Abstract
Theories of embodied cognition suggest that a shared environment and ongoing sensorimotor interaction are central for interpersonal learning and engagement. To investigate the embodied, distributed and hence dynamically unfolding nature of social cognitive capacities, we present a novel laboratory-based coordination task: the BallGame. Our paradigm requires continuous sensing and acting between two players who jointly steer a virtual ball around obstacles towards as many targets as possible. By analysing highly resolved measures of movement coordination and gaming behaviour, game-concurrent experience ratings, semi-structured interviews, and personality questionnaires, we reveal contributions from different levels of observation on social experience. In particular, successful coordination (number of targets collected) and intermittent periods of high versus low movement coordination (variability of relation) emerged as prominent predictors of social experience. Importantly, having the same (but incomplete) view on the game environment strengthened interpersonal coordination, whereas complementary views enhanced engagement and tended to generate more complex interactive behaviour. Overall, we find evidence for a critical balance between similarity and synchrony on the one hand, and variability and difference on the other, for successful engagement in social interactions. Finally, following participant reports, we highlight how interpersonal experience emerges from specific histories of coordination that are closely related to the interaction context in both space and time.
Subject terms: Problem solving, Sensorimotor processing
Introduction
Social cognition involves interactions that span across levels of organisation When humans collaborate to solve a problem, a myriad of things happens. The environment shapes the language, movements and social roles we have available and choose from. The specifics of a task bring certain routines and skills to the foreground. Likewise, our personality, self-confidence and physical condition (fatigue) influence how we experience and behave in social settings.
The complex set of processes at work during collaborative action has inspired a diverse audience of researchers. Here, we present an experimental design and analysis approach that serves the integration of several perspectives on social interaction research. More specifically, we present a task that engages two participants in an interactive computer game, and perform analyses that integrate their gaming behaviour, finger movement coordination, subjective experience and personality traits. At the heart of our approach is the interest in relationality: how do two players co-determine their interaction dynamics? How do different elements of this process, such as personality differences, the interaction context, players' performance levels or their degree of movement coordination, relate?
Our approach is directly inspired by recent proposals to ground social cognition in interactive sensorimotor coordination [1–4, note especially5 in their focus on social connection through interpersonal coordination]. The concept of ‘socialising sensorimotor contingencies’ in particular6 highlights sensing and acting in mutual response as the key organising principle of social cognition. In this regard, we take a pragmatic stance: we locate social cognition in the domain of relationships between individuals and describe social behaviour and experience as the consequence of dynamic cycles of informational and sensorimotor coupling between agents. To bring this perspective into the cognitive science laboratory, we test here whether changes in the experienced quality of interaction are associated with changes in sensorimotor coordination between interacting players. As reviewed by Lübbert and colleagues6, empirical studies from dance and music to classical cognitive science laboratory settings have linked movement synchronisation to neural synchronisation of interacting individuals7,8, to their subjective experience9–11, as well as to contextual factors such as individual differences or task constraints12,13. However, studies that make room for interactive autonomy, generate detailed records across more than two levels of observation, consider changes in time and interaction context, and bridge domains by integrating approaches and findings, remain scarce. To contribute to their development, we present an experimental setting that combines an engaging interactive task with multiple forms of qualitative and quantitative observation: the BallGame.
In the BallGame, two players jointly steer a virtual ball around obstacles and towards as many targets as possible. The BallGame offers participants possibilities for action that are overlapping (both players can steer the ball in any direction with equal maximal force), diverse (at any moment there are many possible ways forward) and stimulating (the game control and task are neither too easy nor too difficult, and present collaborative advantages). Because we are interested in sensorimotor contingencies as a substrate of social cognition, we chose to include continuous movement and ongoing gaming dynamics (instead of discrete actions such as button presses and coordination through turn taking). Additionally, we used a game controller that is unfamiliar to most people: it required steering a virtual ball by bending and stretching one’s index fingers. This allowed participants to start at the same level of experience. Finally, we included trials in our experiment that featured obstacles visible only to one of the two players. This condition both challenged and stimulated interpersonal coordination because players accessed different but overall more information.
Besides recording participants’ finger movements and gaming behaviour, we asked them to rate their experience in terms of their perceived level of ball control, engagement, agreement with and predictability of their partner. Participants also self-assessed their personality traits using questionnaires. To further explore factors influencing interaction dynamics, we assessed personality traits (see for example14), autistic traits (e.g.,15,16) as well as interpersonal reactivity (empathy). Finally, we performed individual interviews at the end of each experiment to hear in more detail about participants’ own account of their experience with the BallGame.
By investigating individual experience as an interactive property—a characteristic of ongoing sensorimotor, interpersonal and situated action—our design reflects current trends towards relationality in the cognitive sciences6,17–21. These strands of research urge us to locate social cognition at interrelating and intersecting levels of organisation: from biological to cultural factors, in individuals, interacting parties as well as their environment. This implies that empirical investigation of social cognitive processes should consider dynamics across multiple levels of observation. We believe that our approach meets this demand. Our participants needed to master a challenging game control (precise index finger movements) and had to coordinate their steering actions with a partner, both of which stimulates engagement and creates room for individual choice. We further considered changes in behaviour and social experience over different periods of time, and assessed the influence of seeing the same versus in part different obstacles compared to one’s partner. In our principal line of investigation, we then predicted participants’ social experience from a combination of multiple operationalisations of interpersonal movement coordination, gaming behaviour, personality differences as well as the interaction context. In line with the concept of socialising sensorimotor contingencies, we hence investigated social cognition as a process that establishes and details itself in embodied and situated action.
The central research question that we pursued with the present study focuses on the relationship between social experience and interpersonal sensorimotor coordination: is social experience (partly) constituted by how we move with our interaction partner? Can we, thus, use measures of interpersonal movement coordination to predict how participants experience their interaction? Our second line of investigation concerns the evolution of participants’ interaction over time and across conditions of joint play. In particular, we tracked changes over blocks (3–4 min of play) and sessions (20 min), and tested for differences in social behaviour and experience at times when participants had the same or partially different views on the game environment. Finally, prompted by unexpected findings in the interviews, we investigated individual differences at the transition from joint back to individual play, as well as the within-trial evolution of the interaction dynamics.
Methods
Participants
23 pairs of players (14 female-female pairs, 8 male-male pairs, 1 female-male pair; mean age 24.7 years, range 20–37) participated in the BallGame. Participants received monetary compensation for their time and a bonus depending on their success at the game (0.7 cents per collected target). Participants had normal or corrected to normal vision and reported no history of neurological or psychiatric illness. The study was approved by the Ethics Committee of the Medical Association Hamburg and conducted in accordance with the Declaration of Helsinki. Prior to the recordings, all participants provided written informed consent.
The BallGame
We designed the BallGame in order to facilitate social engagement, continuous interaction and participant autonomy, while ensuring rigorous multi-level game-concurrent observation. The BallGame is a computer-based task in which two players use their index fingers to steer a virtual ball across a two-dimensional surface, avoiding obstacles to collect as many targets as possible in limited time (Fig. 1A, left presents a screenshot of the game environment). Each trial of the game lasted one minute. At any point in a trial, three targets and six obstacles were visible to each player, and three additional obstacles remained invisible. Players could not talk to each other during the game. To learn about the intentions of their partner, as well as the location of invisible obstacles, they had to keep track of momentary deceleration due to disagreement, versus reliable slowing down of the ball when hitting an invisible obstacle. When players collected a target (when the ball hit one of the three visible coins), this target disappeared, and the previously inactive (fourth) target appeared. The challenge was to learn to steer the ball (first alone, then together), get to know the landscape (remember the location of invisible obstacles) and collect as many targets as possible in limited time: after each one-minute trial, the location of targets and obstacles changed (see also Supplementary Materials A): the three outer targets (visible in Fig. 1A, left) rotated around the equidistant centre, and another 9 of 15 possible obstacle locations were activated (six visible, three invisible). The 9 obstacle locations were pseudo-randomly picked from 15 possible locations, so that all direct lines between the targets were blocked by at least one obstacle. All pairs played the same 60 landscapes, with the order of the landscapes shuffled within each 10 subsequent trials of the same game condition. The game environment was developed in LabView for Windows version 2017 SP1 17.0.1f1, we used IvODE as 3D and physics engine library, and set the order of trial types (baseline, Q&A, break, game) as well as game trial parameters such as obstacle and target locations via Matlab scripts. Note that due to strong dependence on local hardware (a National Instruments PXIe-1083 chassis, a PXI-7842R FPGA card and the bi-metal sensors—for details, please see main text below and Fig. 1), we only make the game program available upon request.
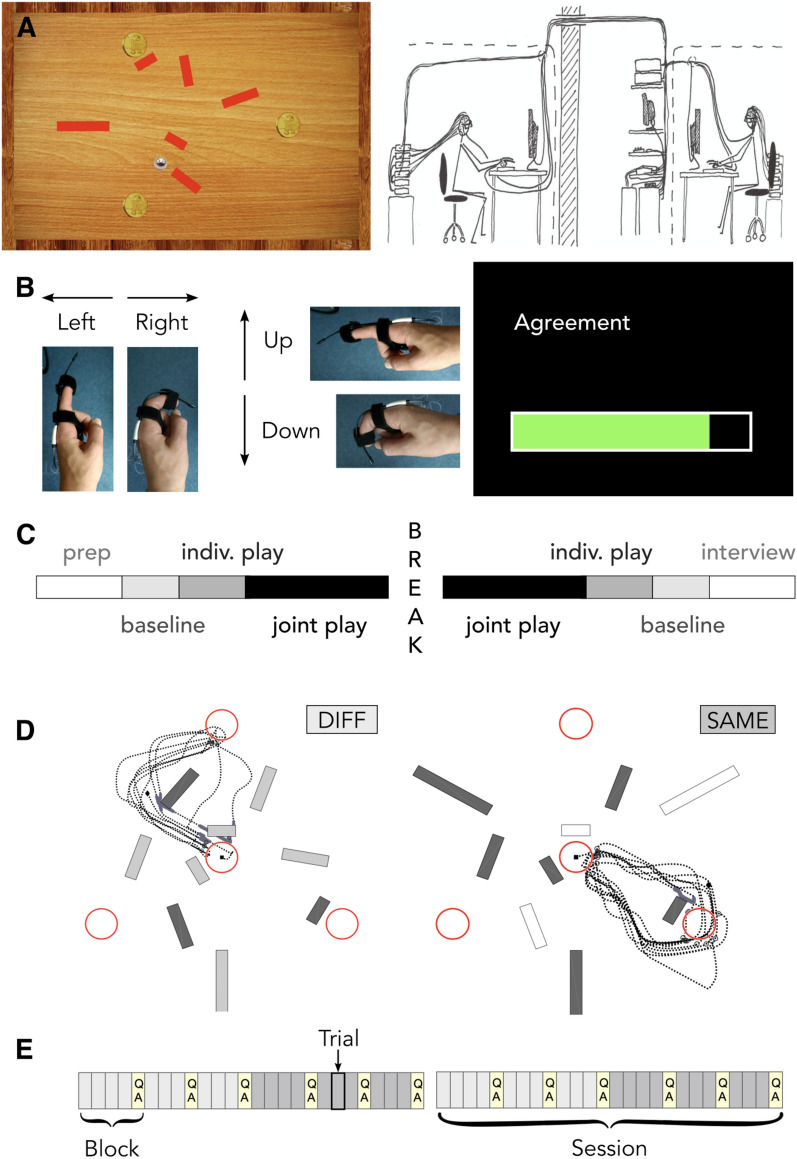
Figure 1.
