transferred into sample vial for Thermo TSQ Quantis MS coupled with a Thermo Vanquish UHPLC (UHPLC-TSQ MS) analysis. For tissue samples, 50 mg of tissue was mixed with 6x volume of 50% methanol-water, added with beads and thoroughly homogenized with a bullet blender. A 30 μL aliquot of tissue homogenate was then added to 200 μL of ice-cold methanol containing IS, vortexed, and centrifuged at 15,000 rcf for 15 min at 4°C. 120 μL of supernatant was transferred into a sample vial for analysis. To quantify metformin in the liquid or tissue samples, methanol stock solutions with different concentrations of metformin were spiked into blank mouse plasma, urine, human amniotic fluid, or tissue homogenate, respectively, and the calibration samples were prepared in the same way as actual samples. The linear ranges of the calibration curves for all liquid or tissue samples were 10–10,000 nmol/L with good linearity (all R2>0.99, weight 1/x2). For urine samples whose metformin concentrations were higher than the upper limit of the calibration curve, the samples were diluted with blank urine for 20 or 100 folds, then prepared according to the previously mentioned method and analyzed. LC-MS/MS analysis: All samples were analyzed with the UHPLC-TSQ MS. The analyte and the IS were separated on a Phenomenex Luna HILIC column (2.1 mm × 100 mm, 1.7 μm), and eluted by a water-acetonitrile mobile phase system (both containing 10 mmol/L of ammonium formate) with isocratic 81% acetonitrile in a 5-min run. The flow rate was set at 0.3 mL/min. The column temperature was 40°C, and the injection volume was 3 μL. Metformin and agomelatine were monitored under the selected reaction monitoring (SRM) mode coupled with a positive electrospray ionization (ESI) source. The quantification ion transition was 130.11→60.05 for metformin (collision energy 13.6 eV), and 244.10→185.05 for agomelatine (collision energy 30.1 eV). The ion spray voltage was 3,500 V. High-purity nitrogen was used as the sheath gas (50 arbitrary unit), auxiliary gas (10 arbitrary unit), sweep gas (1.0 arbitrary unit) and high-purity argon was used as the collision gas. The temperatures of ion transfer tube and vaporizer were 325°C and 350°C, respectively. Signal from 0.5 to 4 minutes elution was recorded by the mass spectrometer.
Fetal skeletal muscle histology.
At fetal necropsy, the mid-belly of several fetal skeletal muscles including medial gastrocnemius (gastroc), soleus, vastus lateralis, was identified, dissected, and embedded in Tissue-Tek® O.C.T. compound (Sakura Finetek, Tokyo, Japan), and slowly frozen in liquid nitrogen-cooled isopentane. Only data from the gastroc has been analyzed herein. All samples were stored at −80°C until processed. Transverse cross-sections (7 μm) were cut from the mid-belly of OCT embedded muscle using a cryostat and mounted onto permafrost glass slides. Slides were warmed to room temperature, washed 3X in PBS, and fixed/permeabilized in 100% ice-cold acetone for 3 minutes. Sections were washed 3X in PBS, incubated with wheat germ agglutinin primary antibody conjugated to AF647 overnight at 4°C with constant rocking. The sections were washed 3X in PBS, mounted in Gold Antifade mounting media and the coverslip sealed with clear nail polish. Images were captured at 10X or 20X magnification using a fluorescent microscope. At least 500 fibers per muscle were used to calculate the average cross-sectional area (CSA) using the Myosoft macro in FIJI (https://github.com/Hyojung-Choo/Myosoft/tree/Myosoft-hub).
Renal histology, serum analytes and electron microscopy.
Upon delivery at G145, the fetal kidneys were weighed, grossly examined for any structural or anatomical abnormalities, and then bivalved to assess for changes to major structures of the kidney including the cortex, medulla, renal pyramids, and calyces. One kidney from each specimen was formalin-fixed and the contralateral was flash frozen before overnight transport to Baylor College of Medicine from ONPRC. A portion of the formalin-fixed tissues were processed in a pediatric clinical pathology lab (Texas Children’s Hospital, Department of Pathology, Division of Anatomic Pediatric Pathology) under the supervision of a pediatric renal pathologist (JH); all personnel were blinded to the allocation group of the animals. Following standardized processing for formalin-fixed tissues, the fetal kidney tissues were paraffin embedded and then sectioned at 4μm. The resultant tissue sections, including the kidney capsule, cortex, corticomedullary junction and medulla, were mounted on glass slides and stained as follows: Hematoxylin & Eosin (H&E): for the general visualization of tissue organization; Periodic acid-Schiff (PAS): for the detection of glycogen, glycoproteins, glycolipids and other polysaccharides; Methenamine silver (Jones): the Jones variation to visualize the basement membranes of renal tissues; and Trichome: to contrast evaluate for fibrous tissue deposition. Note: all processing, tissue sectioning and staining were carried out using well-defined College of American Pathology accredited procedures by trained staff. For electron microscopy, formalin-fixed tissue was submitted to the College of American Pathology accredited electron microscopy clinical laboratory at Texas Children’s Hospital for processing and imaging by transmission electron microscopy to examine podocytes and other ultrastructural features employing well-defined College of American Pathology accredited procedures. All slides and specimens were stripped of group identifiers prior to blinded analyses by two investigators (BG and JH), the latter of which is a senior pediatric renal pathologist with extensive experience in the utilized methodologies.75–82
Statistical analyses.
The study’s unit of analysis was per fetus, and group-wise analyses were performed with the use of the confirmation-of-treatment principle with p<0.05 considered significant. Measures of significance were assessed using Chi-squared, Fisher’s exact or Mann-Whitney U-tests. Data were analyzed by two-way ANOVA with main effects of treatment (vehicle or metformin) and secondary effects of maternal diet (Chow or WSD) and their interaction. Skeletal muscle frequency data were analyzed for difference in best-fit curves across treatment groups. Data with different frequency distribution in fiber size is represented by different curve fits compared to the vehicle/Chow group. Data were analyzed with GraphPad Prism version 10.1.1. (GraphPad Software Inc., La Jolla, CA, USA), R version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria) and STATA 16.0 (STATA Corp, College Station, TX, USA). The funder played no role in data collection, analysis, interpretation, writing of the manuscript or the decision to submit this manuscript for publication.
Data Availability Statement:
Criteria data will be shared by encryption in a de-identified manner with (1) a signed data use/access agreement, and (2) approved data analysis plan.
Results
Inclusion and exclusion of fetal animals.
Of the initial 10 mg/kg twice-daily metformin-dosed or vehicle-control pregnancies a priori destined for fetal necropsy, n=13 continued to viable G145 gestations. Among the n=13 G145 pregnancies with fetal necropsy, two were excluded. One dam and its fetal tissues had detectable metformin levels despite being allocated to the vehicle control group (>1 μmol metformin/kg maternal weight or fetal/placental tissue: Maternal: urine 52.9 μmol/L; Fetal: serum 0.1 μmol/L, urine 4.3 μmol/L, amniotic fluid 0.003 μmol/L, kidney 1.4 μmol/kg, liver 0.1 μmol/L, placenta 0.2 μmol/kg). We assumed that it was most likely the case that this “treat thief” dam had snuck a metformin treat from another dam while located in her temporary domicile on the day of Cesarean delivery. A second fetus allocated to the vehicle control group had severe fetal growth restriction (birthweight 248.32 g, <1% for gestational age) and abnormal prenatal ultrasound inclusive of an irregular, thickened monodiscoid placenta with mild marginal fibrosis, eccentric umbilical cord insertion, and mildly thickened membranes with oligohydramnios.83 Upon necropsy, the prenatal ultrasound findings of low fetal weight and placental abnormalities were confirmed, with additional findings of minimal stomach fluid and absent subcutaneous nor visceral adipose tissue depots indicative of a congenitally anomalous fetus unrelated to the study interventions per prior established criteria in the Rhesus macaque.83 Given our a priori intent to only include non-anomalous Rhesus macaque fetuses, we excluded this second fetus accordingly. After excluding these two fetal gestations from further analyses, 11 fetuses from dams initiated on either vehicle control (n=4, 3 female, 1 male) or 10 mg/kg (twice daily) metformin (n=7, 5 female, 2 male) were available for analyses.
Fetal bioaccumulation of metformin.
Metformin levels were assayed in maternal and fetal fluid and tissues using quantitative triple-quadruple mass spectrometry (UHPLC-QQQ) in SRM mode against standard curves. In preliminary experiments in an adult male Rhesus macaque (n=1), we undertook measurements of metformin administered in treats as described, at 8-hour intervals in a 24-hour pharmacodynamic study. Recapitulating human pharmacology, urine concentrations were at least one-log fold higher than serum or plasma (Fig. 1A) when administered at a clinically relevant standard dose of 10 mg/kg (112.5 mg) orally every 9 hours, with urine concentrations at log-fold higher levels than serum or plasma. Armed with this pharmacodynamic data, we proceeded with our planned studies utilizing dams which were timed-mated bred and initiated on 10 mg/kg twice daily metformin or vehicle control by G30. At the time of breeding, these dams had been initiated on one of two diets: a 16% fat standard chow diet (Chow or CD) or a 36% caloric dense, high fat, Western style diet (WSD) as previously described. 2–7,9–15,68–74 Serum, urine, or amniotic fluid or fetal tissues (Fig. 1C–E) from paired maternal-fetal dyads were measured. Amongst the n=11 included pregnancies, metformin was not detected in animals nor fetal tissues provided treats with vehicle alone, and in the metformin group, metformin levels did not differ by maternal diet (chow vs WSD where Spearman’s correlation, annotated by dashed colored lines for 95% CI, shows high degree of concordance; Fig.1B). Among dams initiated on metformin by G30, we observed bioaccumulation within the fetal kidney (0.78–6.06 μmol/kg, mean 2.48 μmol/kg), fetal liver (0.16–0.73 μmol/kg, mean 0.38 μmol/kg), fetal gut (0.28–1.22 μmol/kg, mean 0.70 μmol/kg ), amniotic fluid (0.43–3.25 μmol/L, mean 1.88 μmol/L), placenta (0.16–1.0 μmol/kg, mean 0.50 μmol/kg) and fetal serum (0–0.66 μmol/L , mean 0.23 μmol/L) and fetal urine (4.1–174.1 μmol/L mean 38.5 μmol/L), the latter of which was near biomolar equivalent to maternal levels (maternal serum 0.18–0.87 μmol/L, mean 0.46 μmol/L; maternal urine 42.6–254.0μM, mean 149.3μM). Metformin levels were measured in the morning of Cesarean delivery and fetal necropsy, approximately 18 hours after the last maternal dose. Fetal accumulation was apparent and as evidenced by the higher than 1:1 ratio correlation of molar concentration of amniotic fluid to maternal and fetal serum (Fig. 1B). These bioaccumulations were irrespective of diet, and WSD feeding neither accelerated nor reduced metformin bioaccumulations in maternal or fetal serum, urine, amniotic fluid, placenta nor fetal tissues.
