Abstract
Fluoroquinolone resistance is a major challenge in treating Multidrug-Resistant Tuberculosis globally. The GenoType MTBDRsl Ver 2.0, endorsed by the WHO, was used to characterize fluoroquinolone resistance. The fluoroquinolone resistance rates in the MDR-TB, Rifampicin-Resistant TB, and non-MDR-TB were 33%, 16.5%, and 5.4%, respectively. The most common mutation found in fluoroquinolone-resistant isolates was D94G (49.5%) in the gyrA gene. Of the 150 MDR-TB isolates, the prevalence of Extensively Drug-Resistant Tuberculosis and pre-XDR-TB was 1.33% and 30%, respectively. Among the 139 RR-TB isolates, pre-XDR-TB prevalence was 15.8%. The fluoroquinolone resistance rates were 5.12% among the 1230 isoniazid-monoresistant isolates. The study found that MDR-TB and RR-TB have higher risk of fluoroquinolone resistance than non-MDR tuberculosis. Rifampicin-resistant isolates with a mutation at codon S450L have a higher risk (RR = 12.96; 95%CI: 8.34–20.13) of developing fluoroquinolone resistance than isolates with mutations at other codons in the rpoB gene. Isoniazid-resistant isolates with a mutation at codon S315T have a higher risk (RR = 2.09; 95%CI: 1.25–3.50) of developing fluoroquinolone resistance. The study concludes that rapid diagnosis of fluoroquinolone resistance before starting treatment is urgently needed to prevent the spread and increase of resistance and to achieve better treatment outcomes in areas where it is higher.
Keywords: Fluoroquinolone, Rifampicin, Isoniazid, Kanamycin, Mycobacterium tuberculosis, Drug-resistant tuberculosis
Subject terms: Genetics, Microbiology, Molecular biology, Diseases, Risk factors
Introduction
The emergence of multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) has become a significant global public health threat. Drug-resistant tuberculosis contributes significantly to the worldwide burden of antimicrobial resistance and consumes a large proportion of health budgets and related resources in many endemic countries. The rise of multidrug-resistant tuberculosis, which is resistant to rifampicin and isoniazid, poses a challenge to global TB control programmes. MDR-TB has become an international public health threat because it is associated with high treatment costs and unfavourable treatment outcomes.
Fluoroquinolone (FQ) such as levofloxacin and moxifloxacin are some of the most important drugs for treating multidrug-resistant tuberculosis. They are also the drug of choice for patients with drug-sensitive tuberculosis who are intolerant to first-line drugs.1,2 The MDR-TB treatment failures have a shorter life expectancy of 9 years, and they can be replicated in the community during this time. Unfortunately, resistance to fluoroquinolones could arise from mutations in the regions in gyrA and gyrB that determine quinolone resistance. The prevalence of FQ resistance in MDR-TB and non-MDR-TB patients was 26.6.2% and 0.8%, respectively. FQ resistance is associated with poor treatment outcomes in MDR-TB patients3. In most cases, patients with unfavourable treatment outcomes are closely associated with the presence of FQ resistance4, which further complicates treatment and eventually leads to the development of extensively drug-resistant tuberculosis.
India ranks first in the world in detecting drug-resistant tuberculosis, and the estimated number of MDR/RR-TB cases in India is 124,000 (9.1/Lkh population) as per the latest Government of India report of March 20215.An estimated 3.3% of new TB cases and 18% of already treated cases had MDR/RR-TB. Three countries account for about half of the global burden of MDR‑TB, namely India (27%), China (14%), and the Russian Federation (8%)6. In the past decades, fluoroquinolones have been used extensively in India for treating bacterial infections of the gastrointestinal tract, respiratory tract, and urinary tract7, and tuberculosis treatment. It has gradually become a core drug in the treatment regimen of patients with drug-resistant tuberculosis8.Fluoroquinolone is associated with a mutation in the quinolone resistance determining region (QRDR) of DNA subunits A (gyrA) and B (gyrB), which encode a type II DNA topoisomerase. Mutations in subunit A result in high-level resistance, while mutations in subunit B result in low-level resistance. During the treatment of TB, multidrug-resistant patients can develop resistance to fluoroquinolones. The development of such resistance is a risk factor that may favour the transition of these patients from MDR to pre-extensively drug-resistant (pre-XDR) TB, and they may become extensively drug-resistant through further resistance to at least one second-line injectable drug. In the gyrA gene, the most frequent mutations occur in codons 88–94 of the QRDR, particularly codons 88, 90, 91, and 94. In the gyrB gene, fluoroquinolone resistance is most frequently associated with codons 500 and 538 mutations. However, there are known geographical differences in the frequency of gyrA mutations. Understanding the frequency and geographic distribution of FQ resistance mutations is crucial for maximizing the sensitivity and specificity of treatment9. The prevalence of FQ resistance was between 27.4% and 29.6% in India in 2018–202010.