Experimental Paradigm. (A, left) Screenshot of the game environment. Participants steered the ball (grey marble) to collect targets (golden coins) and avoid obstacles (red bars that slow down the ball to 10% of its speed). (A, right) Illustration of the BallGame setup: a pair of participants, each equipped with a 128-channel EEG cap, eye-tracker goggles and bimetal sensors attached to index fingers, sitting in adjacent rooms / sound-attenuated cabins. Note that analyses of the the EEG data are not included in the present article. (B, left) Demonstration of the game control—a bimetal sensor attached to the index finger translated bending and stretching of the finger into ball movement on the screen. (B, right) View of an example experience rating (bar filled by ‘left–right’ movement, answer confirmed with long ‘down’ movement). (C) Experimental protocol. After the instructions, participants were prepared (prep) for the game-concurrent data recording. The experiment began with baseline tasks and 10 trials of individual play. After further 20 trials of joint play, we took a longer break. Afterwards, participants played another 20 trials of joint, and 10 trials of individual play, and completed the baseline tasks. Finally, we conducted individual interviews. During this time, the other participant filled in personality questionnaires. (D) The two joint play conditions. In joint play DIFF (different), three of nine obstacles were visible to both players (dark grey bars), three only to player one or two (light grey bars). In joint play SAME, players saw the same six of nine obstacles (dark grey bars)—three obstacles remained invisible to the team (empty bars). The black dotted line indicates the path traveled by the ball in an example one-minute trial. (E) Experimental protocol of the joint play period. Joint play was structured in 12 blocks of three or four trials each, after which participants rated their experience in terms of their level of engagement, agreement and predictability (light-yellow boxes marked ‘QA’ (questions and answers)). In each session, participants played 10 trials of each condition (light grey boxes = joint play DIFF; dark grey boxes = joint play SAME). Note that the starting game condition alternated between pairs.
Throughout a trial, participants continuously influenced the movement of the ball, with either index finger controlling the acceleration of the ball along the x and y axis, respectively. Note that the display sampling rate of the game environment was 60 Hz, while we sampled finger movement at a much higher frequency of 2400 Hz. For each game-frame (of 16.6 ms duration), we hence accelerated the ball according to the average finger movement. During joint play, players’ acceleration was accumulated (up to a maximal force of 0.6 compared to 0.5 during individual play), such that the ball quickly moved right when both players steered right, slowly to the right when players steered at orthogonal directions centred around rightward movement, and not at all, when players’ steering directions were opposite. Our intention was to keep the task as similar as possible across joint and individual play, while allowing for collaborative disturbances as well as advantages. Though prompted by our interest in (continuous) social sensorimotor contingencies, this design feature was particularly inspired by research with a highly reduced space for dyadic interaction: the perceptual crossing paradigm22,23. In this setting, two players move an avatar across a digital line and receive a stimulus (e.g., a vibration on their finger tip) each time they encounter the other, the other’s shadow or a stationary object. This scenario leads players into stable sensorimotor interaction dynamics, allowing them to reliably detect each other’s presence. Findings from the perceptual crossing paradigm convinced us that in spite of limiting the interaction to non-verbal mediated feedback—which boosts controllability as well as our focus on sensorimotor contingencies (embodied cognition)—players could still identify their partner’s actions (within overlapping, continuous game control) and learn to coordinate.
Over the course of the experiment, participants played the BallGame in three different conditions: individual play, joint play with the same obstacle visibility (SAME) and joint play with in part different obstacle visibility (DIFF; see Fig. 1D for an illustration of the two joint play conditions). Note that half of the pairs started the joint play period in joint play SAME, with the other half of the pairs first playing joint play DIFF (the latter case is illustrated in Fig. 1E). Beyond the parallel with natural social engagement (in which interacting partners hold complementary views and information), this design feature was inspired by Vesper and colleagues’(2016) findings about the strong influence of shared perceptual information on how individuals accomplish coordinated action. Overall, joint play presented a collaborative advantage in the form of cumulative acceleration, slightly higher maximal ball speed and access to invisible obstacles via one’s partner (during joint play DIFF). However, players also needed to differentiate hitting an invisible obstacle from disagreeing with their partner (steering in opposite directions), which was particularly challenging during joint play DIFF, where unilaterally (in)visible obstacles were presented.
Experimental protocol
Participants were scheduled to arrive at the institute at the same time. When both participants had finished reading the written game instructions, the experimenter orally summarised the most important points, including a reminder of the joint steering mechanism as well as collaborative advantage of the game: in half of the joint-play trials (joint play ‘DIFF’ condition, see Fig. 1D), their partner would see the three obstacles that remained invisible to themselves. Since participants knew neither of the experimental structure (Fig. 1E) nor which joint play condition they were currently playing, it was advisable for them to always coordinate with their partner, that is to pay attention to their steering directions as potential signals for invisible obstacles.
After clarifying remaining questions, participants took their seats in the two EEG chambers, situated in adjacent rooms (see Fig. 1A, right). With a team of one to three assistants, the experimenter then prepared the game-concurrent data collection: participants were equipped with 128-channel passive electrode EEG caps (EASY CAP BC-128-×7, Herrsching, Germany) to record their brain activity, eye-tracker goggles (Pupil Core, Pupil Labs, Germany) to trace their pupil dilation and gaze-fixation, and bimetal sensors (Finger Twitch Transducer SS61L, BIOPAC Systems, USA) at both index fingers, used as game-control and to answer the questions about their experience of the game (see Fig. 1B). The eye-tracker and bimetal sensors were then calibrated to fit individual movement ranges. After these preparations, participants completed baseline tasks intended to serve as localisers for later EEG analyses (note that the present work does not include analyses of the EEG and eye tracking data). The baseline tasks included two resting conditions (closed eyes, open eyes with fixation) and two active conditions (finger bending, passive viewing of prerecorded game trials). Next, participants performed 10 trials of individual play to familiarise themselves with the BallGame, in particular the game control. We then proceeded with four times 10 trials of joint play, with the order of conditions (joint play SAME and DIFF) counter balanced over pairs. Afterwards, participants played alone again and completed another round of the baseline tasks. Halfway through the joint play period, we took a longer break during which participants could relax, use the bathroom, stretch or step outside. See Fig. 1C for an overview of the experimental protocol. During the play period, we informed participants about transitions between individual and joint play, and asked them to rate their experience every three to five trials (see below, Levels of Observation). We invited participants to use these moments as small breaks. Figure 1E illustrates the experimental protocol of the joint play period. After completing the experiment, we conducted a semi-structured interview with each participant about their experiences playing the game. While one participant was interviewed, the other filled in personality questionnaires.
Levels of observation
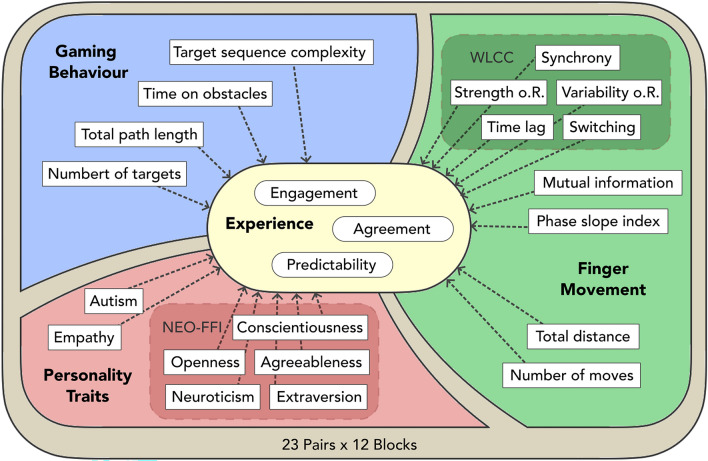
To capture the ongoing interaction dynamics during the BallGame, we organised our analysis along four levels of observation: personality traits, experience, gaming behaviour and finger movement. Below, we describe how we measured and parametrised activity at each level—Fig. 2 gives an overview of all parameters considered in the present analysis.
Figure 2.
Predicting experience from multiple levels of observation: parameters assessed in the BallGame. We considered four levels of observation of interpersonal coordination: personality traits (red), gaming behaviour (blue), finger movement (green) and experience (light-yellow). Each level is described through several parameters. Light-shaded boxes indicate a family relationship between parameters: within personality, this concerns the five traits assessed by the NEO five factor inventory (NEO-FFI); within finger-movement, this concerns five measures of coordination derived from a windowed lagged cross-correlation analysis (WLCC).
Temporal resolution
For all measures except personality traits, we assessed changes over time: across sessions (first vs. second half of joint play), blocks (the first four, second three and last three trials played under the same obstacle visibility condition) and, wherever possible, trial segments (3 × 20 s). There was a short break between the sessions, implying that participants actually experienced a first and a second part of the game. Blocks ran in parallel with the intervals at which participants rated their experience—we hence aggregated data from the three or four trials that preceded a rating. Finally, the rationale for splitting each trial into three segments derived from our findings in the interviews (see below, Results—Within-trial changes in the gaming dynamic).
Personality traits: participants filled in the NEO-FFI24, a general personality questionnaire that allows self-description along the dimensions of neuroticism, extraversion, openness to experience, agreeableness, and conscientiousness. They further completed the Autism Quotient25, and the SPF-IRI26, an interpersonal reactivity index that differentiates four subcomponents (perspective-taking, fantasy, empathic concern, and personal distress) which we aggregated (excluding the last factor) as our ‘Empathy’ measure.
Experience ratings: at fixed moments during the game—that is after trials 5, 10 (individual play); 14, 17, 20; 24, 27, 30; 34, 37, 40; 44, 47, 50 (joint play); 55 and 60 (individual play)—participants provided experience ratings. These ratings assessed whether participants felt able to steer the ball through their finger movements (ball control—only during individual play), how focused and involved they were in the game (engagement—throughout the entire play period), their sense of agreement and smooth performance with their partner (agreement—only during joint play), as well as whether they felt they understood what their partner was doing (predictability—only during joint play). Participants used the game control (the bimetal sensors attached to their index fingers) to provide their answers through a continuous slider. We translated their rating into integers from 0 to 100. After assessing the distributions of the rating data, we used the raw experience ratings for ball control, agreement and predictability ratings, but transformed the engagement ratings using the Arcsine transformation.
