Abstract
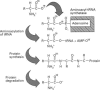
Amino acids labelled with 18(O) on both carboxy oxygen atoms have the potential for use as non-recyclable tracers to measure protein turnover. During protein synthesis one of the labelled oxygen atoms is removed, and thus release of the mono-labelled amino acid could be used to determine proteolysis. Primary cultures of embryonic-chick skeletal-muscle cells were used to test the use of 18(O2)-labelled Leu to measure proteolysis. For 9-day cultures, prelabelled on days 2-8 with medium containing one-half the Leu as [18O2]Leu and one-half as [2H3]Leu, release of [18(O)]Leu was less than 50% that of [2H]Leu over 24 h, suggesting a loss of the 18O label by a mechanism other than protein synthesis. Medium containing [18(O2)]Leu, [2H3]Leu, [18O2]Phe and [13C]Phe was then incubated with 9-day cultures to compare the rate of loss of the 18(O)-label from Leu and Phe with the rate of uptake of the non-carboxy-oxygen-labelled amino acids. Results for Leu demonstrated an 81% loss of the 18(O) label compared with a 33% decrease in [2H]Leu over 12 h. Loss of the 18(O) label was four times as great for Leu as for Phe. Loss of the 18(O) label was not decreased by addition of cycloheximide or by addition of a 3-fold excess of Ile, Val and Tyr; thus the loss of label was not due to protein synthesis alone or to misbinding to incorrect tRNAs. Infusion of the isotopes into pigs showed that the 18(O) label of Leu was not lost during transamination to alpha-ketoisocaproate (alpha-oxoisohexanoate). The most probable explanation is that the 18(O) label is lost as a result of the enzymic deacylation of tRNA, that this process is substantially faster for Leu than for Phe, and that this represents a potentially costly futile cycle for Leu.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beynon R. J., Bond J. S. Catabolism of intracellular protein: molecular aspects. Am J Physiol. 1986 Aug;251(2 Pt 1):C141–C152. doi: 10.1152/ajpcell.1986.251.2.C141. [DOI] [PubMed] [Google Scholar]
- Bonnet J., Ebel J. P. Correction of aminoacylation errors: evidence for a non significant role of the aminoacyl-tRNA synthetase catalysed deacylation of aminoacyl-tRNAs. FEBS Lett. 1974 Mar 1;39(3):259–262. doi: 10.1016/0014-5793(74)80125-0. [DOI] [PubMed] [Google Scholar]
- Bonnet J., Giegé R., Ebel J. P. Lack of specificity in the aminoacyl-tRNA synthetase-catalysed deacylation of aminoacyl-tRNA. FEBS Lett. 1972 Oct 15;27(1):139–144. doi: 10.1016/0014-5793(72)80427-7. [DOI] [PubMed] [Google Scholar]
- Epstein D., Elias-Bishko S., Hershko A. Requirement for protein synthesis in the regulation of protein breakdown in cultured hepatoma cells. Biochemistry. 1975 Nov 18;14(23):5199–5204. doi: 10.1021/bi00694a028. [DOI] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Hedden M. P., Buse M. G. General stimulation of muscle protein synthesis by branched chain amino acids in vitro. Proc Soc Exp Biol Med. 1979 Apr;160(4):410–415. doi: 10.3181/00379727-160-40460. [DOI] [PubMed] [Google Scholar]
- Hong S. O., Layman D. K. Effects of leucine on in vitro protein synthesis and degradation in rat skeletal muscles. J Nutr. 1984 Jul;114(7):1204–1212. doi: 10.1093/jn/114.7.1204. [DOI] [PubMed] [Google Scholar]
- Jones P. J. Stable isotopes in nutrition research: historical perspective and overview. Can J Physiol Pharmacol. 1990 Jul;68(7):935–940. doi: 10.1139/y90-142. [DOI] [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J. Selective control of the degradation of normal and aberrant proteins in Reuber H35 hepatoma cells. Biochem J. 1976 Jun 15;156(3):609–617. doi: 10.1042/bj1560609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Goldberg A. L. Leupeptin, a protease inhibitor, decreases protein degradation in normal and diseased muscles. Science. 1978 Feb 3;199(4328):534–536. doi: 10.1126/science.622552. [DOI] [PubMed] [Google Scholar]
- Mawhinney T. P., Robinett R. S., Atalay A., Madson M. A. Analysis of amino acids as their tert.-butyldimethylsilyl derivatives by gas-liquid chromatography and mass spectrometry. J Chromatogr. 1986 May 16;358(1):231–242. doi: 10.1016/s0021-9673(01)90333-4. [DOI] [PubMed] [Google Scholar]
- OSHIMA T., TAMIYA N. Mechanism of transaminase action. Biochem J. 1961 Jan;78:116–119. doi: 10.1042/bj0780116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridpath J. F., Huiatt T. W., Trenkle A. H., Robson R. M., Bechtel P. J. Growth and differentiation of chicken embryo muscle cell cultures derived from fast- and slow-growing lines. Intrinsic differences in growth characteristics and insulin response. Differentiation. 1984;26(2):121–126. doi: 10.1111/j.1432-0436.1984.tb01384.x. [DOI] [PubMed] [Google Scholar]
- Santi D. V., Webster R. W., Jr, Cleland W. W. Kinetics of aminoacyl-tRNA synthetases catalyzed ATP-PPi exchange. Methods Enzymol. 1974;29:620–627. doi: 10.1016/0076-6879(74)29054-2. [DOI] [PubMed] [Google Scholar]
- Schreier A. A., Schimmel P. R. Transfer ribonucleic acid synthetase catalyzed deacylation of aminoacyl transfer ribonucleic acid in the absence of adenosine monophosphate and pyrophosphate. Biochemistry. 1972 Apr 25;11(9):1582–1589. doi: 10.1021/bi00759a006. [DOI] [PubMed] [Google Scholar]
- Tischler M. E., Desautels M., Goldberg A. L. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J Biol Chem. 1982 Feb 25;257(4):1613–1621. [PubMed] [Google Scholar]
- Vandenburgh H., Kaufman S. Protein degradation in embryonic skeletal muscle. Effect of medium, cell type, inhibitors, and passive stretch. J Biol Chem. 1980 Jun 25;255(12):5826–5833. [PubMed] [Google Scholar]
- Wildenthal K., Griffin E. E. Reduction by cycloheximide of lysosomal proteolytic enzyme activity and rate of protein degradation in organ-cultured hearts. Biochim Biophys Acta. 1976 Sep 24;444(2):519–524. doi: 10.1016/0304-4165(76)90395-0. [DOI] [PubMed] [Google Scholar]
- Woodside K. H. Effects of cycloheximide on protein degradation and gluconeogenesis in the perfused rat liver. Biochim Biophys Acta. 1976 Jan 14;421(1):70–79. doi: 10.1016/0304-4165(76)90170-7. [DOI] [PubMed] [Google Scholar]
- Yarus M. Phenylalanyl-tRNA synthetase and isoleucyl-tRNA Phe : a possible verification mechanism for aminoacyl-tRNA. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1915–1919. doi: 10.1073/pnas.69.7.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarus M. Pseudoverification of mixed aminoacyl transfer ribonucleic acids. The generality of the process. J Biol Chem. 1973 Oct 10;248(19):6755–6758. [PubMed] [Google Scholar]