Figure 1. Metformin does not undergo first pass metabolism and bioaccumulates in maternal-equivalent concentrations in fetal kidney, fetal urine and amniotic fluid as assessed by mass spectrometry.
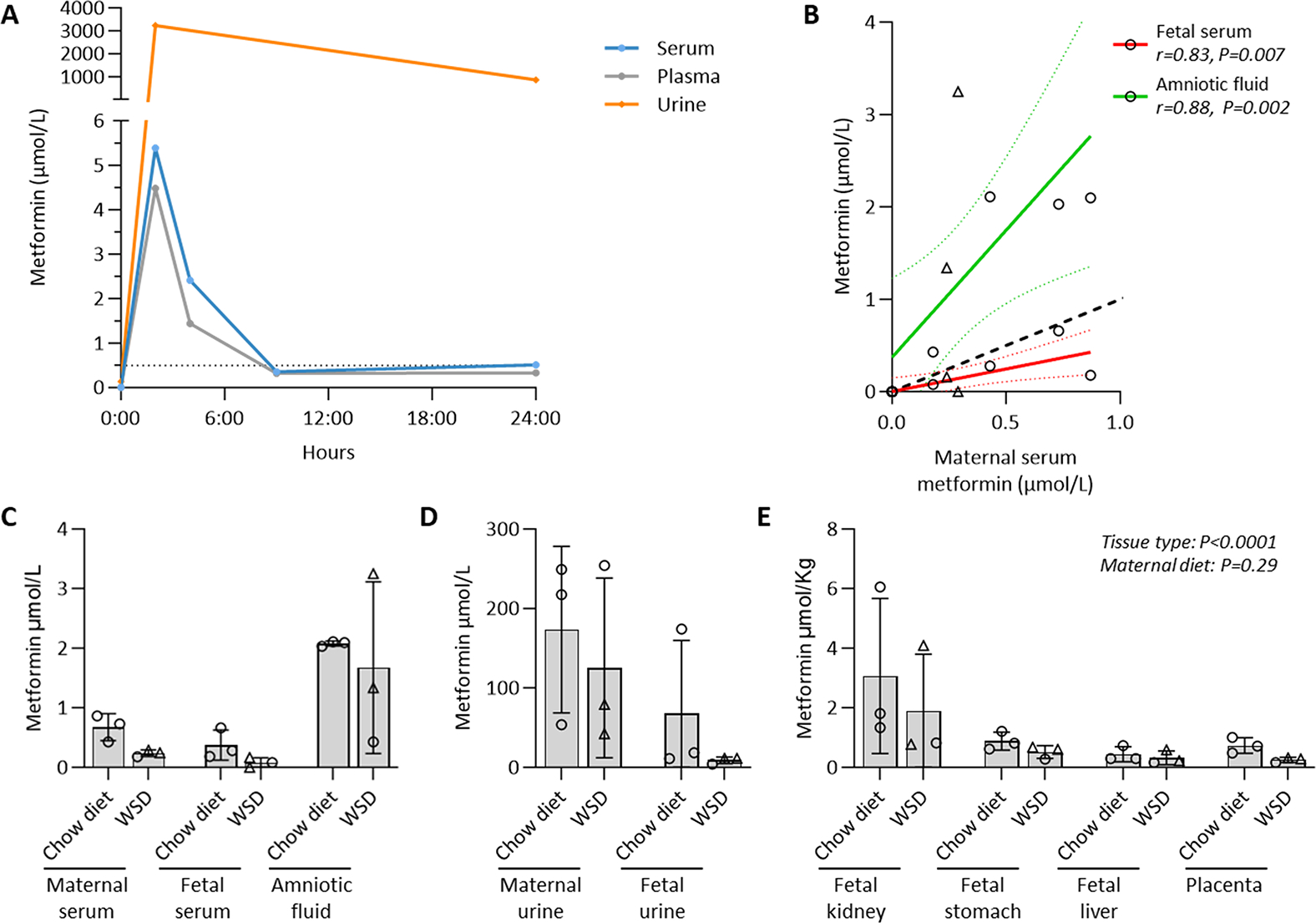
(A) Renal clearance without first-pass metabolism in a non-pregnant male Rhesus macaque. A standard dose of 10 mg/kg was orally administered to an adult male Rhesus macaques every 9 hours, with urine concentrations at log-fold higher than serum or plasma. (B-E) Maternal metformin bioaccumulates in the fetus. Dams were initiated on 10 mg/kg twice daily metformin or vehicle control by gestational day 30 (G30), and fetuses were delivered via Cesarean at G145. Amongst the n=11 included dams, metformin was not detected with vehicle alone (not shown), and levels did not differ by maternal diet (chow diet vs maternal Western style diet; WSD). *Spearman’s correlation, dashed colored lines for 95% CI, shows significant separation from 1:1 line of identity (dashed). Males are represented as triangles, females as circles. Means −/+ SD are shown. Main effects from ANOVA are shown.
Initiation and use of metformin during pregnancy with resultant maternal and fetal plasma and blood analytes and glucose levels.
As anticipated, the individual animal variability of all measured analytes largely reflects fluxes in maternal and fetal physiology that are inherent with the sampling and collection during Cesarean delivery with maternal anesthesia. Umbilical artery hematocrit and blood gasses were measured on immediately clamped cords at Cesarean. There was no difference in fetal hematocrit by virtue of maternal metformin use (Fig. 2A). The fetal pH was higher among fetuses exposed to metformin in utero (Fig. 2B). Fetal pO2 was not significantly different (Fig. 2C), while fetal pCO2 trended lower among fetuses exposed to metformin in utero (Fig. 2D). Blood urea nitrogen (BUN) was 40% lower in fetuses from dams who consumed a WSD compared to those fed a chow diet (Fig. 2E), but there was no difference by maternal metformin use. Importantly, maternal metformin use was not associated with any differences in the fetal lactate nor glucose concentrations (Fig. 2F–G). Additionally, administration of metformin throughout pregnancy did not significantly alter maternal glucose concentrations (Fig. 2H).
Figure 2. Initiation of maternal metformin use by G30 and continued duringdruingduring pregnancy twice daily does not result in maternal nor fetal anemia nor hypoglycemia.
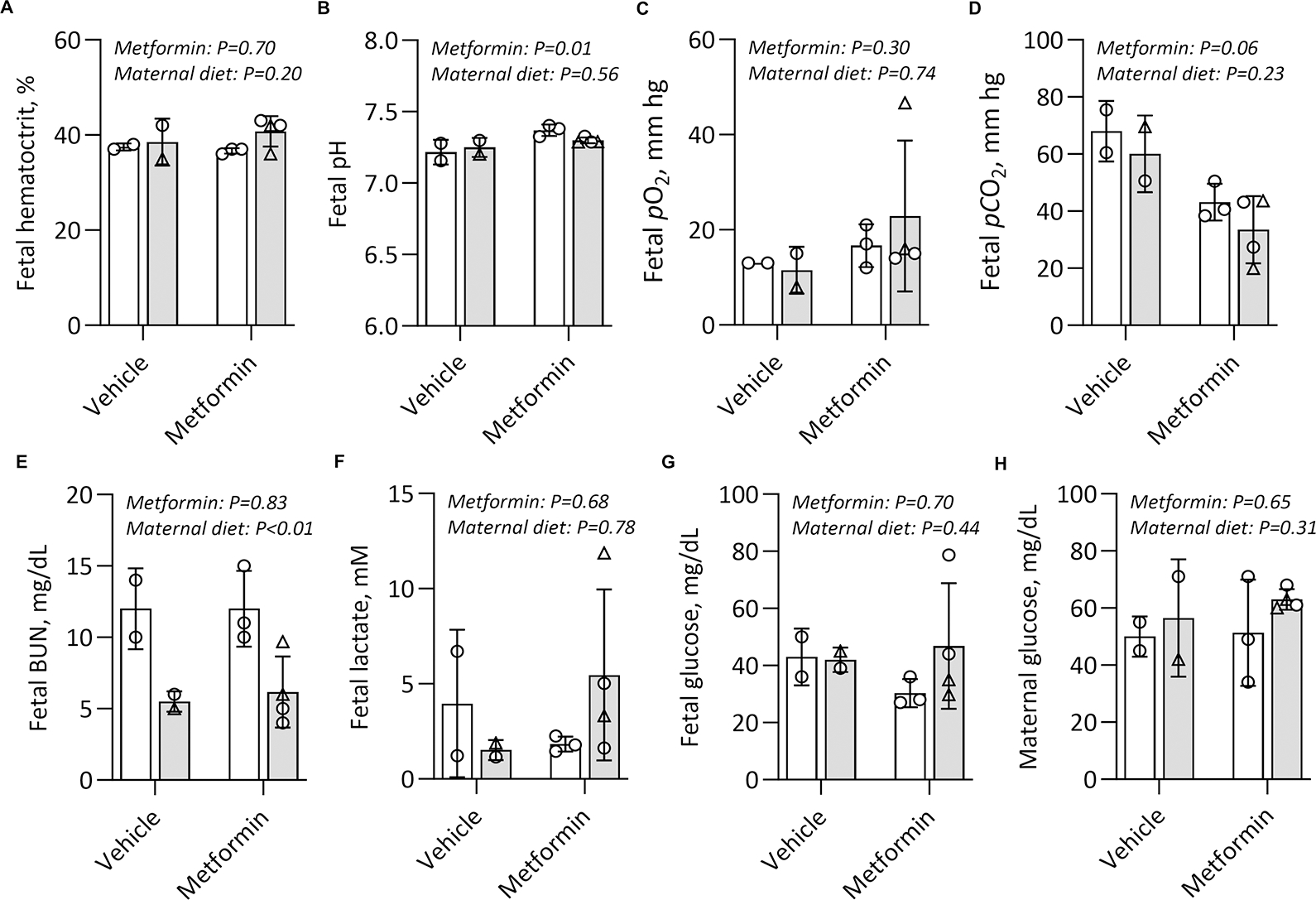
Umbilical artery (fetal) blood was collected at G145 (n=2–4/group). Blood gasses, hematocrit, BUN, lactate, and glucose were measured. Maternal glucose concentrations were collected and measured the day prior to Cesarean surgery prior to fasting. Results were analysed by two-way ANOVA. Main effects and measure of significance shown by p value. Males are represented as triangles, females as circles. Means −/+ SD are shown. Chow diet (CD, white bars) or Western style diet (WSD, grey bars)
Fetal bioaccumulation of metformin and accompanying intrauterine growth.