Participant interviews: We conducted a semi-structured individual interview with each participant at the end of the experiment. This allowed us to systematically and thoroughly assess the nature of participant engagement in the BallGame. We then opened the interview with generic questions ("What comes to mind when you think back to playing the BallGame?”, “Which moments, if any, were exhausting/fun/social?”), in order to avoid biasing participants. After that, we turned to specific aspects of the game, asking questions that directly relate to our research interests (“Was your partner present to you? If so, when and how?”, “On a scale from 0 = ’100% PC game ‘to 10 = ’100% social interaction, how did you experience joint play?”). The full interview sheet is provided in Supplementary Materials C.
Thematic content of the interviews: We performed a thematic content analysis of the individual post-game interviews27,28. Accordingly, we inductively developed a coding scheme that was tested by means of an iterative coding and refining procedure until a quarter of the data could be classified completely and unambiguously. We then continued to code the remainder of the dataset, occasionally merging or refining codes to avoid very small categories (containing less than 5 of the 46 individuals), or to accommodate novel content.
Gaming behaviour: we used four parameters to capture participants’ gaming behaviour (see also Fig. 2)—generating one value per pair during the joint play, and separate values for each player during the individual play period. (1) Number of targets collected: for each third of a trial (i.e. 20 s), we counted the number of targets collected. (2) Time spent on obstacles: for each third of a trial, we divided the number of frames the ball spent on any of the obstacles by the total number of frames. (3) Total path length: for each third of a trial, we calculated the total distance covered by the ball. (4) Target sequence complexity: for each trial, we evaluated how many times the ball went back and forth between two targets. That is, we counted target collection events that did not involve going back and forth between the same two targets, and divided by the total number of targets collected in this trial. The resulting ‘complexity index’ ranges between 0 and 1, with lower values indicating a tendency to stick to a once identified path.
Finger movement (basics): we calculated two basic movement properties. (1) Movement: to generate a simple measure that captures the overall amount of finger movement, we integrated the velocity of both fingers, regardless of the direction of movement, for each trial segment. (2) Number of moves (direction changes): to estimate the stability of steering, we counted how many times participants switched direction on the x- or y-axis in each third of a trial.
Finger movement (coordination): to quantify the degree of coordination between participants’ finger movements, we calculated seven parameters that assess either the relation between players' movements (undirected coordination), or potential leader–follower dynamics (directed coordination). All parameters are calculated based on participants’ combined x- and y-axis movement, that is, the angle into which players steered the ball (‘steering direction’).
Our first set of measures is based on a windowed lagged cross-correlation (WLCC) analysis, in which we calculated the Spearman correlation between participants’ steering direction over short windows of time. In line with previous work29, we generated five measures: we quantified (1) synchrony as the average WLCC coefficient across all lags (see Supplementary Materials B.1 for WLCC parameters), (2) strength of relation as the mean peak-picked WLCC (ppWLCC) coefficient (the largest coefficient of correlation closest to a lag of zero), independent of the lag at which it was observed, (3) variability of relation as the standard deviation across ppWLCC coefficients, (4) time lag as the average absolute ppWLCC lag (ignoring which participant led or lagged, showing only the relative time delay between players’steering directions). Finally, we assessed (5) switching behaviour as the standard deviation over ppWLCC lags. To control for similarities in movement that may have been induced by the game landscape, we calculated surrogate levels of synchrony: here, we used data of players from different pairs that were navigating the same game landscape (see Figure S.1).
We further quantified mutual information (MI) and calculated the phase slope index (PSI) between players’ steering directions. MI quantifies the mutual dependence between two signals and denotes the reduction of uncertainty about one signal that can be achieved by observing the other30,31. PSI is a measure that quantifies the direction of information flow in multivariate time series. Formally, it corresponds to the weighted average of the slope of the phase of cross-spectra between two signals. In our case, these two signals are the steering directions of two players jointly steering a ball32.
See Supplementary Materials B for a more detailed introduction of our measures of movement coordination.
Statistical analyses
Predicting social experience (linear mixed effects models): to test whether participants’ experience ratings can be predicted from finger movement coordination, gaming behaviour and inter-personal differences, we calculated three linear mixed effects models (using R packages ‘lme4’,33, and ‘lmerTest’,34), one for each of our three social experience ratings (engagement, agreement and predictability—always taking the mean value of both players’ answers). In parallel with participants’ experience ratings, we aggregated all data into 12 blocks, yielding 276 observations per measure (23 pairs × 12 blocks). We initiated each model with the complete set of predictors (4 measures of gaming behaviour, 9 measures of finger movement and 7 measures of personality difference as fixed main effects, no interactions were included), a random intercept for pairs, a time parameter that continuously models the 12 blocks of the joint play period, and an autoregressive covariance structure that models the temporal dependence of repeated measures by allowing for greater similarity of observations that are closer in time35. Figure 2 illustrates the initial model. We then used a restricted maximum-likelihood estimator to fit the model and iteratively eliminated non-significant predictors until only significant predictors were left. This hierarchical backwards elimination procedure was not applied to the random intercept and the autoregressive covariance structure. Furthermore, we performed a leave-one-out cross-validation procedure to test the generalisability of our findings: we calculated a repeated measures correlation (using R package ‘rmcorr’,36,37) between the actual (mean) ratings of our players, and the ratings we predicted based on model parameters that were fit to data from all but the present pair. Following the same rationale, we also calculated pair average correlations. In both cases, higher correlations between observed and predicted ratings indicate better generalisability of the model. Note, however, that this procedure only considered fixed effects.
Variance over time and across game conditions (MANOVAs and ANOVAs): to test for general trends in our game-concurrent observations (experience, gaming behaviour, and finger movement), we calculated multivariate repeated measures analyses of variances (MANOVAs) (using the R package ‘MANOVA.RM’,38) with three within-pair factors for each family: session (before vs. after the break in the middle of joint play), condition (SAME vs. DIFFerent obstacle visibility) and block (accumulating data in parallel with the intervals at which we ask questions). We determined p-values based on parametric bootstrapping and calculated the modified ANOVA-type statistics (MATS) that can account for potential heteroscedasticity as well as singular covariance matrices, thus relaxing the assumptions of the model, and providing more reliable results with small sample sizes39. Below, we report MATS instead of parametric statistics such as the F value. Note that in the ANOVA, we tested for differences between the three subsequent blocks played under the same game condition. In our mixed effects models of participants’ experience ratings, in turn, our time parameter considered changes across all 12 subsequent blocks of joint play.
Follow-up analyses in response to unexpected findings from the interviews
Conducting the interviews extended our understanding of how participants played the BallGame. Based on the thematic content analysis, we learned that participants’ experience of the last period of individual play diverged drastically—while some felt relieved of the burden of having to coordinate with their partner, others lost the motivation to play. There were furthermore specific moments in which their interaction partner tended to be especially present to participants: right before and at the beginning of a trial. Finally, the objects in the game environment were omnipresent in participants’ reports about their social experience. To learn more about the interaction dynamic as highlighted by participants, we then conducted three follow-up analyses of our game-concurrent measures of observation:
Individual differences after the transition from joint to individual play: To investigate the differences in participant reports about the second period of individual play, we looked for within-group differences in our measures of observation and used the degree of coordination to split our group of participants into two. We classified pairs as strongly versus weakly coordinated based on their aggregate rank on all seven measures of movement coordination (excluding the median pair from this analysis). We then compared the behaviour and experience of strongly versus weekly coordinated players as they shift from joint back to individual play. First, we performed a repeated measures ANOVA of the number of targets collected during the second session with mode of play (individual vs. joint) as within-pairs, and coordination level as between-pairs factors. As before, we determined p-values based on parametric bootstrapping and calculated the ANOVA-type statistics (ATS). In addition to performance, we looked at experience ratings of the final 10 trials of individual play: do sense of ball control or engagement evolve differently in the two coordination groups? Here, the ANOVA compared sense of control ratings in the early versus late individual play period (ball control was not assessed during joint play), and engagement ratings during joint versus individual play of the second session.
Within-trial changes in the gaming dynamics: To follow up on participants’ reports of differently experiencing the early versus later parts of a trial, we calculated a repeated measures MANOVA with trial segment as the only within-pairs factor for both movement coordination and gaming behavioural measures, followed by individual measure ANOVAs. Note that several measures of observation were excluded from this analysis, because of insufficient or unavailable data at the level of the trial third, namely: experience ratings, target sequence complexity, PSI and MI.
Coordination as a function of target and obstacle proximity: Prompted by participants frequent mention of objects in the game environment, we related the strength of relation (see above) to the time that has passed since the last target was collected—that is, over the target collection cycle. For each moment of the ppWLCC calculation, we identified the fraction of frames that have passed until the next target is collected. We then calculated the mean and standard error of the strength of relation between participants’ finger movements in 20 sub-sections with the same number of entries along the target collection cycle—beginning and ending at the moment a target is collected. We did so separately for the two joint play conditions (SAME versus DIFF), and tested for difference between the conditions in each of the 20 segments along the target cycle, correcting for multiple comparisons using the Benjamini–Hochberg approach40,41 to control false discovery rates (FDR) across the number of bins. We further assessed the influence of nearby obstacles on coordination: for each moment of the ppWLCC, we determined the visibility of the obstacle that was closest to the ball (minimal distance to the borders of any of the nine obstacles active on the current trial), that is, whether the obstacle was visible to both, either or none of the players. We then performed a repeated measures ANOVA of strength of relation with obstacle-visibility and game condition as within-pairs factors.
Post-hoc tests & correction for multiple comparisons (ANOVAs). When we observed a significant effect of the factors block or trial segment (both of which are three-stepped), we used the MANOVA.RM R-package to calculate post-hoc comparisons between individual blocks or trial segments. To correct for multiple comparisons, we used the Benjamini–Hochberg approach40,41 to control false discovery rates (FDR). Note that we corrected in five groups: one ‘meta group’ formed by the four MANOVAs that aggregate measures within each level of observation (A. experience ratings, B. gaming behaviour, C. basic finger movement, and D. finger movement coordination), and four ‘sub-groups’, each accounting for all ANOVAs and post hoc paired comparisons that we calculated within a given level of observation (number of effects = number of parameters at a given level of observation * 7 effects [3 main effects + 3 two-way interactions + 1 three-way interaction] + possible post hoc comparisons for significant effects of block or trial segment]).
Results
Our analyses integrated multiple levels of observation of continuous, engaged social interaction dynamics and focused in particular on interpersonal sensorimotor coordination as a predictor of social experience. This approach was motivated by recent proposals to ground social cognition in interpersonal coupling mechanisms5,6. Overall, our results demonstrated that social experience in the BallGame was influenced by variables from each of our levels of observation: gaming behaviour (especially the number of targets), movement coordination (in particular the variability of relation between players), personality differences and the interaction context (joint play SAME vs. DIFF, time, objects in the game environment).