Widespread or inappropriate use of fluoroquinolones may lead to acquired and transmitted FQ resistance, which could seriously jeopardise the effective treatment and control of MDR tuberculosis. Therefore, effective and accurate diagnosis of these MDR-TB and pre-XDR-TB patients is urgently needed to choose an appropriate treatment regimen and prevent transmission. This retrospective study aims to determine the prevalence of FQ-resistant strains, associated risk factors, and transmission of FQ-resistant strains in first-line drug-resistant tuberculosis in South India and provide valuable insights to policymakers for developing appropriate interventions to reduce the subsequent complications of the disease.
Materials and methods
Sample collection and transportation
Puducherry is one of the tourist cities in South India, with an estimated population of 12.5 lakhs. It also has a relatively well-functioning tuberculosis elimination programme. Tuberculosis hospitals in the ten districts of Tamil Nadu and Pondicherry provide local medical care to tuberculosis patients. Generally, patients with suspected tuberculosis contact the district-level hospital for tuberculosis diagnosis. The Intermediate Reference Laboratory, State TB Training and Demonstration Centre at the Government Hospital for Chest Diseases provides molecular diagnostics, liquid culture, and drug susceptibility testing. All pulmonary tuberculosis patients with microbiologically confirmed drug-resistant tuberculosis in Puducherry and eight adjoining districts of Tamil Nadu between January 2020 and December 2023 were included in this study. The doctors reviewing the medical history of the drug-resistant tuberculosis patients have instructed them to collect sputum samples in a pre-labelled, sterile 50 ml wide-mouthed falcon tube (sputum collection container) before starting treatment. Sputum samples collected at each diagnostic site are packed in a standard three-pack container with an ice pack inserted to maintain a temperature of 2–8 °C and sent to the Intermediate Reference Laboratory with an examination form. The samples are then analysed by fluorescence microscopy and phenotypic and genotypic diagnostics.
Genotype MTBDRsl Ver 2.0 assay for Second-Line TB Drugs Susceptibility Test
Upon receipt, the sputum samples were checked for completeness by ensuring that the examination form was properly completed, the Nikshay number was correct, the specimen tube was correctly labelled and there were no leaks. Once each sputum sample was accepted, a unique laboratory number was assigned for processing. Samples were oriented and processed at the Biosafety Level III facilities. Sputum samples were digested and decontaminated using the NALC-NaOH method and centrifuged at 3000 × g for 15 min. The resulting sediment was re-suspended in 1 mL of phosphate buffer solution and centrifuged at 10,000 × g for 15 min. The supernatant was discarded, and the pellet was further processed for DNA extraction. The pellet was dissolved in 100µL Lysis Buffer and incubated at 95 °C for 5 min. Then 100µL of Neutralisation Buffer was added to the suspension, vortexed for 5 s, and centrifuged at 10,000 × g for 5 min. Approximately 40–80µL of the DNA supernatant was transferred to a sterile PCR tube. The 45µL amplification mix was prepared, and 5µL of the DNA supernatant was added to the PCR tubes, using 5µL of water as a control. The PCR tubes were processed according to the manufacturer's instructions. Each well in the GT blot dish was filled with 20µL of denaturing solution (DEN) and 20µL of the amplified PCR product and incubated for 5 min. The wells were then filled with 1 mL of pre-warmed hybridization buffer, carefully mixed, and a pre-labelled strip was added. After aspirating the hybridization buffer, the tray was incubated at 45 °C for 30 min. It was then washed thoroughly, the conjugate was incubated, and the substrate was added. Finally, the strips were rinsed twice with distilled water, removed, and pasted on an evaluation sheet to analyse the results11.