Fetal growth by direct weight and biometry measurements at Cesarean was primarily compared between offspring of dams initiated and maintained on twice daily metformin and those exposed to vehicle (Fig. 3). We observed a restricted fetal growth phenotype among near term G145 fetuses (87% term gestational length) exposed to metformin, with reduced fetal weight (Fig.3A, metformin 371 g vs vehicle 445 g, metformin main effect p<0.0001) and despite an anticipated increased fetal weight with maternal WSD feeding in this cohort (Fig. 3A, maternal diet main effect p<0.0001). When we sought to parse and ascribe the fetal weight differences to visceral, muscle, or skeletal growth variation, we observed that maternal metformin initiation in early pregnancy and continued 10 mg/kg twice daily dosing until delivery at G145 resulted in reduced fetal liver weight (Fig.3B, metformin 10.64 g vs vehicle 12.6 g, metformin main effect p=0.03), reduced fetal retroperitoneal fat mass (Fig. 3C, metformin 0.078 g vs vehicle 0.1758 g, metformin main effect p=0.02), and fetal heart mass (Fig. 3D, metformin 2.4798 vs vehicle 3.03 g, maternal main effect p=0.02) by G145. There was no difference in fetal kidney weights (Figs. 3E) nor total fetal brain mass (Fig. 3F). Finally, there was no difference in skeletal growth, as determined by the absence of observed differences in the fetal crown-rump length (Fig 3G) nor in the fetal biparietal diameter (BPD Fig. 3H) by either metformin or maternal diet.
Figure 3. Maternal metformin initiation and use during pregnancy reduces fetal visceral and body weight.
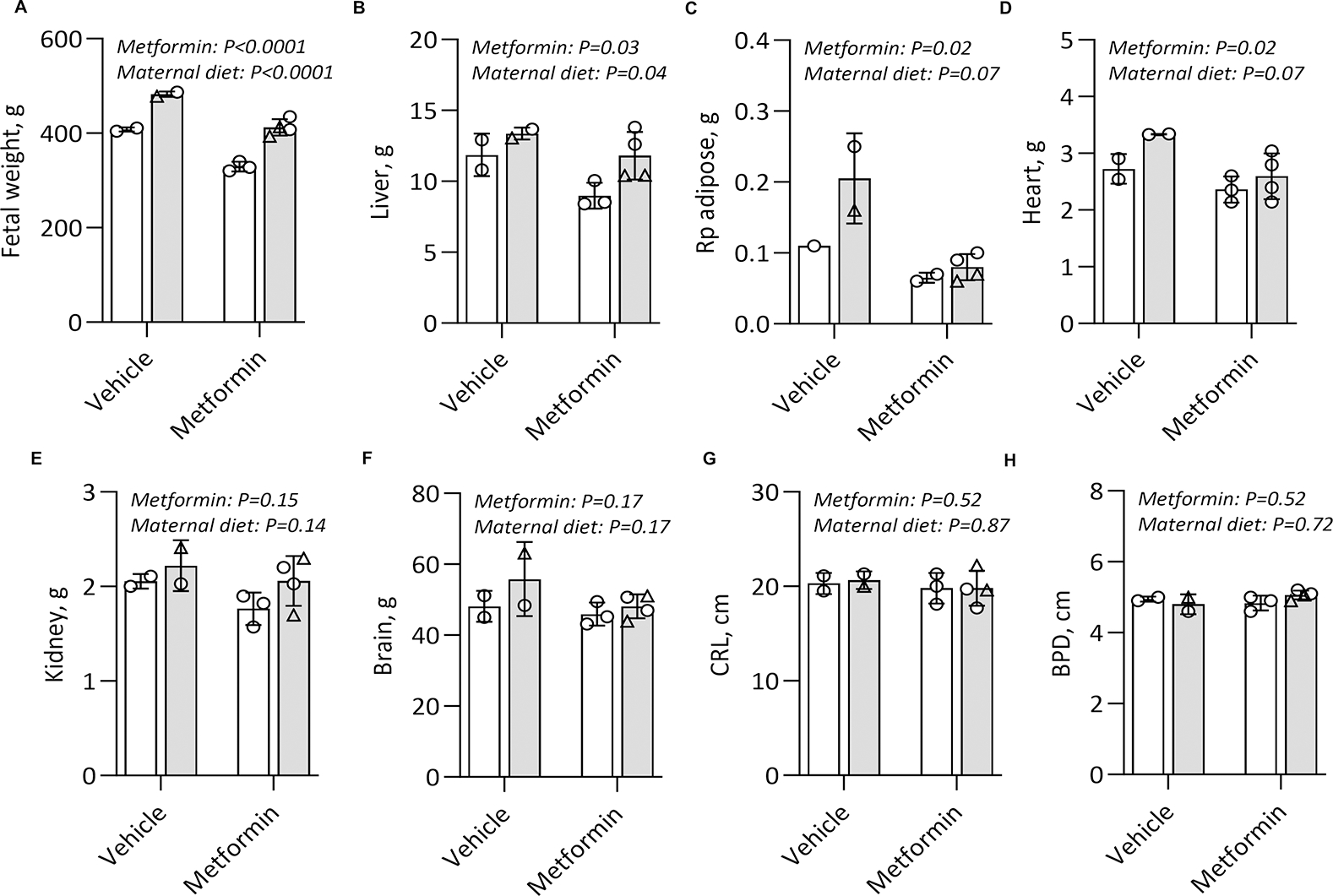
Fetuses were delivered via Cesarean at G145 (n=2–4/group) and (A) fetal weight and fetal organ and tissue weights were measured (B-F). Fetal crown-rump length (CRL) (F) and biparietal diameter (BPD) (G) were also directly measured. Results analysed by two-way ANOVA. Main effects and measure of significance shown by p value. Males are represented as triangles, females as circles. Means −/+ SD are shown. Chow diet (CD, white bars) or Western style diet (WSD, grey bars).
Maternal metformin initiation and continued use in pregnancy and associated fetal skeletal muscle growth as a measure of accretion.
Cross-sectional area was measured in fetal medial gastrocnemius as a marker of fetal muscle growth in each group (Fig. 4A). In Rhesus macaques, the gastrocnemius is a mixed fiber type muscle with a high proportion of type 2a and 2x fibers84 making it optimally representative of whole-body skeletal muscle. The fetal gastrocnemius cross-sectional area (CSA) was reduced by 57% when offspring were exposed to maternal metformin use when compared to vehicle (Fig. 4B; p=0.0001). In vehicle controls but not the metformin group, CSA was reduced by maternal WSD feeding (interaction effect, p=0.03). Frequency distribution graphs revealed a significant left shift in the fetal gastrocnemius fiber size profile with maternal metformin initiation and continued use in pregnancy (Fig. 4C), indicating an overall loss in protein accretion across the range of fiber sizes and serving as a strong indicator of fetal growth restriction. Because maternal weight-based dosing was standard to our protocol, we cannot reliably estimate a dose-response of bioaccumulation of metformin to the gastrocnemius cross-sectional area. When we collectively consider the data presented in Figures 3 and 4, the metformin-associated reduction in total fetal body mass appears to be driven by a restriction of visceral organ mass and skeletal muscle mass accretion, rather than reduced linear or skeletal growth.
Figure 4. Maternal metformin initiation and use during pregnancy reduces fetal skeletal muscle mass.
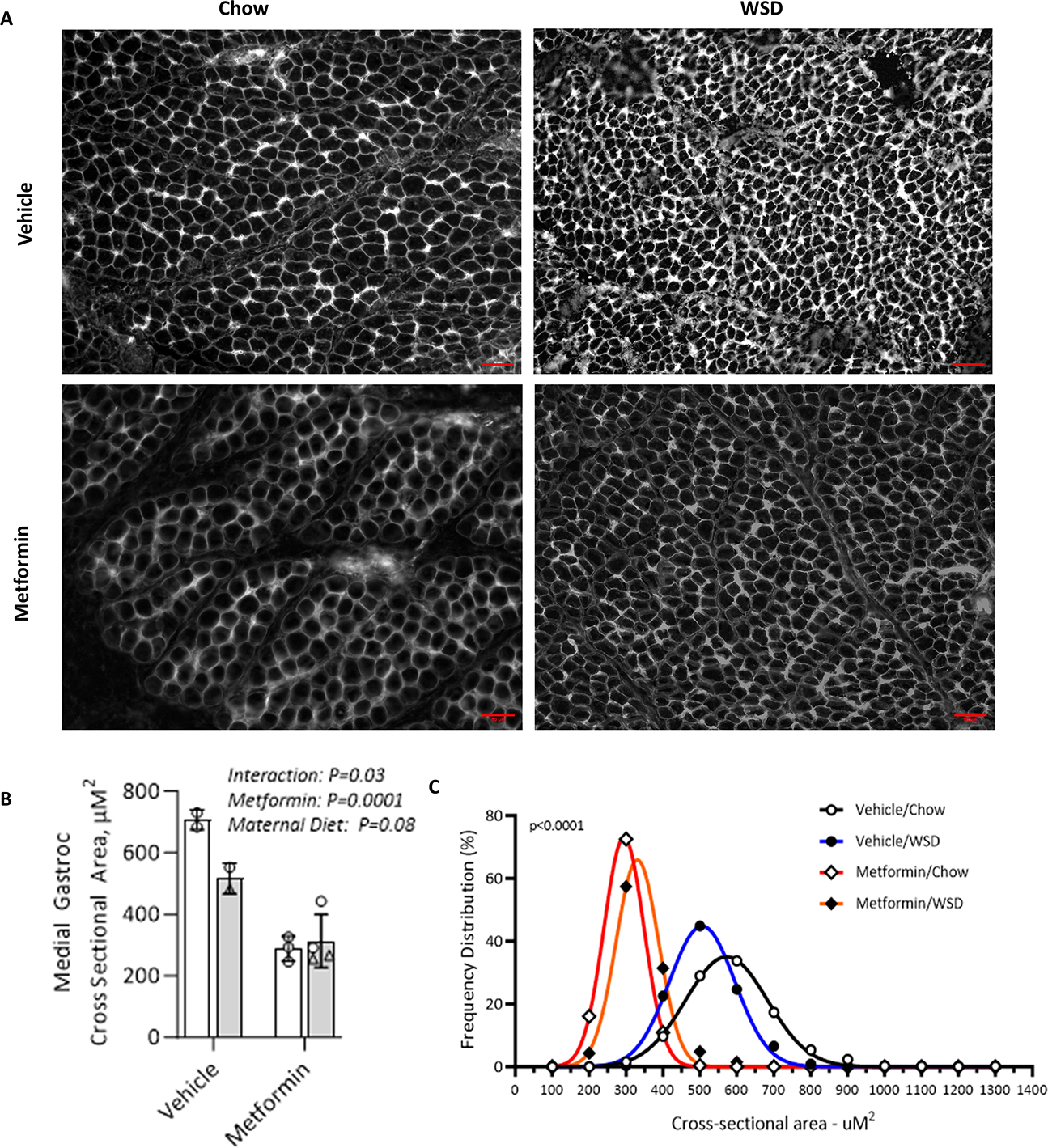
Cross-sectional area (CSA) was measured at the muscle midbelly in the fetal medial gastrocnemius at G145 from dams treated with twice daily metformin or a vehicle control and fed either a chow diet (CD, white bars) or Western style diet (WSD, grey bars), n=2–4 per group. (A) Representative images of muscle from each group taken at 20x magnification with scale bar. (B) CSA was calculated for each animal using a minimum of 500 fibers. Results were analyzed by two-way ANOVA with main effects and measure of significance shown by p value. Data are the mean ± SD. Males are represented as triangles, females as circles. (C) Frequency distribution for fiber size was calculated per animal and frequency per bin averaged by group. Data sets were compared to test if data fit same or different best-fit curves, with the p value indicating that best fit curves are different across groups. Graph shows frequency of data at each 100 unit bin and the best fit curve of the data.