To illustrate the nature of social interaction in the BallGame, we begin with an overview of participant reports. We then present our findings from the linear mixed effects models and analyses of variance grouped along three major themes: (1) predictors of social experience; (2) learning effects: changes in gaming behaviour and finger movement parameters over blocks and sessions; and (3) differences between the joint play conditions: effects of seeing the same versus different obstacles.
Finally, prompted by unexpected findings from the interviews, we present results from follow-up analyses. These focus on: (A) individual differences at the transition from joint to individual play, (B) within-trial changes in the gaming dynamics, and (C) game objects as attractors of attention.
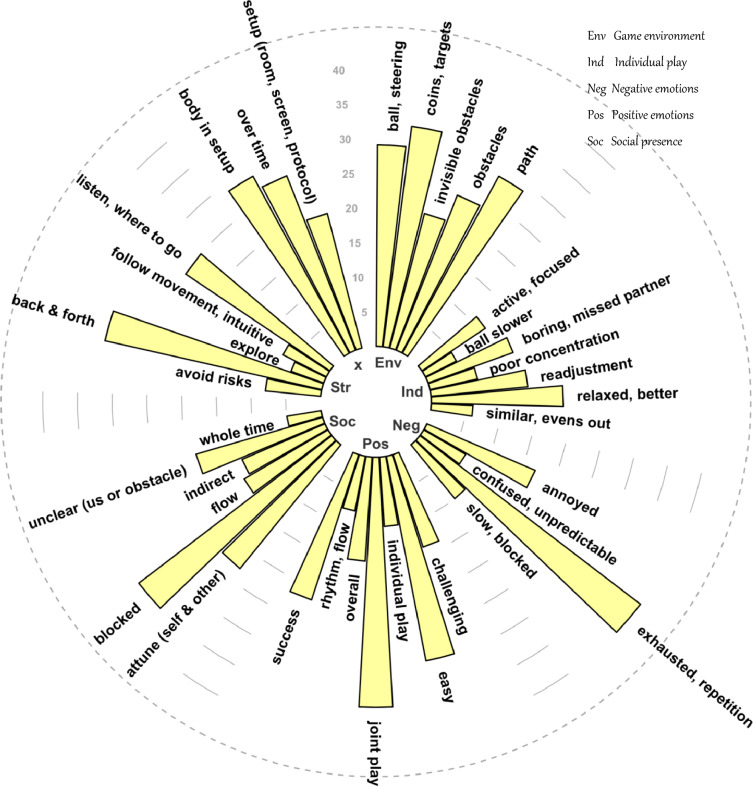
Participant reports: social interaction in the BallGame. The thematic content analysis of participant reports revealed seven major themes (see Fig. 3): game environment, positive emotion, negative emotion, social presence, strategy, individual play and technical comments, each made up of several sub-codes. Figure 3 illustrates how many participants talked about a given code. For a complete summary of the interview contents, consult Supplementary Materials C.2.
Figure 3.
Thematic content of participant interviews. The length of each bar indicates the number of participants that voiced a given code. Grouped bars belong to the same theme (see legend of themes in upper right corner of this figure).
Importantly, the interviews revealed a strong social focus: participants were concerned with figuring out what the partner sees or intends to do (ibid, theme ‘strategy’, sub-category ’listening where to go’, n = 25 participants), in particular during the early trial period (sub-category of ‘listening where to go’, n = 10 participants). Relatedly, participants frequently reported reflecting on whether it was disagreement with their partner or encounter with an invisible obstacle that caused the ball to slow down (theme 'social presence’, sub-category ‘us or obstacle’, n = 19 participants). They also described moments in which difficulties were resolved as particularly pleasant and social (theme 'positive emotion’, sub-categories ‘challenge’ & ‘joint play’, n = 9 participants). What is more, a group of participants experienced a need to re-learn the game control when switching from joint to individual play, possibly indicating strong interpersonal attunement (theme ‘individual play’, sub-category 'readjustment’, n = 14 participants). When asked explicitly, participants also rated their experience of the BallGame as a social interaction rather than a computer game (0 = PC game, 10 = social interaction; mean = 6.45, standard deviation = 1.35). Finally, the interviews confirmed the above mentioned learning effects: participants reportedly learned to coordinate better over time, both concerning steering the ball, as well as interacting with their partner (theme ‘technical comments’, sub-category ‘over time’, n = 14 participants).
Predictors of social experience: successful coordination and interpersonal variability. To integrate our observations of the social interaction dynamics in the BallGame, we calculated linear mixed effects models that assess the influence of parameters from each of our levels of observation on participants’ experience ratings (see Fig. 2). Figure 4 illustrates the final models of participants’ engagement, agreement and predictability ratings, respectively. Our findings demonstrate significant influences from members of each class of observation on participants’ social experience: gaming behaviour (blue boxes/predictors in Fig. 4), movement coordination (green predictors), personality differences (red predictors), as well as the larger interaction context (white predictors).
Figure 4.
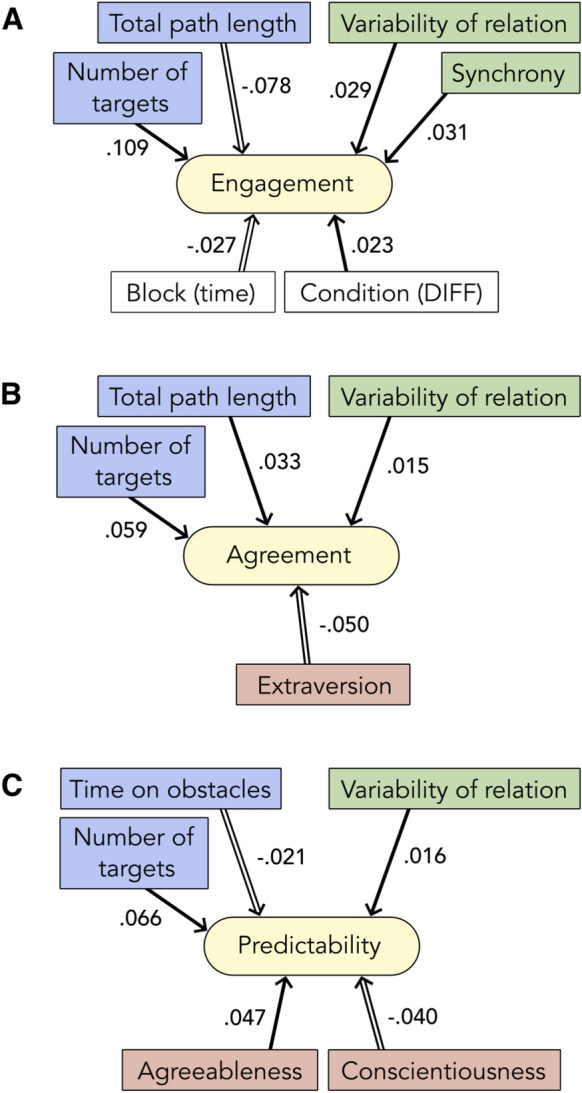
Overview of the final Linear Mixed effects Models of Participants’ Engagement, Agreement and Predictability Ratings (A to C). Filled arrows indicate positive relations (e.g., longer paths predict higher agreement ratings), empty arrows indicate negative relations (e.g. later time predicts lower engagement ratings). In line with the overview presented in Fig. 2, the colour of the boxes/predictors indicates their class of observation: (blue) gaming behaviour, (green) finger movement, (red) personality traits. Time and game condition, as generic contextual factors, are shown in (white). Annotated numbers represent predictor estimates.
Most prominently, we consistently found a higher number of targets collected as well as greater variability in the strength of relation between participants’ finger movements to be associated with enhanced social experience. This is true for the model of participants’ engagement ratings (targets: t = 3.710, p < 0.001, estimate = 0.109; variability of relation: t = 2.397, p = 0.017, estimate = 0.029), as well as the models of participants’ agreement (targets: t = 3.704, p < 0.001, estimate = 0.059; variability of relation: t = 1.971, p = 0.050, estimate = 0.015) and predictability ratings (targets: t = 5.784, p < 0.001, estimate = 0.066; variability of relation: t = 2.363, p = 0.019, estimate = 0.016). The final model of engagement ratings further included synchrony as a positive predictor (t = 2.178, p = 0.030, estimate = 0.031), an indicator of general alignment between participants’ steering directions across interpersonal lags. Path length emerged as a significant predictor of both engagement and agreement ratings—negative in the former (longer paths predict lower engagement ratings; t = − 3.737, p < 0.001, estimate = − 0.078), and positive in the latter case (longer paths predict higher agreement ratings; t = 2.532, p = 0.012, estimate = 0.033). Hence, while travelling long distances together stimulated a sense of agreement with one’s partner, it dampened engagement. Relatedly, we found that the further the time in the experiment had progressed, the lower participants rated their engagement (t = − 7.260, p < 0.001, estimate = − 0.028), in spite of simultaneous improvements in performance and movement coordination over time (see above, learning effects, and Supplementary Figure SF.1). Both effects are likely related to fatigue due to repetition or boredom, as participants unanimously reported in the interviews (Fig. 3, theme ‘negative emotion’, sub-category ‘exhausted, repetition’, n = 38 participants). On the other hand, the model of engagement ratings included the joint play condition as a significant predictor: engagement ratings were higher after joint play DIFF trials (t = − 2.213, p = 0.028, estimate = − 0.023). The increase in engagement in joint play DIFF suggests a stimulating effect of coordinating with a partner that holds a complementary view of one’s environment.
Additional predictors: obstacle collision and personality differences. The final model of predictability ratings also included obstacle time as a significant negative predictor (t = − 2.088, p = 0.038, estimate = − 0.021): spending more time on obstacles reduced participants’ sense of predictability. We relate this finding to statements in the interviews about having to figure out whether the slowdown was caused by the partner or an invisible obstacle. In this sense, more time on obstacles meant greater potential for confusion, or else, a lack of orientation and predictability.
The final models of agreement and predictability ratings included effects of personality difference (see red boxes in Fig. 4B,C). The differences in conscientiousness between players decreased predictability ratings (t = − 2.087, p = 0.049, estimate = − 0.040). The association of smaller differences in conscientiousness with higher interpersonal predictability might suggest that similar levels of ambition and discipline make players predictable to each other in this kind of social interaction. Relatedly, we found that greater similarity in trait extraversion—the tendency to be active, optimistic, interested in communication and exciting stimulation—led to higher agreement ratings (t = − 2.194, p = 0.041, estimate = − 0.050). Accordingly, players may have differed in their tendency to steer the ball through or around obstacles, explore new or repeat old paths, and displayed further more fine-grained differences in steering behaviour. All of these divergences could have caused difficulty to move the ball in a coordinated fashion and thus made it more likely for players to disagree, be stuck on obstacles, and find each other unpredictable. However, we did not find a statistically significant relationship between these personality traits and target sequence complexity (as an indicator of the tendency to explore versus exploit).