Treatment and management of RR/MDR-TB and isoniazid resistant patients
Patients diagnosed with MDR/RR-TB resistance are referred for necessary pre-treatment evaluations. Based on these evaluations, their treatment is initiated at various directly observed treatment short-course (DOTS) plus / Programmatic Management of Drug-resistant Tuberculosis (PMDT) site centres. Eligible patients with confirmed MDR/RR-TB who meet specific criteria are recommended a shorter oral Bedaquiline-containing MDR/RR-TB regimen lasting 9–11 months. The regimen includes an initial phase (IP) of 4–6 months (Bdq, Lfx, Cfz, Z, E, Hh, Eto) and a continuation phase (CP) of 5 months (Lfx, Cfz, Z, E). Eligible MDR/RR-TB Patients with fluoroquinolone resistance are recommended a longer oral Bedaquiline-containing MDR/RR-TB regimen lasting 18–20 months. In this case, the regimen consists of (18–20) Bdq, Lfx, (6 months or longer)Lzd, Cfz, and Cs. Suppose H mono/poly DR-TB is detected. In that case, the treating doctor conducts appropriate clinical evaluation, and an H mono/poly DR-TB regimen is initiated at the respective health facility while waiting for the SL-LPA and Liquid Culture and drug susceptibility test results. H mono/poly DR-TB regimen is of 6 or 9 months Lfx R E Z with no separate IP/CP. Patients with adverse drug events or clinical deterioration are treated accordingly and, if needed, admitted to the hospital. Psychologists at PMDT sites provide psychological support to the patients. Patients are treated as outpatients and must have monthly follow-up visits for treatment compliance, detection of adverse drug events, and clinical response evaluation. Patient samples are collected monthly, and positive sputum cultures are sent for DST every two months to the Intermediate Reference Laboratory (IRL). If any additional resistance to Z/Cfz is found in the Culture and Drug Susceptibility Test of the baseline sample, or if FQ/InhA and katG mutations are detected in the 4th-month sample, the patient should be reassessed at the DOTS plus / PMDT site centers. If necessary, the patient should discontinue the shorter oral Bedaquiline-containing MDR/RR-TB regimen and start a longer oral M/XDR-TB regimen immediately upon receiving the report12.
Ethical consideration
The Ethics and Scientific Review Committee of the Indira Gandhi Government General Hospital and postgraduate (No.GHIEC/2020/243 dt.08–06-2020) Institute of the Directorate of Health and Family Welfare Services, Puducherry, approved this study. All methods were performed according to the relevant guidelines and regulations stipulated by the World Health Organization (WHO) and the National Tuberculosis Elimination Program (NTEP), Government of India. This research involves retrospective analysis using previously collected sputum samples for diagnostic purposes. The Committee permitted the preceding written informed consent, already obtained during sample collection. The samples were given unique study codes and were uncoupled from the patients, while age and sex were the only socio-demographic data retained. It is worth noting that the study samples did not affect the original patient outcomes.
Statistical analysis
We used MedCalc software (version 22.026), 2024 MedCalc Software Ltd, Begium for all statistical analyses. We used logistic regression analysis to determine the relative risk associated with FQ resistance and transmission of FQ resistance. Statistical results were expressed as relative risk (RR) and 95% confidence intervals (Cl). All tests were two-sided, and a p-value of < 0.05 was considered statistically significant13.
Results
Characteristics of patients and strains
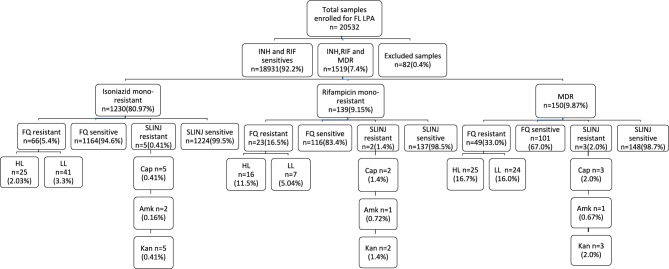
A total of 1519 patients with microbiologically confirmed drug-resistant tuberculosis were included in this retrospective study, with 20,532 samples processed for the MTBDRplus version 2 assay. Of the 1519 Drug-Resistant Tuberculosis isolates, 78.0% (1185) were male, and 22.0% (334) were female. Of the 1519 isolates with drug-resistant tuberculosis, 1230 were mono-resistant to isoniazid (Mono-resistant tuberculosis is a form of tuberculosis where the M.tuberculosis bacteria are resistant to just one treatment drug for TB), 139 were resistant to rifampicin alone, and 150 were multidrug-resistant to tuberculosis (MDR TB is caused by M. tuberculosis bacteria that are resistant to at least isoniazid and rifampicin, which are the most effective first-line TB treatment drugs). Of the 1230 isoniazid mono-resistant isolates, 25 (2.03%) were resistant to high-dose moxifloxacin, levofloxacin resistant, 41 (3.3%) were resistant to low-dose moxifloxacin, levofloxacin resistant and 5 were resistant to second-line injectable drugs. Of the 139 monoresistant rifampicin patient samples processed for the MTBDRsl version 2 assay, 16 (11.5%) showed high-level resistance to moxifloxacin and levofloxacin. In contrast, 7 (5.04%) showed low-level resistance to moxifloxacin and levofloxacin, and 3 (2.0%) were resistant to the second-line injectable drug amikacin. Of the 150 patient samples with multidrug resistance tested using the MTBDRsl version 2 assay, 25 (16.7%) showed high-level resistance to moxifloxacin and levofloxacin, 24 (16.0%) showed low-level resistance to these drugs, and 2 (1.3%) were resistant to the second-line injectable drug amikacin, as shown in Fig. 1.