Fetal metformin exposure and histologic measures of renal developmental and function.
Our analysis of renal cortical histology extending from the renal capsule adjacent nephrogenic zone to the corticomedullary junction revealed that metformin-exposed fetuses display a significant delay in glomerular migration, as evidenced by a reduction in the number of glomerular generations when compared to the control group (Fig. 5 panels A, D, G & K). Specifically, metformin-exposed fetuses on CD showed a 30% decrease in the average number of glomerular generations compared to vehicle controls (Fig. 6A; metformin main effect, p<0.0001). Maternal WSD diet feeding neither potentiated nor exacerbated these findings, given that WSD exposed fetal kidneys demonstrated a 45% reduction in glomerular generation when also exposed to metformin, compared to CD without exposure to metformin nor WSD (Fig. 6A; diet effect, p=0.004). There was no significant interaction of diet and metformin.
Figure 5. Representative images of fetal renal histology and ultrastructure with maternal metformin initiation and use during pregnancy.
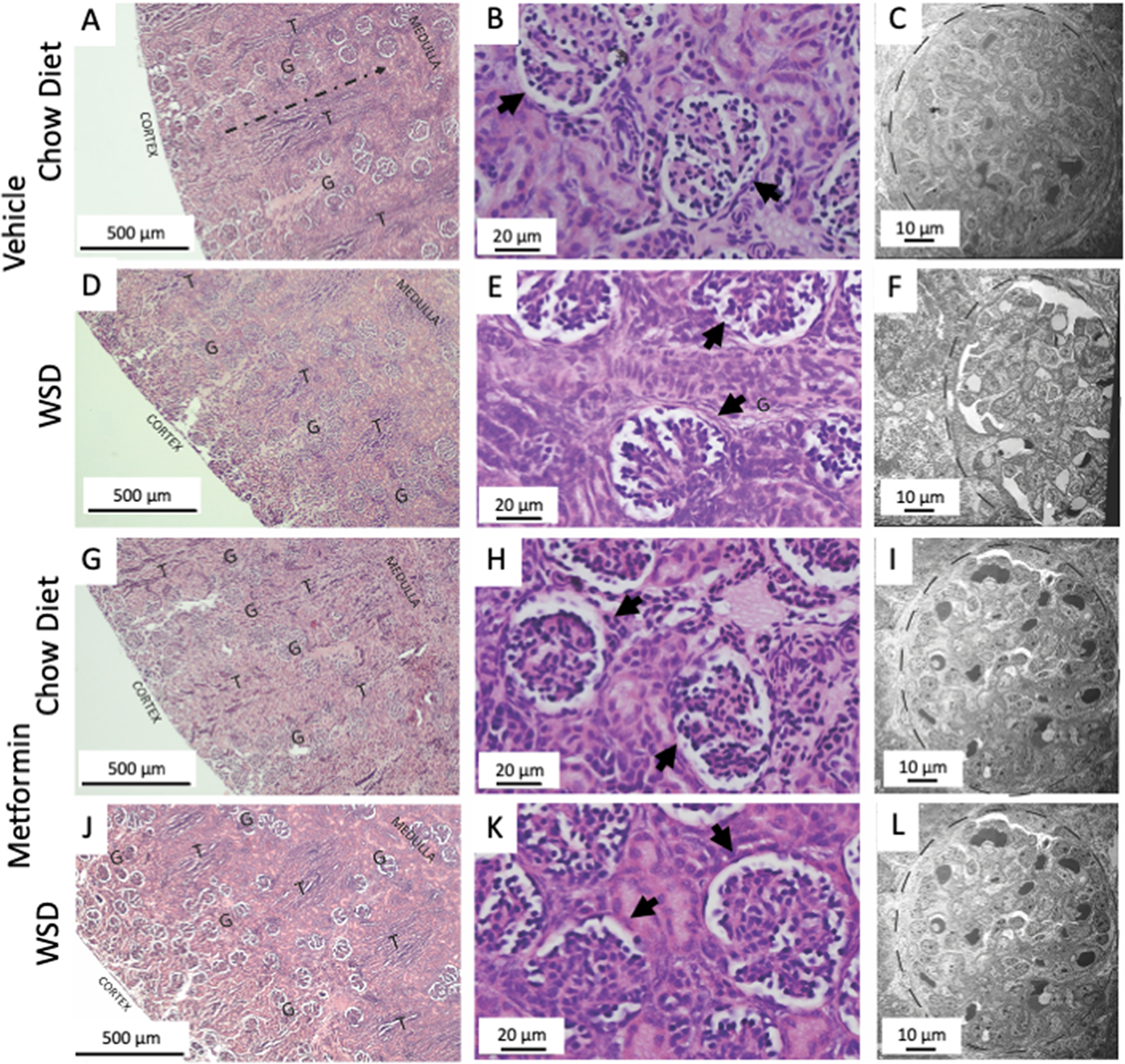
(A, D, G, J) H&E staining of the renal cortex from capsule (outer edge) to the corticomedullary junction. Glomeruli (G) develop from the capsular edge and travel toward the corticomedullary junction during development, following the path indicated in panel A (dashed line) between tubule bundles (T). Animals exposed to metformin exhibited greater disorganization, reduced generations, and greater proliferation of glomeruli near the renal capsule. (B,E,H, K) H&E stains at greater magnification to increase mesangial proliferation within the glomeruli of metformin exposed fetuses (arrows). (C,F,I,L) Electron microscopy ultrastructural images of renal glomeruli (dashed line) including podocytes, with no evidence of ultrastructural disruptions with fetal metformin exposure in utero.
Figure 6. Quantitative analyses of fetal renal histology.
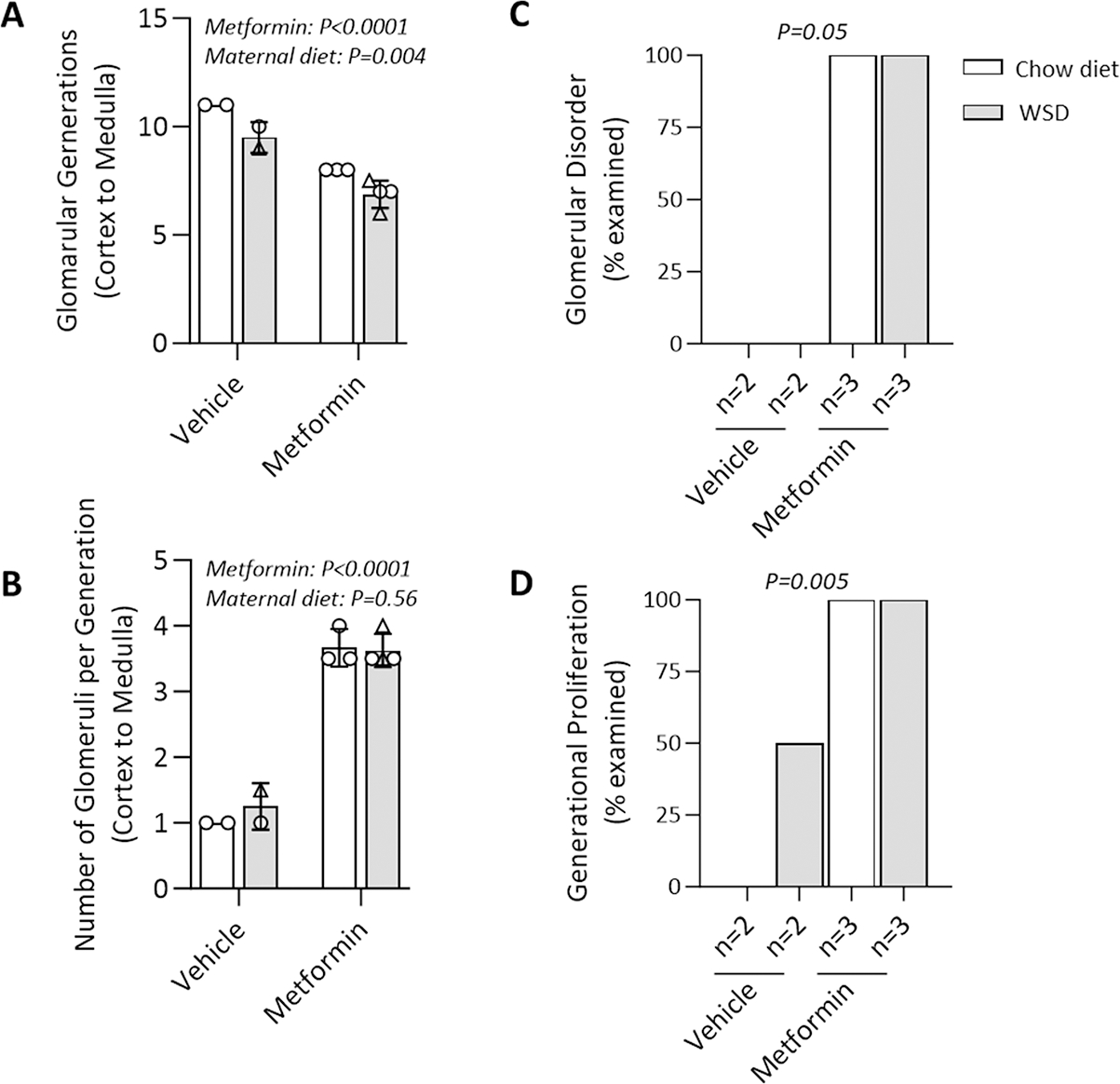
(A) The number of fetal glomerular generations present varied significantly in association with both maternal metformin use and WSD feeding. (B) Within each generation, there was increased proliferation of glomeruli in association with maternal metformin exposure, but not with maternal WSD. The disordered patterning (C) and generational proliferation (D) (as compared to absence of proliferation) of fetal glomeruli was observed with maternal metformin exposure during pregnancy independent of maternal diet as blindly assessed by a pediatric pathologist. The significance was assessed by two-way ANOVA (A&B) and Fisher’s exact test (C&D).