Opposite to the effects we observed for extraversion and conscientiousness, differences in trait agreeableness benefited social experience: teams of players with different tendencies for altruistic, empathic, understanding or benevolent behaviour gave higher predictability ratings (t = 2.424, p = 0.024, estimate = 0.047). When plotting agreeableness differences against interpersonal time lag, we found evidence for a positive relation between these two characteristics at small to intermediate levels of agreeableness differences (see Supplementary Figure SD.2B). This is congruent with the development of more prominent leader–follower relations in pairs with moderate differences in agreeableness. Note however, that we could more evidently relate directed interpersonal lags to performance differences (the better player tends to be the leader, see Supplementary Figure SD.2D).
Supplementary Materials D present a complete overview of the initial and final model parameters, as well as the leave-one-out cross-validation that we performed to assess our final models’ generalisability. Predicted and observed ratings correlated significantly in all three cross-validations, speaking to the generalisability of our findings.
Learning effects: changes in experience and behaviour over blocks and sessions. To assess changes in experience, behaviour and movement coordination over shorter (blocks of 3–4 min duration) and longer time intervals (sessions of 20 min), we calculated an ANOVA. We found that participants’ performance improved over both blocks (targets: ATS = 8.43, p = 0.003, obstacle time: ATS = 9.078, p = 0.005, and path length: ATS = 13.911, p < 0.001) and sessions (targets: ATS = 29.146, p < 0.001, obstacle time: ATS = 7.17, p = 0.024, path length: ATS = 10.296, p = 0.008), that is, over both short (one block = 3–4 min) and intermediate periods of time (one session = 20 min). We also saw changes in our measures of movement coordination: mutual information (MI) increased over blocks (ATS = 13.027, p < 0.001), and both synchrony and strength of relation increased from the first to the second session (synchrony: ATS = 18.647, p < 0.001, strength of relation: ATS = 20.249, p < 0.001). Supplementary Figure F1 visualises these effects, Supplementary Materials E provide an overview of statistics, including post-hoc comparisons between individual blocks.
Differences between the two joint play conditions: effects of seeing the same versus different obstacles. In our ANOVAs we also compared observations across the two joint play conditions, i.e. at the times when participants saw exactly the same or partially different obstacles.
When participants saw the same obstacles, they collected more targets (ATS = 6.807, p = 0.030), but spent more time on obstacles (ATS = 5.896, p = 0.046). We also found differences in measures of movement coordination between the two joint play conditions: synchrony (ATS = 34.137, p < 0.001), strength of relation (ATS = 19.913, p < 0.001) and MI (ATS = 40.549, p < 0.001) are all higher in joint play SAME. Supplementary Figure SF.2 visualises these effects of condition for both performance and interpersonal movement coordination measures. Thus, while performance is balanced across the two joint play conditions, movement coordination is higher in joint play SAME.
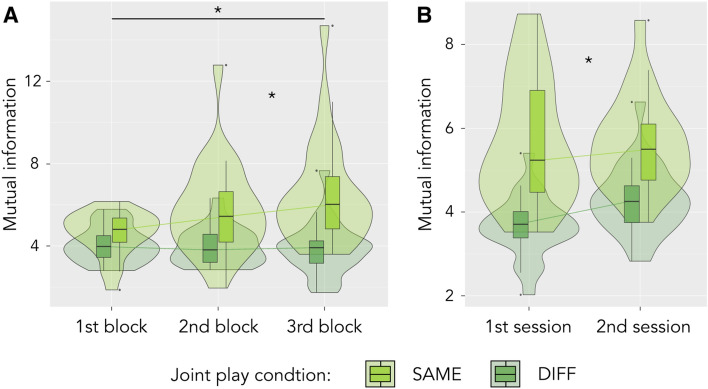
When considering changes over time, we further saw that MI evolved differently over time under the two joint play conditions, with significant interaction effects of the joint play condition with both block and session, respectively: MI increased over blocks in joint play SAME, but not DIFF (ATS = 9.912, p = 0.020; see Fig. 5A), and MI increased more strongly in joint play DIFF, versus SAME, over sessions (ATS = 7.77, p = 0.046; see Fig. 5B). Relatedly, we saw that target sequence complexity tended to increase over blocks in joint play DIFF, with an opposite trend in joint play SAME (ATS = 3.75, p = 0.079; see Supplementary Figure SF.4D).
Figure 5.
Mutual Information (MI) evolves differently in periods when players see the same (joint play SAME) versus different obstacles (joint play DIFF). Both plots display significant interaction effects as revealed by an ANOVA. Significance levels (FDR-corrected) are indicated by * (p < .05) and n.s. (p > = .05). (A) Interaction effect of condition and block: MI increased more strongly in joint play SAME from the first and second to the third block. (B) Interaction effect of condition and session: MI increased more strongly in joint play DIFF over sessions.
These results provide evidence for slower learning in conditions of different obstacle visibility between players, or else, a faster transition into stable modes of playing and coordinating in joint play SAME. Having different obstacle visibility furthermore led to higher engagement ratings (see above, Fig. 4A). Overall, our findings suggest that a complementary view supports the development of more complex and involving behavioural and coordination dynamics.
Individual differences and within-trial dynamics: following up on participant reports.
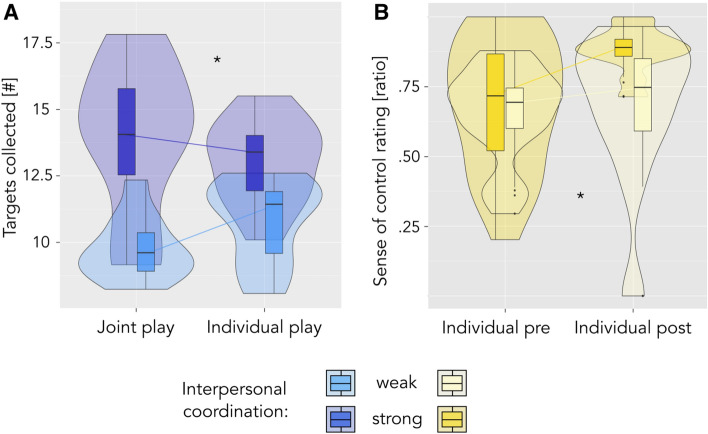
Individual differences at the transition from joint to individual play. When asked what it was like to play alone again after the joint play period, participants’ comments ranged from clearly negative (‘boring’, ‘just working it off’, ‘missed my partner’) to rather positive (‘I felt more active’, ‘now I knew how to control the ball and could just to do my thing’). To follow up on these reports, we calculated an ANOVA of performance in highly versus weakly coordinated players. Our results show that coordinated players overall collected more targets (ATS = 17.378, p < 0.001), and displayed a drop in performance at the shift from joint to individual play—the opposite was true for weakly coordinated players, whose lower performance increased when they shifted to individual play (ATS = 9.903, p = 0.002; see Fig. 6A; for parallel developments of ball velocity, see Supplementary Figure SC.1). When comparing the sense of ball control as rated by participants before and after joint play (ball control was only assessed in periods of individual play), we further found that the sense of ball control increased more strongly for players from strongly coordinated pairs (ATS = 4.423, p = 0.035; see Fig. 6B). Importantly, players from strongly coordinated pairs nevertheless talked more negatively about the final period of individual play (see Supplementary Table SC.1).
Figure 6.
Players from strongly versus weakly coordinated teams at the shift from joint to individual play. Plots display interaction effects of coordination level (strong versus weak) and time. Significant effects are indicated by ** (p < .01) and * (p < .05). (A) Players from highly coordinated pairs collect fewer targets in the final period of individual play compared to their joint play performance in the second session—the opposite holds for players from weakly coordinated pairs. (B) The sense of ball control increases more strongly from the first to the last 10 trials of individual play for players from strongly coordinated pairs.
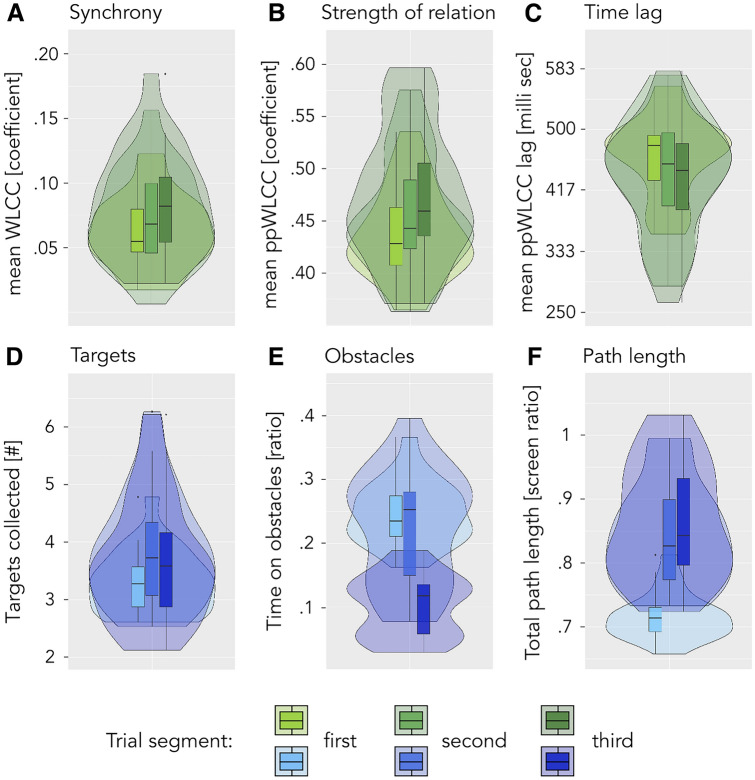
Within-trial changes in the gaming dynamics. Participants described a marked shift in their experience from the early trial, during which they were deeply involved with resolving coordination issues and understanding what their partner wanted, to the late trial, which was more about performing and could even feel like playing alone. To trace these within-trial changes in game-concurrent observations, we calculated an ANOVA of performance and movement coordination measures within trials, cutting the trial into three segments of 20 s each. Our results show that coordination improved significantly and rather continuously throughout the trial (see Fig. 7A–C; synchrony: p = 0.006, ATS = 8.51; strength of relation: p < 0.001, ATS = 13.26; time lag: p = 0.006, ATS = 8.48). Performance measures, however, evolved in a less regular fashion: while generally improving over trial segments (see Fig. 7D–F; targets: p < 0.001, ATS = 19.73; obstacle time: p < 0.001, ATS = 155; path length: p < 0.001, ATS = 102.81), the number of targets collected as well as the total path length increased mostly from the first to the second trial segment, whereas obstacle time only dropped in the final trial-third (see Table SE.3 for exact post-hoc pair-wise comparison statistics). These findings agree with participant statements about changes in the gaming dynamics across the trial. They also indicate that participants succeeded relatively quickly at reaching more targets, whereas reducing obstacle collisions was only accomplished late during the trial.