Fig. 1.
Flowchart of SL-LPA testing for the samples received from 2020 to 2023. INH Isoniazid, RIF-Rifampicin, MDR Multidrug-resistant, FQ Fluoroquinolone, HL High level, LL Low level, FL LPA First-Line Line Probe Assay, Cap Capreomycin, Amk Amikacin, Kan Kanamycin, SLINJ Second-line injectable.
Detection of FQ resistance
To detect fluoroquinolone resistance, we use the GenoType MTBDRsl version 2 assay to detect significant mutations in the DNA gyrase genes gyrA and gyrB. The assay determines the resistance level by detecting the presence or absence of wild-type and mutant probes. If all wild-type probes of a gene are present, this means that no mutation is detectable, i.e. no resistance is detected. If a wild-type probe is missing, this indicates a detectable mutation and the results show that resistance has been detected. If all wild-type probes of a gene are present, but a prominent mutation probe is expressed, this indicates heteroresistance.One or more wild-type probes are absent without corresponding mutant probes, indicating resistance. In our study, out of 1230 isoniazid mono-resistant tuberculosis, 63 (5.12%) were found to be resistant to fluoroquinolones, 3 (0.24%) to SLID (second-line injectable) and 2 (0.16%) to FQ and SLID. In addition, one strain showed dual resistance to the gyrA and gyrB genes. Of the 139 rifampicin-monoresistant tuberculosis, 22 (15.83%) were resistant to FQ, 2 (1.44%) were resistant to SLID, and 1 had dual resistance to the gyrA and gyrB genes. Of the 150 multidrug-resistant tuberculosis, 45 (30.0%) were resistant to FQ, 1 (0.67%) was resistant to SLID, and 2 (1.33%) showed resistance to both FQ and SLID. In addition, two multidrug-resistant strains exhibited dual resistance to gyrA and gyrB, as indicated in Table 1. Of the 150 rifampicin monoresistant and 139 MDR-TB resistant isolates, the prevalence of pre-XDR, XDR, and SLID-resistant isolates was 25.26% (73/289), 0.69% (2/289) and 2.1% (6/289), respectively. Extensively drug-resistant tuberculosis (XDR TB) is a rare form of multi-drug resistant TB caused by M.tuberculosis bacteria that are resistant to isoniazid, rifampicin, a fluoroquinolone, and a second-line injectable (amikacin, capreomycin, and kanamycin) or isoniazid, rifampin, a fluoroquinolone, and bedaquiline or linezolid.
Table 1.
Transmission of first-line drug resistant to fluoroquinolones resistance and its pattern.