Additionally, at G145 the fetal kidneys exposed to metformin exhibited a marked increase in glomerular proliferation within the nephrogenic zone of the kidney capsule (Fig. 5 panels A, D, G & K). This was characterized by a disorderly appearance and excessive generational proliferation largely absent in the unexposed group (Fig.6 panels C & D, p=0.05 and p=0.005, respectively). At each glomerular generation, there were more than 3 times the number of glomeruli present within the metformin exposed group compared to vehicle control (Fig. 6B; metformin effect p<0.0001). Metformin exposed glomeruli also had increases in mesangial cellularity (Fig. 5 panels B, E, H & I) and decreased cortical thickness. However, despite these structural alterations, ultrastructural examination with electron microscopy revealed no glomerular abnormalities with podocytes, mesangial cell, mesangial matrix or glomerular basement membrane architecture (Fig.5 panels C,F, I & L). Collectively, these studies of the fetal renal tissues suggest that effects of maternal metformin use during pregnancy may be limited to disruptions in fetal renal glomerular development rather than ultrastructural dysgenesis or changes in glomerular function.
Discussion
Principal findings
Among dams initiated on metformin by G30, we observed bioaccumulation within the fetal kidney, liver, and gut, as well as in the amniotic fluid, placenta, and fetal serum and fetal urine, the latter of which was near biomolar equivalent to maternal levels. These findings were associated with growth restriction observed in the fetal heart, liver, and retroperitoneal fat masses (p<0.05), driving lower birthweight. Skeletal muscle CSA, a marker of muscle growth and protein accretion, was significantly reduced in the fetal gastrocnemius with maternal metformin initiation and use during pregnancy. Kidneys from metformin-exposed fetuses demonstrated delayed maturation, with disorganized glomerular generations and increased cortical thickness; this renal dysmorphology was not accompanied by structural changes indicative of renal insufficiency. Consistent with studies of fetal growth restriction in utero in primates and other mammals, these findings may suggest a potential link to an elevated likelihood of obesity and insulin resistance in childhood. These conditions could, in turn, contribute to a higher risk of cardiometabolic diseases in the adult years of the offspring. However, since the current studies were terminal fetal necropsies, neither neonatal nor longer term outcomes were measured. As we continue the overall study, the impact of metformin exposure during pregnancy will become more apparent.
Our results in the context of what is known.
Maternal pharmacology studies aimed at identifying risks of fetal drug toxicity have, historically, primarily relied on the detection of malformations or harm observed during fetal development or at birth and may overlook more nuanced findings related to fetal growth or metabolism that pose potential long-term health risks. Additionally, risk of fetal drug bioaccumulation occurs with classes of drugs that do not undergo first pass hepatic metabolism and are transplacentally transported. Metformin, a mainstay of oral glycemic and insulin-sensitizing therapy, is one such drug. Metformin is not known to cause congenital anomalies, and our studies support that conclusion. However, the absence of congenital anomalies neither predicts nor signals short nor long term safety among exposed offspring. Historically, it was thought that it would be unlikely that metformin would have bioactive effects in the fetus based on two assumptions: first, that mitochondrial metabolism, a key target of metformin action, was not recognized as being mature in the fetus, and second, that metformin transporters were not known to be abundant in human or primate fetal tissues. Our published data have refuted those assumptions. In addition, because metformin does not undergo first pass metabolism and does undergo native placental transport, we and others recognize that there is a real risk for fetal bioaccumulation and subsequent harm.37,51–59 We undertook these terminal fetal studies in the non-human primate because they could not be undertaken in humans, and rodents fail to recapitulate either human pregnancy physiology or metformin functional pharmacology. Employing a rigorous study design benefitting from our use of timed-mated breeding pairs, our findings are of significance, are intrinsically causal, and our results and their interpretation lend potential insight into rather heterogeneous results arising from secondary analyses of several large clinical trials.38–40,44–50
Inadequate maternal nutrition and/or fetal malperfusion results in fetal growth restriction and reprogramming.1,4,11,12,14 These adaptations may include alterations in placental size and function, changes in fetal metabolism, and modifications of hormone levels that influence growth and development.2,3,5,7,9 The complex interaction between maternal nutrition and placental function has been demonstrated to modify key development outcomes.89 Similar to studies of preterm and growth restricted fetuses, metformin exposure in utero may lead to developmental changes that, while beneficial in the context of managing maternal hyperglycemia, could be detrimental to fetal metabolic programming and renal development.
The adverse perinatal and fetal consequences of maternal hyperglycemia are well-characterized, and optimizing maternal glycemic control is a relevant and achievable goal. The lingering unanswered question is what oral medication(s) offer near equivalent maternal efficacy, without risk of harm to the exposed offspring. Our findings of reduced fetal growth are consistent with findings from several human clinical trials. Feig and colleagues published the results of the international MiTy trial, a randomized controlled trial of insulin plus metformin or placebo in pregnant participants with overt diabetes.85 Consistent with our findings, the MiTy investigators reported that neonates born to participants exposed to metformin were more likely to be small-for-gestational age compared to those exposed to placebo, with neonatal adiposity but no significant reduction in composite neonatal morbidity.85 The most recent follow-up of these former neonates, now at 24 months of age, demonstrated that while anthropometric analyses failed to show a significant difference between metformin and placebo-exposed cohorts, the BMI was significantly higher in the metformin-exposed offspring at 6–24 months of age.86 Although our primate model plans to do so, we have not yet examined the impact of maternal metformin use on later childhood growth and metabolism outcomes in juvenile offspring. However, other secondary analysis of childhood outcomes following maternal randomization to metformin for diabetes and other metabolic disorders, including the MiG and PregMet trials, have shown that by 4 and 9 years of age, metformin exposed offspring demonstrate significantly higher BMI, waist-to-height ratio, and waist circumference z-scores with proximal measures of insulin resistance.37–39,44,45,87 Our results in the context of these longer term outcome studies in children collectively suggest that the ‘anti-macrosomic’ effect of metformin attributed to improved maternal glycemic control may actual be more insidious, and represent metformin-mediated inhibition of cellular mechanisms crucial for healthy fetal and early growth trajectories, resulting in accelerated obesity & insulin resistance in early childhood. 37–39,44,45,85–87
Strengths and limitations.
This study has numerous strengths. First, it was conducted in a primate model which recapitulates human pregnancy and fetal physiology. Second, we took advantage of our unique TMB resource to precisely date pregnancies and initiate maternal metformin in early gestation and not in a highly variable preconception period. Third, we tested the effect of metformin on fetal bioaccumulation and accompanying tissue-specific disruptions on two maternal diets inclusive of a control chow and a Western style, calorically dense diet. Fourth, we developed a highly sensitive mass spectrometry assay to directly measure metformin levels in fetal and maternal fluids and tissues. Fifth, we leveraged the expertise of senior pediatric renal pathologists (blinded to animal allocation group) in identifying and characterizing metformin-related dysmorphology in the fetal kidney.
Our study is not without limitations. First, we are reporting the initial findings in a limited number of animals in an experimental cohort of well-characterized pregnancies. While we reached statistical significance in our findings when testing for the main effect of metformin compared to vehicle placebo, across both diet groups, we could not report our analyses stratified by fetal gender and thus cannot account for fetal gender as a biologic factor. Second, we were unable to include two fetuses, either due to an unknown duration of “treat thieving” in a vehicle control dam, or a suspected congenital growth anomaly in a second fetus. Third, the focus of the current report is on the fetal bioaccumulation and outcomes and does not include our ongoing work in postnatal offspring. Thus, claims relating to insulin resistance and obesity from the current cohort cannot be made and would be premature. Fourth, the impact of both metformin and maternal diet is limited by our small sample size. and secondary conclusions drawn from groups where the n is less than 3 are limited to conditional analyses.
Clinical implications of our findings.
We demonstrate fetal bioaccumulation of metformin with associated fetal growth restriction in the viscera and skeletal muscle, and significant renal dysmorphology, following maternal initiation of the drug within 30 days of conception. Given these results and the prevalence of metformin therapy, additional investigation of any potential immediate and enduring effects of prenatal metformin use is warranted.
Supplementary Material
Supplemental Figure 1. Treats used in the delivery of metformin (dosed to each individual dam at 10 mg/kg twice daily)or vehicle control. (A) An example of the variety of treats offered. The full spectrum of treat types include: fig newton or banana and cereal balls, honey graham cracker balls, graham or saltine cracker sandwiches with jam or peanut butter, nilla wafer and jam sandwiches, fruit loop marshmallow balls, yogurt pudding cups, orange gummies, jello shots, gingersnap and marshmallow fluff sandwiches, injected orange slice, monkey morsel (trail mix) patty, banana and cereal saltine sandwiches, pumpkin cup with marshmallows, pumpkin or sweet potato cups, and mashed banana mixes. (B) Number of treats typically required for two days of therapy. Average time spent administering and observing adherence with non-sharing of treats approximates two hours per day. As noted in results, one dam (“treat thief”) allocated to the vehicle control group did have detectable levels of metformin on assay and that fetal offspring was excluded from analyses. Because of the mechanism of observed treat intake, the “treat thief” was thought to have taken the metformin treat approximating the timing of delivery and was not representative of chronic “treat swapping” nor “treat thievery” among allocated group animals in the cohort.