Figure 7.
Within-trial changes in movement coordination and gaming behaviour. Plots display significant main effects of trial segment as revealed by ANOVAs. Significance levels (within-class FDR-corrected) are indicated by *** (p < .001), ** (p < .01) and * (p < .05). Top row, significant effects in movement coordination measures: (A and B) Synchrony and Strength of Relation increased from the first and second to the final trial segment. (C) Interpersonal time-lag was lower in the second and third, compared to the first trial segment. Bottom row, significant effects in performance measures: (D) The number of targets collected was lowest in the first and highest in the second trial segment, after which it decreased again from the second to the third trial segment. (E) Obstacle time was only reduced in the third, compared to the first and second trial segments. (F) Path length increased from the first to the second, as well as from second to third trial segment. See tables SE.4 and SE.3 for an overview of statistics.
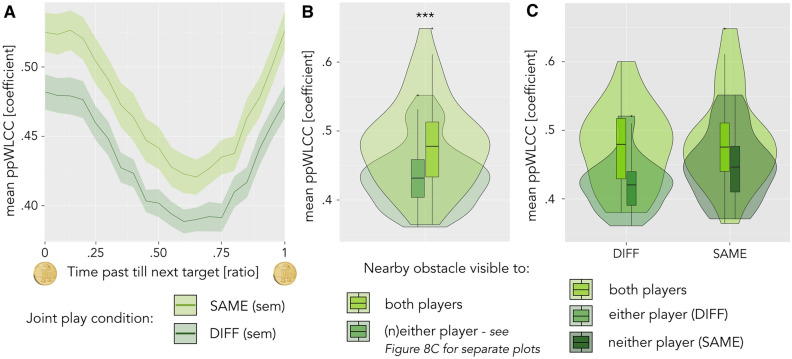
Game objects as attractors of attention. Participants frequently mentioned all objects present in the game environment—to recall their experience, describe strategies used in the game or voice emotions. This prompted us to investigate coordination in the vicinity of targets and obstacles. More specifically, we compared the strength of relation between players’ steering movements across the target cycle, as a function of how long ago the last target was collected and how quickly they would reach the next one. As can be seen in Fig. 8A, the strength of relation between participants’ steering movements was highest during and immediately after target collection. This pattern was remarkably consistent across both joint play conditions (plotted in light versus dark green, Fig. 8A), with a significant, consistent offset in the strength of relation between the two conditions. We also compared the strength of relation at times when the ball was closest to an obstacle that both players could see, to moments when one or no player could see the most proximal obstacle. Here, we find that players’ steering movements were more strongly related when the ball was closest to an obstacle that both of them could see (ATS = 57.247, p < 0.001; see Fig. 8B). Interestingly, coordination appears higher around completely invisible obstacles, compared to obstacles that only one player can see (Fig. 8C). In short, objects in the game environment showed to have a strong influence on interpersonal coordination.
Figure 8.
Objects in the game environment influence interpersonal movement coordination. FDR-corrected significance levels are indicated by *** (p < .001). (A) Coordination across the target cycle: lines and shaded areas represent mean and standard error of the mean strength of relation from one target collection event to the next, separate for the two joint play conditions (joint play SAME = light green, joint play DIFF = dark green). (B) Coordination as a function of obstacle visibility for the average of SAME and DIFF: players’ steering movements are more strongly related when the ball is closest to an obstacle that both players can see (light green bar and violin plot), versus neither or only one of them (dark green bar and violin plot). (C) Coordination as a function of obstacle visibility, separate plots for joint play DIFF and SAME: while the interaction effect between visibility and joint play condition is not significant (p = .105), coordination appears higher around obstacles that no one can see (SAME, dark green bar and violin) compared to obstacles that only one player can see (DIFF, medium green bar and violin).
Discussion
The present work contributes to social interaction research in three distinct ways. First, we present a novel paradigm for the laboratory-based study of motivated and continuous interaction that accomplishes both genuine social engagement and a remarkable degree of controllability. Second, our analyses integrated multiple levels of observation, including several operationalisations of sensorimotor coupling in a social context. Besides highlighting moments of target collection and variability in the strength of interpersonal coordination as predictors of enhanced social experience, this allowed us to describe the influence of movement coordination, gaming behaviour, personality traits and the interaction context on social experience. Third, following participant reports, we identified the degree of coordination as a marker of individual differences in experience at the transition from joint back to individual play, were able to locate the evolution of interpersonal coordination dynamics in the game environment, and revealed marked and divergent changes in gaming behaviour and movement coordination over short periods of time (within the trial). Overall, our findings emphasise the specific temporal and spatial contexts for interpersonal coordination, and point to a critical balance between interpersonal synchrony and difference for positive social engagement.
Social engagement requires interpersonal synchrony and variability. Our findings strongly suggest that both ‘synchrony’ (e.g. strength of relation, a shared view, collecting targets) and ‘difference’ (e.g. variability of relation, a complementary view) are important for positive social experiences. Good performance and synchrony are straightforward predictors of experience—both contribute directly to the task at hand (‘coordinate with your partner and collect as many targets as possible’). An increase in the variability of the peak strength of relation between players ‘steering movements (independent of the time-lag between them) is a more surprising predictor of experience. It concurs with the opposite effects of path length on engagement and agreement, the decrease in engagement over time and its increase after joint play DIFF trials, to indicate an important balance between predictability (successful coordination) and stimulating difference (fun, challenge, surprise) for engaging social interactions. The motivating effect of such a balanced (successful) social interaction might also explain the drop in performance that we observed for players from strongly coordinated pairs at the transition from joint to individual play (see Fig. 6A): when interacting works, its absence becomes decidedly demotivating—in spite of an increased sense of ball control (Fig. 6B) and steady ball velocity (see Supplementary Figure SC.1). A special role for variability in social interactions—next to a positive impact of synchronous or otherwise coordinated behaviour—is in line with Proksch and colleagues’42 finding of a parallel increase in stability and variability over the course of an orchestra performance explicitly designed to transition from uncoordinated to coordinated behaviour. The authors applied recurrence quantification analysis to sound recordings of the performance—variability in this case referred to the variable length of recurrent sound (amplitude) sequences. Early work on interactional synchrony in the movements and vocalisations of a conversing group further points out variations in interpersonal synchrony as a key mechanism for coordinating switches in communicative roles43. We also find support for a contribution of both alignment and difference in the theoretical literature. De Jaegher’s44 account of cognition as participatory sense making, for example, puts variability centre stage: “social interactional timing is a variable affair, and not rigid”. Similar to the suggested balance between synchrony and difference, authors from diverse fields have emphasised the importance to both exploit proven and explore new strategies: from healthy psychological attachment45, to successful foraging behaviour46,47, creativity48, persistence and having fun49. The suggested balance between synchrony and difference can also be compared to a co-existence of integrative and segregative tendencies that is emphasised in dynamical systems theory50. Importantly, when studying the creativity of joint productions as a function of the groups ‘conversational style (instructive vs. inclusive vs. integrative), Bjørndahl and colleagues51 found that creativity is high when group members allow (and synthesise, integrate) different viewpoints by engaging in frequent repair of own and others ‘contributions. Relatedly, research on interpersonal synergies emphasises the need to study social behaviour at higher (emergent) orders that engender reciprocal compensation and dimensional reduction at lower levels (e.g. individual behaviour)52. Overall, we find strong evidence for an important role of variation in social sensorimotor coordination: successful interpersonal engagement seems to require room and sensitivity for differences just as much as it relies on the capacity to integrate them in synchronised forms of acting and shared understanding (see also53).
Rhythms of coordination: invariant features in the environment as guidelines for interpersonal attention. Participant reports and our follow-up analyses revealed strong local modulations of interpersonal coordination and experience in space (around targets and obstacles) and time (within the trial and at the transition from joint back to individual play). In particular, our findings suggest several scales of recurrence through which the link between external structures and ongoing interpersonal coordination is established and maintained. The notion of several time-scales of entrainment is in line with the distinction between modality- and object-specific sensorimotor contingencies17,54. Applied to the social domain, this principle suggests that successful engagement in social coordination requires participants to align their attention through a variety of shared features in the environment: from immediate sensory (e.g. approaching a target or obstacle, steering a particular curve) to longer-term frames of reference (e.g. typical course of a trial, gaming behavioural strategies, roles in the interaction; see also55). In other words, they need to develop social sensorimotor contingencies6, or else interpersonal synergies (5,52; see also56 for a study of the emergence of interpersonal haptic signals). As emphasised by Krabben and colleagues57, this requires brinkmanship58: the propensity to never become fully settled, paired with the ability to navigate critical space where multiple courses of acting and understanding are possible. Taking a very similar approach, predictive coding theories of neural and cognitive function20 suggest that our organism continuously (re-)generates schemas and strategies to help us approach meaningful aspects of our self and our environment (predictions, hypotheses), and engage in exploratory behaviour that probes, fine-tunes, extends or repurposes them. In successful social interactions, this balancing between reliability and effective exploration becomes a shared process, a collective rhythm that allows us to adapt to our environment in a coordinated or co-creative fashion. Besides emphasising local (situated, unique) histories of coordination, a rhythmic perspective strongly suggests the investigation of recurrent as well as progressively changing features of coordination (see also59–62): how does coordination evolve from one instance to the next (in our case e.g. the evolution of coordination between subsequent target collections, obstacle collisions, trials, blocks, etc.)?
A call for future work on the particular kind of dynamics that sustain interpersonal coordination. Our approach does not yield simple (nor final) answers about the relationship between interactive movement coordination and social experience.
First, the analysis and report of the game-concurrent eye-tracking and EEG recordings is beyond the scope of this article. However, these data present a well-suited extension to answer our research questions on a neurophysiological level. For example, in light of previous findings that show enhanced movement driven modulation of neural activity in followers (especially8, who observed repetitive hand opening and closing; see also7, who studied spontaneous imitation of hand movements), it would be interesting to assess whether ‘follower-typical’ neural modulation is enhanced in those participants in our study who show lower performance or higher levels of agreeableness, as well as in close proximity to (unilaterally) invisible obstacles. Likewise, we plan to look for neural correlates of the experiential and behavioural changes we observed within the trial, to explore potential substrates of a shift in focus from social to performative. Analyses of our EEG data will also be an opportunity to investigate the particular, pair-specific learning histories that we largely left aside in the current manuscript, such as the emergence and evolution of leader–follower roles. Beyond that, the consideration of eye movement and pupil dilation data is likely to complement our understanding of social interaction in the BallGame.
