Abstract
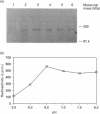
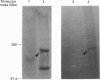
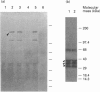
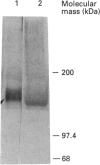
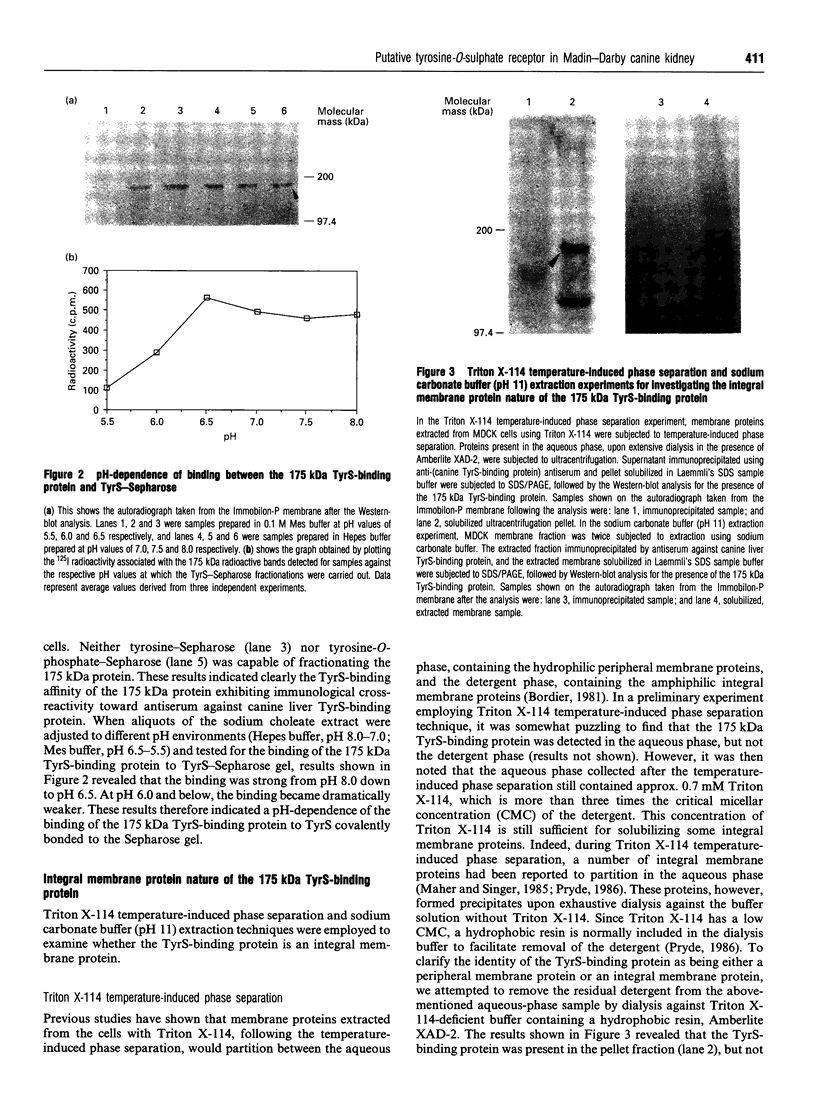
By employing an affinity-gel fractionation technique coupled to Western-blot analysis, we have identified a 175 kDa tyrosine-O-sulphate (TyrS)-binding protein present in Madin-Darby canine kidney (MDCK) cells. The binding of this TyrS-binding protein to TyrS covalently bonded to Sepharose gel was found to be pH-dependent, being strong from pH 8.0 down to pH 6.5 and increasingly weak at pH 6.0 and below. Results obtained from Triton X-114 temperature-induced phase separation and sodium carbonate buffer (pH 11) extraction experiments indicated that the TyrS-binding protein is an integral membrane protein. This 175 kDa TyrS-binding protein was found to be present in association with a major tyrosine-sulphated protein, the apically secreted 80 kDa glycoprotein (gp 80), in cell lysate prepared from MDCK cells maintained under normal growth conditions. When the cell lysate used was prepared from MDCK cells pretreated with 20 mM sodium chlorate, a metabolic sulphation inhibitor, the complex formed between the two proteins could no longer be detected, indicating that the binding of the TyrS-binding protein is through the TyrS residue(s) of gp 80. Both cell-surface biotinylation and cell-surface trypsinization studies demonstrated the predominantly, if not exclusively, intracellular location of the TyrS-binding protein. Furthermore, radioactive pulse-chase experiments revealed that the newly synthesized radiolabelled fibronectin and gp 80 were present in complexes with the TyrS-binding protein in MDCK cells pulse-labelled with [35S]methionine or [35S]sulphate. Exogenous [35S]methionine-labelled gp 80 added to the medium, on the other hand, was not found to be present in association with the TyrS-binding protein in MDCK cells over a 2-h time course. These results strongly suggested the identity of the 175 kDa TyrS-binding protein as a putative 'TyrS receptor', possibly functioning in the biosynthetic transport of tyrosine-sulphated proteins in MDCK cells.