AJOG at a Glance:
Why was this study conducted?
While maternal metformin use is increasingly prevalent, targeted studies examining fetal bioaccumulation are limited.
Key findings.
Among the n=11 G145 pregnancies with confirmed exposure to drug or vehicle and normal fetal necropsy, we observed significant metformin bioaccumulation in kidney, liver, gut, placenta, amniotic fluid, serum and urine of drug-exposed fetuses. Levels in fetal urine neared biomolar equivalence to maternal levels following initiation by G30. Bioaccumulation of metformin in the fetus was associated with growth restriction in liver, skeletal muscle, heart and retroperitoneal fat masses, driving lower fetal body weight. Sagittal sections of fetal kidneys demonstrated delayed maturation, with disorganized glomerular generations and increased cortical thickness.
What does this add to what is known?
We demonstrate fetal bioaccumulation of metformin with associated fetal growth restriction and renal dysmorphology following maternal initiation of the drug within 30 days of conception. Given these results and the prevalence of metformin use during pregnancy, additional investigation of potential immediate and enduring effects of prenatal metformin use is warranted.
ACKNOWLEDGMENTS
This research was supported by NIH grant RO1DK128187 (K.M.A, communicating PI; P.K. and J.E.F co-PIs). The ONPRC Rhesus colony and TMB resources are supported by NIH P51 OD011092. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. K.M.A, P.K., M.G., C.E.M, J.E.F., S.R.W., T.A.D., B.T.G., M.A.S. and M.D.S are the guarantors of this work and, as such, had full access to the data included in this study and take collective responsibility for the integrity of the data and the accuracy of the data analysis. We also wish to thank the Baylor College of Medicine Advanced Technology Core for their support of the NMR and Drug Metabolism Core, where the metformin quantitative assay was developed and validated.
Funding.
This work was supported by NIH (NIDDK R01DK128187 to KMA, PK, JEF).
Footnotes
COMPETING INTERESTS STATEMENT
No authors have any conflicts of interest to declare.
Paper Presentation Information. Data from this paper was presented as an oral presentation (abstract #25) at the Society of Maternal-Fetal Medicine’s 44th Annual Meeting-The Pregnancy Meeting™, February 2024.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008. Jul 3;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aagaard-Tillery KM, Grove K, Bishop J, et al. Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. J Mol Endocrinol 2008;41(2):91–102. DOI: 10.1677/JME-08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox J, Williams S, Grove K, Lane RH, Aagaard-Tillery KM. A maternal high-fat diet is accompanied by alterations in the fetal primate metabolome. Am J Obstet Gynecol 2009;201(3):281 e1–9. DOI: 10.1016/j.ajog.2009.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayward AD, Rickard IJ, Lummaa V. Influence of early-life nutrition on mortality and reproductive success during a subsequent famine in a preindustrial population. Proc Natl Acad Sci U S A. 2013. Aug 20;110(34):13886–91. doi: 10.1073/pnas.1301817110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suter MA, Chen A, Burdine MS, et al. A maternal high-fat diet modulates fetal SIRT1 histone and protein deacetylase activity in nonhuman primates. FASEB J 2012;26(12):5106–14. DOI: 10.1096/fj.12-212878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suter MA, Sangi-Haghpeykar H, Showalter L, et al. Maternal high-fat diet modulates the fetal thyroid axis and thyroid gene expression in a nonhuman primate model. Mol Endocrinol 2012;26(12):2071–80. DOI: 10.1210/me.2012-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suter MA, Takahashi D, Grove KL, Aagaard KM. Postweaning exposure to a high-fat diet is associated with alterations to the hepatic histone code in Japanese macaques. Pediatr Res 2013;74(3):252–8. DOI: 10.1038/pr.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wapner RJ, Sorokin Y, Mele L, Johnson F, Dudley DJ, Spong CY, Peaceman AM, Leveno KJ, Malone F, Caritis SN, Mercer B, Harper M, Rouse DJ, Thorp JM, Ramin S, Carpenter MW, Gabbe SG; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Long-term outcomes after repeat doses of antenatal corticosteroids. N Engl J Med. 2007. Sep 20;357(12):1190–8. doi: 10.1056/NEJMoa071453. [DOI] [PubMed] [Google Scholar]
- 9.Ma J, Prince AL, Bader D, et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun 2014;5:3889. DOI: 10.1038/ncomms4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suter MA, Ma J, Vuguin PM, et al. In utero exposure to a maternal high-fat diet alters the epigenetic histone code in a murine model. Am J Obstet Gynecol 2014;210(5):463 e1–463 e11. DOI: 10.1016/j.ajog.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seferovic MD, Goodspeed DM, Chu DM, et al. Heritable IUGR and adult metabolic syndrome are reversible and associated with alterations in the metabolome following dietary supplementation of 1-carbon intermediates. FASEB J 2015;29(6):2640–52. DOI: 10.1096/fj.14-266387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodspeed D, Seferovic MD, Holland W, et al. Essential nutrient supplementation prevents heritable metabolic disease in multigenerational intrauterine growth-restricted rats. FASEB J 2015;29(3):807–19. DOI: 10.1096/fj.14-259614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suter MA, Abramovici AR, Griffin E, et al. In utero nicotine exposure epigenetically alters fetal chromatin structure and differentially regulates transcription of the glucocorticoid receptor in a rat model. Birth Defects Res A Clin Mol Teratol 2015;103(7):583–8. DOI: 10.1002/bdra.23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, McNamara J, Metcalfe NB, Monaghan P, Spencer HG, Sultan SE. Developmental plasticity and human health. Nature. 2004. Jul 22;430(6998):419–21. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 15.Prince AL, Pace RM, Dean T, et al. The development and ecology of the Japanese macaque gut microbiome from weaning to early adolescence in association with diet. Am J Primatol 2019;81(10–11):e22980. DOI: 10.1002/ajp.22980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cilvik SN, Wesolowski SR, Anthony RV, Brown LD, Rozance PJ. Late gestation fetal hyperglucagonaemia impairs placental function and results in diminished fetal protein accretion and decreased fetal growth. J Physiol. 2021. Jul;599(13):3403–3427. doi: 10.1113/JP281288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulsen ME, Rosario FJ, Wesolowski SR, Powell TL, Jansson T. Normalizing adiponectin levels in obese pregnant mice prevents adverse metabolic outcomes in offspring. FASEB J. 2019. Feb;33(2):2899–2909. doi: 10.1096/fj.201801015R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dumolt J, Powell TL, Jansson T, Rosario FJ. Normalization of maternal adiponectin in obese pregnant mice prevents programming of impaired glucose metabolism in adult offspring. FASEB J. 2022. Jul;36(7):e22383. doi: 10.1096/fj.202200326R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wesolowski SR, Kasmi KC, Jonscher KR, and Friedman JE. Developmental origins of NAFLD: a womb with a clue. Nat Rev Gastroenterol Hepatol 14: 81–96, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carroll D, Sassin A, Aagaard K, Gannon M. Developmental effects of in utero metformin exposure. Trends Dev Biol. 2021;14:1–17. [PMC free article] [PubMed] [Google Scholar]
- 21.Meek CL. An unwelcome inheritance: childhood obesity after diabetes in pregnancy. Diabetologia. 2023. Nov;66(11):1961–1970. doi: 10.1007/s00125-023-05965-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholtens DM, Kuang A, Linder B, Lawrence JM, Lebenthal Y, McCance D, Hamilton J, Nodzenski M, Talbot O, Brickman WJ, Clayton P, Ma RC, Tam WH, Dyer AR, Catalano PM, Lowe LP, Metzger BE; HAPO follow-up study cooperative research group. hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): Maternal Gestational Diabetes Mellitus and Childhood Glucose Metabolism. Diabetes Care. 2019. Mar;42(3):372–380. doi: 10.2337/dc18-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furse S, Koulman A, Ozanne SE, Poston L, White SL, Meek CL. Altered lipid metabolism in obese women with gestational diabetes and associations with offspring adiposity. J Clin Endocrinol Metab. 2022. Jun 16;107(7):e2825–e2832. doi: 10.1210/clinem/dgac206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Josefson JL, Catalano PM, Lowe WL, Scholtens DM, Kuang A, Dyer AR, Lowe LP, Metzger BE. The joint associations of maternal BMI and glycemia with childhood adiposity. J Clin Endocrinol Metab. 2020. Jul 1;105(7):2177–88. doi: 10.1210/clinem/dgaa180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bianco ME, Josefson JL. Hyperglycemia during pregnancy and long-term offspring outcomes. Curr Diab Rep. 2019. Nov 21;19(12):143. doi: 10.1007/s11892-019-1267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simmons D, Immanuel J, Hague WM, Teede H, Nolan CJ, Peek MJ, Flack JR, McLean M, Wong V, Hibbert E, Kautzky-Willer A, Harreiter J, Backman H, Gianatti E, Sweeting A, Mohan V, Enticott J, Cheung NW; TOBOGM Research Group. Treatment of Gestational Diabetes Mellitus Diagnosed Early in Pregnancy. N Engl J Med. 2023. Jun 8;388(23):2132–2144. doi: 10.1056/NEJMoa2214956. [DOI] [PubMed] [Google Scholar]
- 27.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Jeffrie Seley J, Stanton RC, Gabbay RA, on behalf of the American Diabetes Association. 15. Management of diabetes in pregnancy: Standards of care in diabetes-2023. Diabetes Care. 2023. Jan 1;46(Suppl 1):S254–S266. doi: 10.2337/dc23-S015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 2000;343(16):1134–8. (In eng). DOI: 10.1056/NEJM200010193431601. [DOI] [PubMed] [Google Scholar]