The BallGame presents an example of combining quantitative and qualitative methods to trace continuous interaction dynamics. Future research is needed to refine such multi-level approaches to studying social engagement. Efforts into this direction can, for example, be found in Hall and Stevens’63 Interaction Analysis, a method for reconstructing gestural and conversational interactions in a group of people. Similarly, Kalaydjian and colleagues’64 investigation of free play in groups of children highlights gestures of suggestion, recognition and confirmation as different phases of a joint (distributed) transition between making, following and breaking rules. Incorporating both momentary and aggregated measures of behaviour and experience appears to be key, as well as paying attention to how coordination unfolds through recurrent, progressively changing patterns that attune to the local context. This implies that future work should deliver extended modelling approaches, for example to estimate mediating relations between individual predictors and consider a greater variety of temporal dependencies so as to, ideally, trace the emergence and evolution of social dependencies over time. In this regard, it would be interesting to construct a dynamic version of the Uncontrolled Manifold method frequently used to assess interpersonal synergies65. Relatedly, we plan to assess changes in sensorimotor processing during passive viewing before and after participants have played the game (see Methods, baseline tasks).
Our main finding about a positive contribution of variability and difference to engaging social interactions likewise merits greater attention. One strategy for pursuing this could be especially designed experience assessment (such as questions about challenging, creative or surprising moments; see also the dynamic ratings of togetherness in66; or the phenomenological investigation of interaction dynamics in67). Another approach could be to ease the contribution of difference to social interactions in the laboratory. This could be accomplished by measures such as starting the experiment with a simple activity that allows participants to notice and express their experience, or by using an experimental task that provides a clear frame but invites creative contribution.
The BallGame as a novel paradigm in social interaction research. The BallGame can be located between tasks that require rhythmic interpersonal coordination7–9,13,65,68–70 and experimental approaches that focus on natural interactions10,11,63,64. Our design is explicitly set up at their intersection: interactional synchrony provides an advantage but is not the only or explicit goal in the BallGame. Communicating and finding agreement based on individual preferences and complementary viewpoints and actions is equally relevant. Importantly, the BallGame involves continuous interpersonal sensing and acting, overlapping possibilities for action and shared as well as complementary information between players. As such, this task encourages social engagement and leaves room for individual and interactional autonomy around the development of interpersonal coordination. Such an approach entails analytical challenges: greater freedom implies greater potential for genuine social engagement, but also more complex interpersonal dynamics that are harder to capture in simple measures of behaviour and interpersonal coordination. Therefore, we have chosen an iterative approach that goes beyond generating detailed multi-level records and assessing changes over time: we followed up on participants’ specific descriptions of their interpersonal experience. We argue that this combination provides a powerful tool and a novel approach in laboratory studies of social learning and engagement. In conclusion, we urge future studies to leave room for participant autonomy and invest in thorough evaluation of commonalities and differences in their experience. Analytic approaches can then be designed to integrate what is learnt about the specific histories of coordination that unfold between participants.
Supplementary Information
Acknowledgements
This work was supported by grants from the EU (project ‘socSMCs,’ H2020-641321) and the DFG (SFB936-178316478-A3 and TRR169-261402652-B1/B4). Karin Reimann provided invaluable support with participant recruitment and preparation. Hanna Krause contributed to the development of the semi-structured interview.
Author contributions
AL and FG developed the experimental paradigm and analytic strategy in exchange with TS and AKE. MS, AL and FG implemented the hard- and software of the game environment and hyper scanning setup. AL and FG conducted the study and analysed the data in exchange with TS. AL and KH worked on the qualitative analysis. AL wrote the article in close collaboration with FG. AKE acquired the funding and supervised the study. All authors reviewed the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets and scripts used in the main analyses of the present study are publicly available via the Reserach Data Repository of the University of Hamburg, FDR@UHH, https://www.fdr.uni-hamburg.de/record/14631. For requests to work with our quantitative data that go beyond these specific analyses, please contact the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Andreas K. Engel, Florian Göschl.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-69678-9.
References
- 1.Varela, F. J., Thompson, E. & Rosch, E. The embodied mind: Cognitive science and human experience (MIT Press, 1991). 10.7551/mitpress/6730.001.0001. [Google Scholar]
- 2.Clark, A. Being there: Putting brain, body, and world together again (MIT Press, 1997). 10.7551/mitpress/1552.001.0001. [Google Scholar]
- 3.Menary, R. Introduction to the special issue on 4E cognition. Phenom. Cogn. Sci.9, 459–463. 10.1007/s11097-010-9187-6 (2010). 10.1007/s11097-010-9187-6 [DOI] [Google Scholar]
- 4.Engel, A. K., Maye, A., Kurthen, M. & König, P. Where’s the action? The pragmatic turn in cognitive science. Trends. Cogn. Sci.17, 202–209. 10.1016/j.tics.2013.03.006 (2013). 10.1016/j.tics.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 5.Marsh, K. L., Richardson, M. J. & Schmidt, R. C. Social connection through joint action and interpersonal coordination. Top. Cognitive Sci.1, 320–339. 10.1111/j.1756-8765.2009.01022.x (2009). 10.1111/j.1756-8765.2009.01022.x [DOI] [PubMed] [Google Scholar]
- 6.Lübbert, A. et al. Socializing sensorimotor contingencies. Front. Hum. Neurosci.15, 624610. 10.3389/fnhum.2021.624610 (2021). 10.3389/fnhum.2021.624610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumas, G., Nadel, J., Soussignan, R., Martinerie, J. & Garnero, L. Interbrain synchronization during social interaction. PLoS One5, e12166. 10.1371/journal.pone.0012166 (2010). 10.1371/journal.pone.0012166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou, G., Bourguignon, M., Parkkonen, L. & Hari, R. Neural signatures of hand kinematics in leaders vs. followers: A dual-MEG study. Neuroimage125, 731–738. 10.1016/j.neuroimage.2015.11.002 (2016). 10.1016/j.neuroimage.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llobera, J. et al. The subjective sensation of synchrony: An experimental study. PLoS One11, e0147008. 10.1371/journal.pone.0147008 (2016). 10.1371/journal.pone.0147008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakubowski, K. et al. Multimodal perception of interpersonal synchrony: Evidence from global and continuous ratings of improvised musical duo performances. Psychomusicol. Music Mind Brain30(4), 159–177. 10.1037/pmu0000264 (2020). 10.1037/pmu0000264 [DOI] [Google Scholar]
- 11.Ramseyer, F. & Tschacher, W. Movement coordination in psychotherapy: Synchrony of hand movements is associated with session outcome. A single-case study. Nonlinear Dyn. Psychol. Life Sci.20(2), 145–166 (2016). [PubMed] [Google Scholar]
- 12.Feniger-Schaal, R., Hart, Y., Lotan, N., Koren-Karie, N. & Noy, L. The body speaks: Using the mirror game to link attachment and non-verbal behavior. Front. Psychol.9, 1560. 10.3389/fpsyg.2018.01560 (2018). 10.3389/fpsyg.2018.01560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vesper, C., Schmitz, L., Safra, L., Sebanz, N. & Knoblich, G. The role of shared visual information for joint action coordination. Cognition153, 118–123. 10.1016/j.cognition.2016.05.002 (2016). 10.1016/j.cognition.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao, K. & Smillie, L. D. The role of interpersonal traits in social decision making: Exploring sources of behavioral heterogeneity in economic games. Pers. Soc. Psychol. Rev.19(3), 277–302. 10.1177/1088868314553709 (2015). 10.1177/1088868314553709 [DOI] [PubMed] [Google Scholar]
- 15.Cheng, M., Kato, M. & Tseng, C. H. Gender and autistic traits modulate implicit motor synchrony. PLoS One12(9), e0184083. 10.1371/journal.pone.0184083 (2017). 10.1371/journal.pone.0184083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curioni, A., Minio-Paluello, I., Sacheli, L. M., Candidi, M. & Aglioti, S. M. Autistic traits affect interpersonal motor coordination by modulating strategic use of role-based behavior. Mol. Autism.8, 23. 10.1186/s13229-017-0141-0 (2017). 10.1186/s13229-017-0141-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Regan, J. K. & Noë, A. A sensorimotor account of vision and visual consciousness. Behav. Brain Sci.24(5), 883–917. 10.1017/s0140525x01000115 (2001). 10.1017/s0140525x01000115 [DOI] [PubMed] [Google Scholar]
- 18.De Jaegher, H. & Di Paolo, E. Participatory sense-making. Phenom. Cogn. Sci.6, 485–507. 10.1007/s11097-007-9076-9 (2007). 10.1007/s11097-007-9076-9 [DOI] [Google Scholar]
- 19.Konvalinka, I. & Roepstorff, A. The two-brain approach: How can mutually interacting brains teach us something about social interaction?. Front. Hum. Neurosci.6, 215. 10.3389/fnhum.2012.00215 (2012). 10.3389/fnhum.2012.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark, A. Surfing Uncertainty: Prediction, Action and the Embodied Mind (Oxford University Press, 2016). 10.30965/9783957437907_015. [Google Scholar]
- 21.Durt, C., Fuchs, T. & Tewes, C. Embodiment, enaction and culture: Investigating the constitution of the shared world (MIT Press, 2017). 10.7551/mitpress/9780262035552.001.0001. [Google Scholar]
- 22.Auvray, M. & Rohde, M. Perceptual crossing: The simplest online paradigm. Front. Hum. Neurosci.6, 181. 10.3389/fnhum.2012.00181 (2012). 10.3389/fnhum.2012.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Froese, T., Iizuka, H. & Ikegami, T. Embodied social interaction constitutes social cognition in pairs of humans: A minimalist virtual reality experiment. Sci. Rep.4, 3672. 10.1038/srep03672 (2014). 10.1038/srep03672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borkenau, P. & Ostendorf, F. NEO-FFI: NEO-Fünf-Faktoren-Inventar nach Costa und McCrae, Manual 2nd edn. (Hogrefe, 2008). [Google Scholar]