Full text
PDF




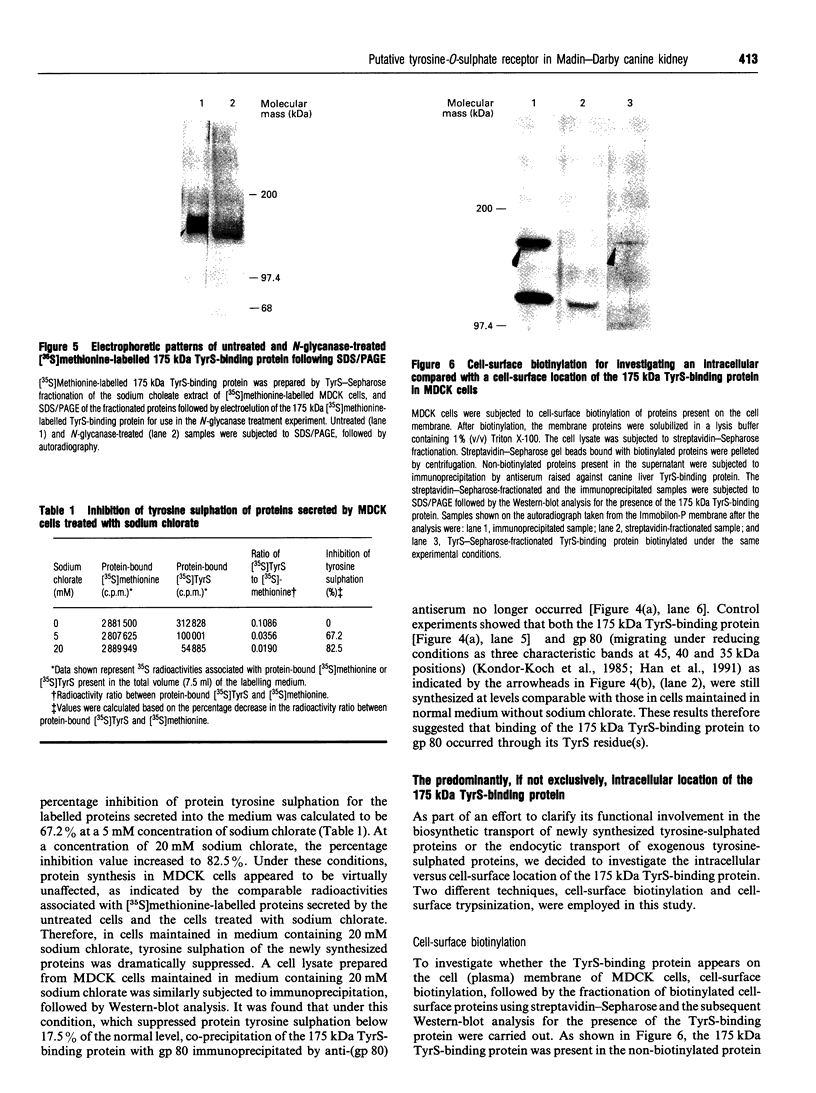
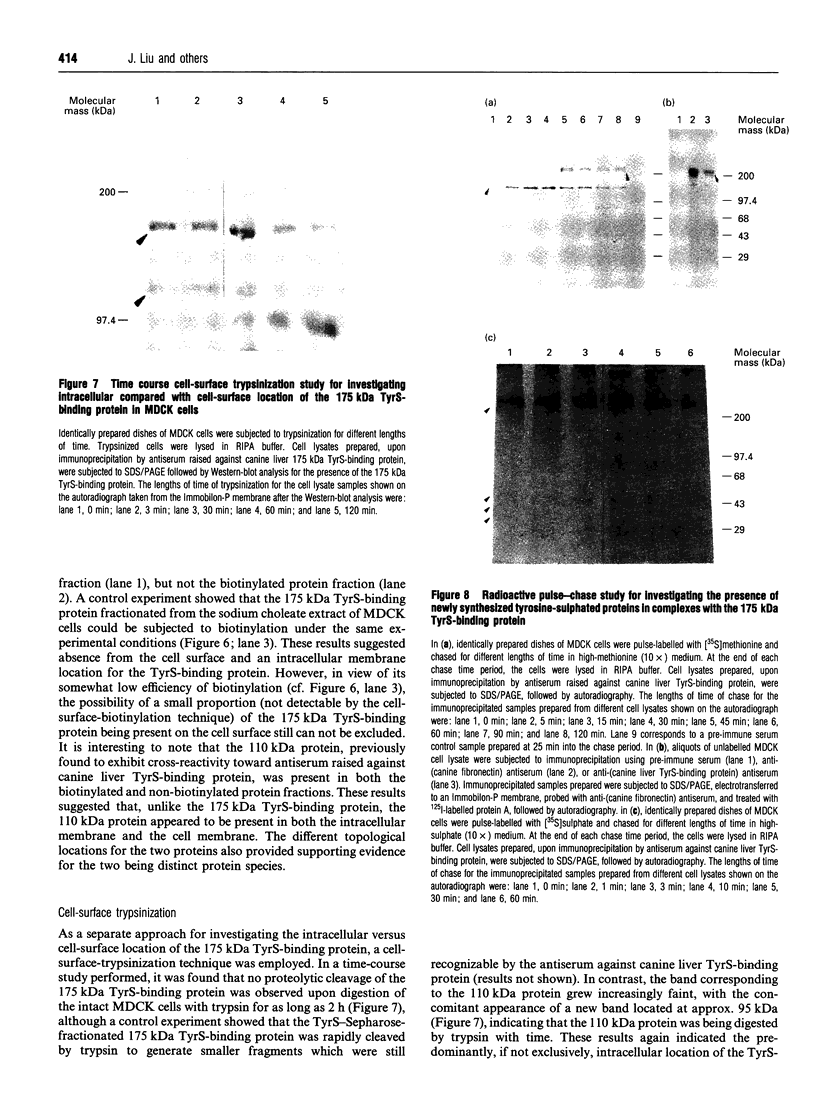



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Huttner W. B. Chlorate--a potent inhibitor of protein sulfation in intact cells. Biochem Biophys Res Commun. 1986 Dec 15;141(2):870–877. doi: 10.1016/s0006-291x(86)80253-4. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Huttner W. B. Tyrosine sulfation is a trans-Golgi-specific protein modification. J Cell Biol. 1987 Dec;105(6 Pt 1):2655–2664. doi: 10.1083/jcb.105.6.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartles J. R., Hubbard A. L. Plasma membrane protein sorting in epithelial cells: do secretory pathways hold the key? Trends Biochem Sci. 1988 May;13(5):181–184. doi: 10.1016/0968-0004(88)90147-8. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Caplan M. J., Stow J. L., Newman A. P., Madri J., Anderson H. C., Farquhar M. G., Palade G. E., Jamieson J. D. Dependence on pH of polarized sorting of secreted proteins. Nature. 1987 Oct 15;329(6140):632–635. doi: 10.1038/329632a0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Gilead Z., Jeng Y. H., Wold W. S., Sugawara K., Rho H. M., Harter M. L., Green M. Immunological identification of two adenovirus 2-induced early proteins possibly involved in cell transformation. Nature. 1976 Nov 18;264(5583):263–266. doi: 10.1038/264263a0. [DOI] [PubMed] [Google Scholar]
- Gottlieb T. A., Beaudry G., Rizzolo L., Colman A., Rindler M., Adesnik M., Sabatini D. D. Secretion of endogenous and exogenous proteins from polarized MDCK cell monolayers. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2100–2104. doi: 10.1073/pnas.83.7.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. R., Liu M. C. Polarized secretion of tyrosine-sulphated proteins and free tyrosine O-sulphate by filter-grown Madin-Darby canine kidney (MDCK) cells. Biochem J. 1991 Oct 1;279(Pt 1):289–295. doi: 10.1042/bj2790289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. R., Suiko M., Liu C. C., Liu M. C. Post-translational modifications and binding properties of the apically secreted 80-kDa glycoprotein from Madin-Darby canine kidney cells: similarities to the C-terminal portion of the basolaterally secreted fibronectin. Arch Biochem Biophys. 1991 May 1;286(2):337–345. doi: 10.1016/0003-9861(91)90049-o. [DOI] [PubMed] [Google Scholar]