- 29.Gui J, Liu Q, Feng L. Metformin vs insulin in the management of gestational diabetes: a meta-analysis. PloS One 2013;8(5):e64585. DOI: 10.1371/journal.pone.0064585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo L, Ma J, Tang J, Hu D, Zhang W, Zhao X. Comparative Efficacy and safety of metformin, glyburide, and insulin in treating gestational diabetes mellitus: A meta-analysis. J Diab Research 2019;2019:9804708. DOI: 10.1155/2019/9804708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American College of O, Gynecologists’ Committee on Practice B-O. ACOG Practice Bulletin No. 201: Pregestational Diabetes Mellitus. Obstet Gynecol 2018;132(6):e228–e248. DOI: 10.1097/AOG.0000000000002960. [DOI] [PubMed] [Google Scholar]
- 32.American Diabetes Association Professional Practice C. 15. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2022. Diabetes care 2022;45(Suppl 1):S232–S243. DOI: 10.2337/dc22-S015. [DOI] [PubMed] [Google Scholar]
- 33.Kanda S, Chatha U, Odoma VA, Pitliya A, AlEdani EM, Bhangu JK, Javed K, Manshahia PK, Nahar S, Hamid P. Effect of Metformin (MTF) intervention during pregnancy in women with Polycystic Ovarian Syndrome (PCOS): A systematic review. Cureus. 2023. Aug 26;15(8):e44166. doi: 10.7759/cureus.44166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan X, Li S, Chang Y, Fang C, Liu H, Zhang X, Wang Y. Effect of metformin treatment during pregnancy on women with PCOS: a systematic review and meta-analysis. Clin Invest Med. 2016. Sep 11;39(4):E120–31. doi: 10.25011/cim.v39i4.27091. [DOI] [PubMed] [Google Scholar]
- 35.Beyuo T, Obed SA, Adjepong-Yamoah KK, Bugyei KA, Oppong SA, Marfoh K. Metformin versus insulin in the management of pre-gestational diabetes mellitus in pregnancy and gestational diabetes mellitus at the Korle Bu Teaching Hospital: A Randomized Clinical Trial. PLoS One. 2015. May 6;10(5):e0125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cluver CA, Hiscock R, Decloedt EH, Hall DR, Schell S, Mol BW, Brownfoot F, Kaitu’u-Lino TJ, Walker SP, Tong S. Use of metformin to prolong gestation in preterm pre-eclampsia: randomised, double blind, placebo controlled trial. BMJ. 2021. Sep 22;374:n2103. doi: 10.1136/bmj.n2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbour LA, Scifres C, Valent AM, et al. A cautionary response to SMFM statement: pharmacological treatment of gestational diabetes. Am J Obstet Gynecol 2018;219(4):367 e1–367 e7. DOI: 10.1016/j.ajog.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarry-Adkins JL, Aiken CE, Ozanne SE. Neonatal, infant, and childhood growth following metformin versus insulin treatment for gestational diabetes: A systematic review and meta-analysis. PLoS Med 2019;16(8):e1002848. DOI: 10.1371/journal.pmed.1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanem LGE, Stridsklev S, Juliusson PB, et al. Metformin use in PCOS pregnancies increases the risk of offspring overweight at 4 years of age: follow-up of two RCTs. J Clin Endocrinol Metab 2018;103(4):1612–1621. DOI: 10.1210/jc.2017-02419. [DOI] [PubMed] [Google Scholar]
- 40.Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): Body composition and metabolic outcomes at 7–9 years of age. BMJ Open Diabetes Res Care 2018;6(1):e000456. DOI: 10.1136/bmjdrc-2017-000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang S, Teague AM, Tryggestad JB, Jensen ME, Chernausek SD. Role of metformin in epigenetic regulation of placental mitochondrial biogenesis in maternal diabetes. Sci Rep 2020;10(1):8314. DOI: 10.1038/s41598-020-65415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faure MC, Khoueiry R, Quanico J, et al. In utero exposure to metformin reduces the fertility of male offspring in adulthood. Front Endocrinol (Lausanne) 2021;12:750145. DOI: 10.3389/fendo.2021.750145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eyal S, Easterling TR, Carr D, Umans JG, Miodovnik M, Hankins GD, Clark SM, Risler L, Wang J, Kelly EJ, Shen DD, Hebert MF. Pharmacokinetics of metformin during pregnancy. Drug Metab Dispos. 2010. May;38(5):833–40. doi: 10.1124/dmd.109.031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hjorth-Hansen A, Salvesen Ø, Engen Hanem LG, Eggebø T, Salvesen KÅ, Vanky E, Ødegård R. Fetal growth and birth anthropometrics in metformin-exposed offspring born to mothers with PCOS. J Clin Endocrinol Metab. 2018. Feb 1;103(2):740–747. doi: 10.1210/jc.2017-01191. [DOI] [PubMed] [Google Scholar]
- 45.Carlsen SM, Martinussen MP, Vanky E. Metformin’s effect on first-year weight gain: a follow-up study. Pediatrics. 2012. Nov;130(5):e1222–6. doi: 10.1542/peds.2012-0346. [DOI] [PubMed] [Google Scholar]
- 46.Rowan JA, Rush EC, Obolonkin V, Battin M, Wouldes T, Hague WM. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): Body composition at 2 years of age. Diabetes Care 2011;34(10):2279–84. DOI: 10.2337/dc11-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo L, Ma J, Tang J, Hu D, Zhang W, Zhao X. Comparative Efficacy and Safety of metformin, glyburide, and insulin in treating Gestational Diabetes Mellitus: A meta-analysis. J Diab Res 2019;2019:9804708. DOI: 10.1155/2019/9804708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Weelden W, Wekker V, de Wit L, et al. Long-Term effects of oral antidiabetic drugs during pregnancy on offspring: A systematic review and meta-analysis of follow-up studies of RCTs. Diabetes Ther 2018;9(5):1811–1829. DOI: 10.1007/s13300-018-0479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Q, Xie Q. Long-term effects of prenatal exposure to metformin on the health of children based on follow-up studies of randomized controlled trials: A systematic review and meta-analysis. Arch Gynecol Obstet 2019;299(5):1295–1303. DOI: 10.1007/s00404-019-05124-w. [DOI] [PubMed] [Google Scholar]
- 50.Landi SN, Radke S, Engel SM, Boggess K, Stürmer T, Howe AS, Funk MJ. Association of long-term child growth and developmental outcomes with metformin vs insulin treatment for Gestational Diabetes. JAMA Pediatr. 2019. Feb 1;173(2):160–168. doi: 10.1001/jamapediatrics.2018.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hufnagel A, Fernandez-Twinn DS, Blackmore HL, Ashmore TJ, Heaton RA, Jenkins B, Koulman A, Hargreaves IP, Aiken CE & Ozanne SE. (2022). Maternal but not fetoplacental health can be improved by metformin in a murine diet-induced model of maternal obesity and glucose intolerance. The Journal of physiology 600, 903–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lofthouse EM, Cleal J, Lewis RM, Sengers BG. Computational modelling of paracellular diffusion and OCT3 mediated transport of metformin in the perfused human placenta. J Pharm Sci. 2023. Sep;112(9):2570–2580. doi: 10.1016/j.xphs.2023.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Lee N, Hebert MF, Wagner DJ, Easterling TR, Liang CJ, Rice K, Wang J. Organic Cation Transporter 3 facilitates fetal exposure to metformin during pregnancy. Mol Pharmacol. 2018. Oct;94(4):1125–1131. doi: 10.1124/mol.118.112482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hemauer SJ, Patrikeeva SL, Nanovskaya TN, Hankins GD, Ahmed MS. Role of human placental apical membrane transporters in the efflux of glyburide, rosiglitazone, and metformin. Am J Obstet Gynecol. 2010. Apr;202(4):383.e1–7. doi: 10.1016/j.ajog.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alshahrani MY, Ebrahim HA, Alqahtani SM, Bayoumy NM, Kamar SS, ShamsEldeen AM, Haidara MA, Al-Ani B, Albawardi A. Metformin suppresses thioacetamide-induced chronic kidney disease in association with the upregulation of ampk and downregulation of oxidative stress and inflammation as well as dyslipidemia and hypertension. Molecules. 2023. Mar 18;28(6):2756. doi: 10.3390/molecules28062756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Owen MD, Baker BC, Scott EM, Forbes K. Interaction between metformin, folate and vitamin B12 and the potential impact on fetal growth and long-term metabolic health in diabetic pregnancies. Int J Mol Sci. 2021. May 28;22(11):5759. doi: 10.3390/ijms22115759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ozdemir Kutbay N, Biray Avci C, Sarer Yurekli B, Caliskan Kurt C, Shademan B, Gunduz C, Erdogan M. Effects of metformin and pioglitazone combination on apoptosis and AMPK/mTOR signaling pathway in human anaplastic thyroid cancer cells. J Biochem Mol Toxicol. 2020. Oct;34(10):e22547. doi: 10.1002/jbt.22547. [DOI] [PubMed] [Google Scholar]
- 58.Amara VR, Surapaneni SK, Tikoo K. Metformin attenuates cardiovascular and renal injury in uninephrectomized rats on DOCA-salt: Involvement of AMPK and miRNAs in cardioprotection. Toxicol Appl Pharmacol 2019;362:95–104. DOI: 10.1016/j.taap.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 59.Forteath C, Mordi I, Nisr R, Gutierrez-Lara EJ, Alqurashi N, Phair IR, Cameron AR, Beall C, Bahr I, Mohan M, Wong AKF, Dihoum A, Mohammad A, Palmer CNA, Lamont D, Sakamoto K, Viollet B, Foretz M, Lang CC, Rena G. Amino acid homeostasis is a target of metformin therapy. Mol Metab. 2023. Aug;74:101750. doi: 10.1016/j.molmet.2023.101750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swenson KS, Wang D, Jones AK, Nash MJ, O’Rourke R, Takahashi DL, Kievit P, Hennebold JD, Aagaard KM, Friedman JE, Jones KL, Rozance PJ, Brown LD, Wesolowski SR. Metformin disrupts signaling and metabolism in fetal hepatocytes. Diabetes. 2023. Sep 1;72(9):1214–1227. doi: 10.2337/db23-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015;528(7581):262–266. DOI: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hurley-Kim K, Vu CH, Dao NM, Tran LC, McBane S, Lee J, Sepassi A. Effect of metformin use on vitamin B12 deficiency over time (EMBER): A real-world evidence database study. Endocr Pract. 2023. Nov;29(11):862–867. doi: 10.1016/j.eprac.2023.06.013. [DOI] [PubMed] [Google Scholar]