- 25.Baron-Cohen, S., Wheelwright, S., Skinner, R., Matin, J. & Clubley, E. The AUTISM-SPECTRUM QUotient (AQ): Evidence from Asperger Syndrome/High-Functioning Autism, males and females, scientists and mathematicians. J. Autism. Dev. Disord.31, 5–17. 10.1023/a:1005653411471 (2001). 10.1023/a:1005653411471 [DOI] [PubMed] [Google Scholar]
- 26.Paulus, C. The Saarbrueck Personality Questionnaire on Empathy: Psychometric evaluation of the German version of the Interpersonal Reactivity Index (2009). 10.23668/psycharchives.9249
- 27.Elo, S. & Kyngäs, H. The qualitative content analysis process. J. Adv. Nurs.62(1), 107–115. 10.1111/j.1365-2648.2007.04569.x (2008). 10.1111/j.1365-2648.2007.04569.x [DOI] [PubMed] [Google Scholar]
- 28.Kuckartz, U. Qualitative Inhaltsanalyse. Methoden, Praxis, Computerunterstützung (Beltz Juventa, 2012). [Google Scholar]
- 29.Moulder, R. G., Boker, S. M., Ramseyer, F. & Tschacher, W. Determining synchrony between behavioral time series: An application of surrogate data generation for establishing falsifiable null-hypotheses. Psychol. Methods23, 757–773. 10.1037/met0000172 (2018). 10.1037/met0000172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen, M. X. Analyzing neural time series data: Theory and practice (MIT Press, 2014). 10.7551/mitpress/9609.001.0001. [Google Scholar]
- 31.Quian Quiroga, R. & Panzeri, S. Extracting information from neuronal populations: information theory and decoding approaches. Nat. Rev. Neurosci.10(3), 173–185. 10.1038/nrn2578 (2009). 10.1038/nrn2578 [DOI] [PubMed] [Google Scholar]
- 32.Nolte, G. et al. Robustly estimating the flow direction of information in complex physical systems. Phys. Rev. Lett.100(23), 234101. 10.1103/physrevlett.100.234101 (2008). 10.1103/physrevlett.100.234101 [DOI] [PubMed] [Google Scholar]
- 33.Bates, D., Maechler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw.67(1), 1–48. 10.18637/jss.v067.i01 (2015). 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 34.Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw.82(13), 1–26. 10.18637/jss.v082.i13 (2017). 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- 35.De Haan-Rietdijk, S., Kuppens, P. & Hamaker, E. L. What’s in a day? A guide to decomposing the variance in intensive longitudinal data. Front. Psychol.7, 891. 10.3389/fpsyg.2016.00891 (2016). 10.3389/fpsyg.2016.00891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bakdash, J.Z. & Marusich, L.R. rmcorr: Repeated measures correlation. R package version 0.4.3. (2021). https://CRAN.R-project.org/package=rmcorr
- 37.Bakdash, J. Z. & Marusich, L. R. Repeated measures correlation. Front. Psychol.8, 456. 10.3389/fpsyg.2017.00456 (2017). 10.3389/fpsyg.2017.00456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedrich, S., Konietschke, F. & Pauly, M. MANOVA.RM: Resampling-based analysis of multivariate data and repeated measures designs. R package version 0.5.1. (2021). https://CRAN.R-project.org/package=MANOVA.RM
- 39.Friedrich, S. & Pauly, M. MATS: Inference for potentially singular and heteroscedastic MANOVA. J. Multivar. Anal.165, 166–179. 10.1016/j.jmva.2017.12.008 (2018). 10.1016/j.jmva.2017.12.008 [DOI] [Google Scholar]
- 40.Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodological)57(1), 289–300. 10.1111/j.2517-6161.1995.tb02031.x (1995). 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 41.Haynes, W. Benjamini-Hochberg method. In Encyclopedia of Systems Biology (eds Dubitzky, W. et al.) (Springer, 2013). 10.1007/978-1-4419-9863-7_1215. [Google Scholar]
- 42.Proksch, S., Reeves, M., Spivey, M. & Balasubramaniam, R. Coordination dynamics of multi-agent interaction in a musical ensemble. Sci. Rep.12, 421. 10.1038/s41598-021-04463-6 (2022). 10.1038/s41598-021-04463-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kendon, A. Movement coordination in social interaction: Some examples described. Acta Psychologica32, 101–125. 10.1016/0001-6918(70)90094-6 (1970). 10.1016/0001-6918(70)90094-6 [DOI] [PubMed] [Google Scholar]
- 44.De Jaegher, H. Social interaction rhythm and participatory sense-making: an embodied, interactional approach to social understanding, with some implications for autism [unpublished doctoral thesis]. University of Sussex (2007).
- 45.Bowlby, J. Attachment and loss: Retrospect and prospect. Am. J. Orthopsychiatr.52, 664–678. 10.1111/j.1939-0025.1982.tb01456.x (1982). 10.1111/j.1939-0025.1982.tb01456.x [DOI] [PubMed] [Google Scholar]
- 46.Stephens, D. W., Brown, J. S. & Ydenberg, R. C. Foraging: Behavior and ecology (University of Chicago Press, 2008). [Google Scholar]
- 47.Hills, T. T., Todd, P. M. & Jones, M. N. Foraging in semantic fields: How we search through memory. Top. Cognit. Sci.7(3), 513–534. 10.1111/tops.12151 (2015). 10.1111/tops.12151 [DOI] [PubMed] [Google Scholar]
- 48.Hart, Y. et al. Creative foraging: An experimental paradigm for studying exploration and discovery. PLOS ONE12(8), e0182133. 10.1371/journal.pone.0182133 (2017). 10.1371/journal.pone.0182133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCullough, S. E. Mechanical intuitions: The origins and growth of mountain biking [unpublished doctoral thesis] (University of California, 2013). [Google Scholar]
- 50.Tognoli, E. & Kelso, J. The metastable brain. Neuron81, 35–48. 10.1016/j.neuron.2013.12.022 (2014). 10.1016/j.neuron.2013.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bjørndahl, J. S., Fusaroli, R., Østergaard, S. & Tylén, K. Agreeing is not enough: The constructive role of miscommunication. Interact. Stud. Soc. Behav. Commun. Biol. Artif. Syst.16(3), 495–525. 10.1075/is.16.3.07fus (2015). 10.1075/is.16.3.07fus [DOI] [Google Scholar]
- 52.Riley, M. A., Richardson, M., Shockley, K. & Ramenzoni, V. C. Interpersonal synergies. Front. Psychol.2, 7933. 10.3389/fpsyg.2011.00038 (2011). 10.3389/fpsyg.2011.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sebanz, N., Bekkering, H. & Knoblich, G. Joint action: Bodies and minds moving together. Trends Cognitive Sci.10, 70–76. 10.1016/j.tics.2005.12.009 (2006). 10.1016/j.tics.2005.12.009 [DOI] [PubMed] [Google Scholar]
- 54.Maye, A. & Engel, A. K. Time scales of sensorimotor contingencies. In Advances in Brain Inspired Cognitive Systems (eds Zhang, H. et al.) 240–249 (Springer Berlin Heidelberg, 2012). 10.1007/978-3-642-31561-9_27. [Google Scholar]
- 55.Silva, P., Garganta, J., Araújo, D., Davids, K. & Aguiar, P. Shared knowledge or shared affordances? Insights from an ecological dynamics approach to team coordination in sports. Sports Med.43, 765–772. 10.1007/s40279-013-0070-9 (2013). 10.1007/s40279-013-0070-9 [DOI] [PubMed] [Google Scholar]
- 56.Thorne, N., Honisch, J. J., Kondo, T., Nasuto, S. & Hayashi, Y. Temporal structure in haptic signalling under a cooperative task. Front. Hum. Neurosci.13, 372. 10.3389/fnhum.2019.00372 (2019). 10.3389/fnhum.2019.00372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krabben, K., Orth, D. & van der Kamp, J. Combat as an interpersonal synergy: An ecological dynamics approach to combat sports. Sports Med.49(12), 1825–1836 (2019). 10.1007/s40279-019-01173-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kimmel, M. & Rogler, C. R. Affordances in interaction: The case of Aikido. Ecol. Psychol.10.1080/10407413.2017.1409589.5 (2018). 10.1080/10407413.2017.1409589.5 [DOI] [Google Scholar]
- 59.Jones, M. R. Time, our lost dimension: Toward a new theory of perception, attention, and memory. Psychol. Rev.83, 323–355. 10.1037/0033-295X.83.5.323 (1976). 10.1037/0033-295X.83.5.323 [DOI] [PubMed] [Google Scholar]
- 60.Large, E. W. & Jones, M. R. The dynamics of attending: How people track time-varying events. Psychol. Rev.106(1), 119–159. 10.1037/0033-295X.106.1.119 (1999). 10.1037/0033-295X.106.1.119 [DOI] [Google Scholar]
- 61.Lefebvre, H. Rhythmanalysis: Space time and everyday life (Continuum, 2004). 10.5040/9781472547385. [Google Scholar]
- 62.Wöllner, C. & London, J. Performing time: Synchrony and temporal flow in music and dance (Oxford University Press, 2023). 10.1093/oso/9780192896254.001.0001. [Google Scholar]
- 63.Hall, R. & Stevens, R. Interaction Analysis approach to knowledge in use. In Knowledge and Interaction. A Synthetic Agenda for the Learning Sciences (eds diSessa, A. A. et al.) 72–108 (Routledge, 2015). 10.4324/9781315757360. [Google Scholar]
- 64.Kalaydjian, J., Laroche, J., Noy, L. & Bachrach, A. A distributed model of collective creativity in free play. Front. Educ.7, 902251. 10.3389/feduc.2022.902251 (2022). 10.3389/feduc.2022.902251 [DOI] [Google Scholar]
- 65.Romero, V., Kallen, R., Riley, M. A. & Richardson, M. J. Can discrete joint action be synergistic? Studying the stabilization of interpersonal hand coordination. J. Exp. Psychol. Human Percept. Perform.41(5), 1223. 10.1037/xhp0000083 (2015). 10.1037/xhp0000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Noy, L., Levit-Binun, N. & Golland, Y. Being in the zone: Physiological markers of togetherness in joint improvisation. Front. Hum. Neurosci.9, 187. 10.3389/fnhum.2015.00187 (2015). 10.3389/fnhum.2015.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kimmel, M., Hristova, D. & Kussmaul, K. Sources of embodied creativity: Interactivity and ideation in contact improvisation. Behav. Sci.8, 52. 10.3390/bs8060052 (2018). 10.3390/bs8060052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Konvalinka, I., Vuust, P., Roepstorff, A. & Frith, C. D. Follow you, follow me: Continuous mutual prediction and adaptation in joint tapping. Q. J. Exp. Psychol.63, 2220–2230. 10.1080/17470218.2010.497843 (2010). 10.1080/17470218.2010.497843 [DOI] [PubMed] [Google Scholar]
- 69.Dumas, G., de Guzman, G. C., Tognoli, E. & Kelso, J. A. S. The human dynamic clamp as a paradigm for social interaction. Proc. Natl. Acad. Sci. USA111, 3726–3734. 10.1073/pnas.1407486111 (2014). 10.1073/pnas.1407486111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Varlet, M., Nozaradan, S., Nijhuis, P. & Keller, P. E. Neural tracking and integration of “self” and “other” in improvised interpersonal coordination. NeuroImage206, 116303. 10.1016/j.neuroimage.2019.116303 (2020). 10.1016/j.neuroimage.2019.116303 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets and scripts used in the main analyses of the present study are publicly available via the Reserach Data Repository of the University of Hamburg, FDR@UHH, https://www.fdr.uni-hamburg.de/record/14631. For requests to work with our quantitative data that go beyond these specific analyses, please contact the corresponding author.