- Hirani S., Bernasconi R. J., Rasmussen J. R. Use of N-glycanase to release asparagine-linked oligosaccharides for structural analysis. Anal Biochem. 1987 May 1;162(2):485–492. doi: 10.1016/0003-2697(87)90424-6. [DOI] [PubMed] [Google Scholar]
- Howell K. E., Palade G. E. Hepatic Golgi fractions resolved into membrane and content subfractions. J Cell Biol. 1982 Mar;92(3):822–832. doi: 10.1083/jcb.92.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries D. E., Silbert J. E. Chlorate: a reversible inhibitor of proteoglycan sulfation. Biochem Biophys Res Commun. 1988 Jul 15;154(1):365–371. doi: 10.1016/0006-291x(88)90694-8. [DOI] [PubMed] [Google Scholar]
- Hurley W. L., Finkelstein E., Holst B. D. Identification of surface proteins on bovine leukocytes by a biotin-avidin protein blotting technique. J Immunol Methods. 1985 Dec 17;85(1):195–202. doi: 10.1016/0022-1759(85)90287-x. [DOI] [PubMed] [Google Scholar]
- Ingalls H. M., Goodloe-Holland C. M., Luna E. J. Junctional plasma membrane domains isolated from aggregating Dictyostelium discoideum amebae. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4779–4783. doi: 10.1073/pnas.83.13.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEVONS F. R. TYROSINE O-SULPHATE IN FIBRINOGEN AND FIBRIN. Biochem J. 1963 Dec;89:621–624. doi: 10.1042/bj0890621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondor-Koch C., Bravo R., Fuller S. D., Cutler D., Garoff H. Exocytotic pathways exist to both the apical and the basolateral cell surface of the polarized epithelial cell MDCK. Cell. 1985 Nov;43(1):297–306. doi: 10.1016/0092-8674(85)90035-2. [DOI] [PubMed] [Google Scholar]
- LIPMANN F. Biological sulfate activation and transfer. Science. 1958 Sep 12;128(3324):575–580. doi: 10.1126/science.128.3324.575. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lisanti M. P., Le Bivic A., Sargiacomo M., Rodriguez-Boulan E. Steady-state distribution and biogenesis of endogenous Madin-Darby canine kidney glycoproteins: evidence for intracellular sorting and polarized cell surface delivery. J Cell Biol. 1989 Nov;109(5):2117–2127. doi: 10.1083/jcb.109.5.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. W., Lu R. L., Han J. R., Liu M. C. Purification and characterization of the apically secreted 80 KDa glycoprotein from Madin-Darby canine kidney (MDCK) cells. Biochem Int. 1991 Sep;25(1):109–121. [PubMed] [Google Scholar]