- 63.Fituri S, Akbar Z, Ganji V. Impact of metformin treatment on cobalamin status in persons with type 2 diabetes. Nutr Rev. 2023. May 11:nuad045. doi: 10.1093/nutrit/nuad045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Owen MD, Baker BC, Scott EM, Forbes K. Interaction between metformin, folate and vitamin B12 and the potential impact on fetal growth and long-term metabolic health in diabetic pregnancies. Int J Mol Sci. 2021. May 28;22(11):5759. doi: 10.3390/ijms22115759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.D’Mello RJ, Lo JO, Hagen OL, Castro JN, Graham JA, Frias AE, Roberts VHJ. Ultrasound evaluation of normal rhesus macaque fetal biometry and uteroplacental hemodynamics. Am J Primatol. 2023. Jul;85(7):e23504. doi: 10.1002/ajp.23504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tarantal AF, Hendrickx AG. Use of ultrasound for early pregnancy detection in the rhesus and cynomolgus macaque (Macaca mulatta and Macaca fascicularis). J Med Primatol. 1988;17(2):105–12. [PubMed] [Google Scholar]
- 67.Nyland TG, Hill DE, Hendrickx AG, Farver TB, McGahan JP, Henrickson R, Anderson J, Phillips HE. Ultrasonic assessment of fetal growth in the nonhuman primate (Macaca mulatta). J Clin Ultrasound. 1984. Sep;12(7):387–95. doi: 10.1002/jcu.1870120703. [DOI] [PubMed] [Google Scholar]
- 68.Chan-Ling T, Hu P, Li Calzi S, Warner J, Uddin N, DuPont M, Neuringer M, Kievit P, Renner L, Stoddard J, Ryals R, Boulton ME, McGill T, Grant MB. Glial, neuronal, vascular, retinal pigment epithelium, and inflammatory cell damage in a new Western diet-induced primate model of diabetic retinopathy. Am J Pathol. 2023. Nov;193(11):1789–1808. doi: 10.1016/j.ajpath.2023.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bishop CV, Takahashi DL, Luo F, Sidener H, Martin LD, Gao L, Fei SS, Hennebold JD, Slayden OD. The combined impact of testosterone and Western-style diet on endometriosis severity and progression in rhesus macaques†. Biol Reprod. 2023. Jan 14;108(1):72–80. doi: 10.1093/biolre/ioac183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ravisankar S, Murphy MJ, Redmayne-Titley N, Davis B, Luo F, Takahashi D, Hennebold JD, Chavez SL. Long-term hyperandrogenemia and/or Western-style diet in Rhesus macaque Females impairs preimplantation embryogenesis. Endocrinology. 2022. Apr 1;163(4):bqac019. doi: 10.1210/endocr/bqac019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suter M, Bocock P, Showalter L, Hu M, Shope C, McKnight R, Grove K, Lane R, Aagaard-Tillery K. Epigenomics: maternal high-fat diet exposure in utero disrupts peripheral circadian gene expression in nonhuman primates. FASEB J. 2011. Feb;25(2):714–26. doi: 10.1096/fj.10-172080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harris RA, Alcott CE, Sullivan EL, Takahashi D, McCurdy CE, Comstock S, Baquero K, Blundell P, Frias AE, Kahr M, Suter M, Wesolowski S, Friedman JE, Grove KL, Aagaard KM. Genomic Variants associated with resistance to high fat diet induced obesity in a primate model. Sci Rep. 2016. Nov 4;6:36123. doi: 10.1038/srep36123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73..Nash MJ, Dobrinskikh E, Soderborg TK, Janssen RC, Takahashi DL, Dean TA, Varlamov O, Hennebold JD, Gannon M, Aagaard KM, McCurdy CE, Kievit P, Bergman BC, Jones KL, Pietras EM, Wesolowski SR, Friedman JE. Maternal diet alters long-term innate immune cell memory in fetal and juvenile hematopoietic stem and progenitor cells in nonhuman primate offspring. Cell Rep. 2023. Apr 25;42(4):112393. doi: 10.1016/j.celrep.2023.112393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009. Feb;119(2):323–35. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alge JL, Wenderfer SE, Hicks J, et al. Hemolytic uremic syndrome as the presenting manifestation of WT1 mutation and Denys-Drash syndrome: a case report. BMC Nephrol 2017;18(1):243. DOI: 10.1186/s12882-017-0643-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Askenazi D, Myones B, Kamdar A, et al. Outcomes of children with proliferative lupus nephritis: the role of protocol renal biopsy. Pediatr Nephrol 2007;22(7):981–6. DOI: 10.1007/s00467-007-0447-9. [DOI] [PubMed] [Google Scholar]
- 77.Banerjee K, Zhao N, Srinivasan A, et al. An Adaptive Multivariate Two-Sample Test With Application to Microbiome Differential Abundance Analysis. Front Genet 2019;10:350. DOI: 10.3389/fgene.2019.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ben Moshe Y, Bekheirnia N, Smith RJH, Hicks J, Braun MC, Bekheirnia MR. Genetic diagnosis and renal biopsy findings in the setting of a renal genetics clinic. Am J Med Genet C Semin Med Genet 2022;190(3):302–308. DOI: 10.1002/ajmg.c.32009. [DOI] [PubMed] [Google Scholar]
- 79.Maliakkal JG, Hicks MJ, Michael M, et al. Renal Survival in Children with Glomerulonephritis with Crescents: A Pediatric Nephrology Research Consortium Cohort Study. J Clin Med 2020;9(8). DOI: 10.3390/jcm9082385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Orjuela A, Suwanichkul A, Canter D, et al. High titer anti-basement membrane antibodies in a subset of patients with pediatric systemic lupus erythematosus. Am J Nephrol 2015;41(3):241–7. DOI: 10.1159/000381965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patel P, de Guzman M, Hicks MJ, et al. Utility of the 2018 revised ISN/RPS thresholds for glomerular crescents in childhood-onset lupus nephritis: a Pediatric Nephrology Research Consortium study. Pediatr Nephrol 2022;37(12):3139–3145. DOI: 10.1007/s00467-022-05524-2. [DOI] [PubMed] [Google Scholar]
- 82.Pereira M, Muscal E, Eldin K, et al. Clinical presentation and outcomes of childhood-onset membranous lupus nephritis. Pediatr Nephrol 2017;32(12):2283–2291. DOI: 10.1007/s00467-017-3743-z. [DOI] [PubMed] [Google Scholar]
- 83.Roberts VHJ, Castro JN, Wessel BM, Conrad DF, Lewis AD, Lo JO. Rhesus macaque fetal and placental growth demographics: A resource for laboratory animal researchers. Am J Primatol. 2023. Aug;85(8):e23526. doi: 10.1002/ajp.23526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grichko VP, Gettelman GJ, Widrick JJ, Fitts RH. Substrate and enzyme profile of fast and slow skeletal muscle fibers in rhesus monkeys. J Appl Physiol (1985). 1999. Jan;86(1):335–40. doi: 10.1152/jappl.1999.86.1.335. [DOI] [PubMed] [Google Scholar]
- 85.Feig DS, Donovan LE, Zinman B, et al. Metformin in women with type 2 diabetes in pregnancy (MiTy): a multicentre, international, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2020;8(10):834–844. DOI: 10.1016/S2213-8587(20)30310-7. [DOI] [PubMed] [Google Scholar]
- 86.Feig DS, Sanchez JJ, Murphy KE, et al. Outcomes in children of women with type 2 diabetes exposed to metformin versus placebo during pregnancy (MiTy Kids): a 24-month follow-up of the MiTy randomised controlled trial. Lancet Diabetes Endocrinol 2023;11(3):191–202. DOI: 10.1016/S2213-8587(23)00004-9 [DOI] [PubMed] [Google Scholar]
- 87.Hanem LGE, Salvesen Ø, Juliusson PB, Carlsen SM, Nossum MCF, Vaage MØ, Ødegård R, Vanky E. Intrauterine metformin exposure and offspring cardiometabolic risk factors (PedMet study): a 5–10 year follow-up of the PregMet randomised controlled trial. Lancet Child Adolesc Health. 2019. Mar;3(3):166–174. doi: 10.1016/S2352-4642(18)30385-7. [DOI] [PubMed] [Google Scholar]
- 88.Boggess KA, Valint A, Refuerzo JS, Zork N, Battarbee AN, Eichelberger K, Ramos GA, Olson G, Durnwald C, Landon MB, Aagaard KM, Wallace K, Scifres C, Rosen T, Mulla W, Valent A, Longo S, Young L, Marquis MA, Thomas S, Britt A, Berry D. Metformin plus insulin for preexisting diabetes or gestational diabetes in early pregnancy: The MOMPOD Randomized Clinical Trial. JAMA. 2023. Dec 12;330(22):2182–2190. doi: 10.1001/jama.2023.22949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fransen HP, Peeters PHM, Beulens JWJ, Boer JMA, de Wit GA, Onland-Moret NC, van der Schouw YT, Bueno-de-Mesquita HB, Hoekstra J, Elias SG, May AM. Exposure to Famine at a Young Age and Unhealthy Lifestyle Behavior Later in Life. PLoS ONE. 2016;11(5):e0156609. doi.org/ 10.1371/journal.pone.0156609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rodríguez MM, Gómez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo GE. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol. 2004;7(1):17–25. doi: 10.1007/s10024-003-3029-2 [DOI] [PubMed] [Google Scholar]
- 91.Callaway D, McGill-Vargas L, Quinn A et al. Prematurity disrupts glomeruli development, whereas prematurity and hyperglycemia lead to altered nephron maturation and increased oxidative stress in newborn baboons. Pediatr Res 83, 702–711 (2018). 10.1038/pr.2017.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Treats used in the delivery of metformin (dosed to each individual dam at 10 mg/kg twice daily)or vehicle control. (A) An example of the variety of treats offered. The full spectrum of treat types include: fig newton or banana and cereal balls, honey graham cracker balls, graham or saltine cracker sandwiches with jam or peanut butter, nilla wafer and jam sandwiches, fruit loop marshmallow balls, yogurt pudding cups, orange gummies, jello shots, gingersnap and marshmallow fluff sandwiches, injected orange slice, monkey morsel (trail mix) patty, banana and cereal saltine sandwiches, pumpkin cup with marshmallows, pumpkin or sweet potato cups, and mashed banana mixes. (B) Number of treats typically required for two days of therapy. Average time spent administering and observing adherence with non-sharing of treats approximates two hours per day. As noted in results, one dam (“treat thief”) allocated to the vehicle control group did have detectable levels of metformin on assay and that fetal offspring was excluded from analyses. Because of the mechanism of observed treat intake, the “treat thief” was thought to have taken the metformin treat approximating the timing of delivery and was not representative of chronic “treat swapping” nor “treat thievery” among allocated group animals in the cohort.
Data Availability Statement
Criteria data will be shared by encryption in a de-identified manner with (1) a signed data use/access agreement, and (2) approved data analysis plan.