- Liu M. C., Lipmann F. Decrease of tyrosine-O-sulfate-containing proteins found in rat fibroblasts infected with Rous sarcoma virus or Fujinami sarcoma virus. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3695–3698. doi: 10.1073/pnas.81.12.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. C., Lipmann F. Isolation of tyrosine-O-sulfate by Pronase hydrolysis from fibronectin secreted by Fujinami sarcoma virus-infected rat fibroblasts. Proc Natl Acad Sci U S A. 1985 Jan;82(1):34–37. doi: 10.1073/pnas.82.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. C., Lu R. L., Han J. R., Tang X. B., Suiko M., Liu C. C. Identification of complexes between the tyrosine-O-sulphate-binding protein and tyrosine-sulphated proteins in bovine liver membrane lysates. Biochem J. 1991 Apr 1;275(Pt 1):259–262. doi: 10.1042/bj2750259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. C., Suiko M., Tang X. B. Isolation and characterization of a bovine liver tyrosine-O-sulfate-binding protein--a putative receptor molecular for tyrosine-sulfated proteins? Biochem Biophys Res Commun. 1988 Oct 31;156(2):964–969. doi: 10.1016/s0006-291x(88)80938-0. [DOI] [PubMed] [Google Scholar]
- Lu R. L., Tang X. B., Han J. R., Suiko M., Liu M. C. Purification and characterization of a membrane-bound tyrosine-O-sulfate-binding protein from bovine liver. Arch Biochem Biophys. 1991 May 1;286(2):481–487. doi: 10.1016/0003-9861(91)90069-u. [DOI] [PubMed] [Google Scholar]
- Maher P. A., Singer S. J. Anomalous interaction of the acetylcholine receptor protein with the nonionic detergent Triton X-114. Proc Natl Acad Sci U S A. 1985 Feb;82(4):958–962. doi: 10.1073/pnas.82.4.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Separation of the two enzymatic phases in active sulfate synthesis. J Biol Chem. 1958 Sep;233(3):681–685. [PubMed] [Google Scholar]
- Sargiacomo M., Lisanti M., Graeve L., Le Bivic A., Rodriguez-Boulan E. Integral and peripheral protein composition of the apical and basolateral membrane domains in MDCK cells. J Membr Biol. 1989 Mar;107(3):277–286. doi: 10.1007/BF01871942. [DOI] [PubMed] [Google Scholar]
- Simons K., Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990 Jul 27;62(2):207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]