| Total resistant | FL drug target gene | SL drug target gene | Type of resistance | Nos | Mutation Probe Pattern | Mutation site (codons) | Total isolates n (144) | Frequency (%) |
|---|---|---|---|---|---|---|---|---|
| Isoniazid mono resistant n(69) | katG n(50) | gyrA n(27) | True resistant | 11 | MUT1 + | A90V | 3 | 2.1 |
| MUT1 + MUT3C + | A90V + D94G | 1 | 0.7 | |||||
| MUT2 + | S91P | 1 | 0.7 | |||||
| MUT3C + | D94G | 6 | 4.2 | |||||
| Inferred resistant | 2 | WT2, WT3, MUT | 89–96 | 1 | 0.7 | |||
| WT3, MUT | 92–96 | 1 | 0.7 | |||||
| Hetero resistant | 14 | WT + MUT1 + | A90V | 2 | 1.4 | |||
| WT + MUT1 + MUT3B + | A90V + D94N/Y | 1 | 0.7 | |||||
| WT + MUT3B + | D94N/Y | 1 | 0.7 | |||||
| WT + MUT3C + | D94G | 9 | 6.3 | |||||
| WT + MUT3D + | D94H | 1 | 0.7 | |||||
| gyrB n(18) | Inferred resistant | 18 | WT, MUT | 536–541 | 18 | 12.5 | ||
| gyrA + gyrB n(1) | True resistant | 1 | MUT1 + | A90V | 1 | 0.7 | ||
| Inferred resistant | WT, MUT | 536–541 | ||||||
| rrs n(2) | Inferred resistant | 1 | WT1, ΔMUT | region 1400 | 1 | 0.7 | ||
| Hetero resistant | 1 | WT + , MUT1 + | a1401g | 1 | 0.7 | |||
| gyrA + rrs n(2) | Hetero resistant | 1 | WT + MUT1 + | A90V | 1 | 0.7 | ||
| True resistant | MUT1 + | a1401g | ||||||
| Hetero resistant | 1 | WT + MUT3C + | D94G | 1 | 0.7 | |||
| True resistant | MUT2 + | g1484t | ||||||
| inhA n(18) | gyrA n(7) | True resistant | 2 | MUT1 + | A90V | 2 | 1.4 | |
| Inferred resistant | 1 | WT2, WT3, MUT | 89–96 | 1 | 0.7 | |||
| Hetero resistant | 4 | WT + MUT3C + | D94G | 3 | 2.1 | |||
| WT + MUT3D + | D94H | 1 | 0.7 | |||||
| gyrB n(10) | Inferred resistant | 10 | WT, MUT | 536–541 | 10 | 6.9 | ||
| rrs n(1) | Inferred resistant | 1 | WT1, MUT | region 1400 | 1 | 0.7 | ||
| katG + inhA n(1) | gyrA n(1) | Hetero resistant | 1 | WT + MUT3D + | D94H | 1 | 0.7 | |
| Rifampicin mono resistant n(25) | rpoB n(25) | gyrA n(20) | True resistant | 8 | MUT1 + | A90V | 2 | 1.4 |
| MUT3B + | D94N/D94Y | 1 | 0.7 | |||||
| MUT3C + | D94G | 5 | 3.5 | |||||
| Inferred resistant | 2 | WT1, WT2, MUT | 85–93 | 1 | 0.7 | |||
| WT3, MUT | 92–96 | 1 | 0.7 | |||||
| Hetero resistant | 10 | WT + MUT2 + | S91P | 1 | 0.7 | |||
| WT + MUT3B + | D94N/D94Y | 1 | 0.7 | |||||
| WT + MUT3C + | D94G | 7 | 4.9 | |||||
| WT + MUT3D + | D94H | 1 | 0.7 | |||||
| gyrB n(2) | Inferred resistant | 2 | WT,MUT | 536–541 | 2 | 1.4 | ||
| gyrA + gyrB n(1) | Hetero resistant | 1 | WT + MUT3D + | D94H | 1 | 0.7 | ||
| Inferred resistant | WT,MUT | 536–541 | ||||||
| rrs n(2) | True resistant | 1 | MUT2 + | g1484t | 1 | 0.7 | ||
| Inferred resistant | 1 | WT1, MUT | region 1400 | 1 | 0.7 | |||
| Multidrug resistant tuberculosis n(50) | rpoB + katG n(39) | gyrA n(31) | True resistant | 19 | MUT1 + | A90V | 6 | 4.2 |
| MUT2 + MUT3A + | S91P + D94A | 1 | 0.7 | |||||
| MUT3B + | D94N/D94Y | 2 | 1.4 | |||||
| MUT3C + | D94G | 8 | 5.6 | |||||
| MUT3D + | D94H | 2 | 1.4 | |||||
| Inferred resistant | 4 | WT1, MUT | 85–89 | 1 | 0.7 | |||
| WT2, MUT | 89–93 | 3 | 2.1 | |||||
| Hetero resistant | 8 | WT + MUT1 + | A90V | 1 | 0.7 | |||
| WT + MUT3A + | D94A | 1 | 0.7 | |||||
| WT + MUT3B + | D94N/D94Y | 1 | 0.7 | |||||
| WT + MUT3C + | D94G | 5 | 3.5 | |||||
| gyrB n(4) | Inferred resistant | 4 | WT, MUT | 536–541 | 4 | 2.8 | ||
| gyrA + gyrB n(2) | True resistant | 1 | MUT3B + | D94N/D94Y | 1 | 0.7 | ||
| Hetero resistant | WT1 + MUT1 + | N538D | ||||||
| Hetero resistant | 1 | WT + MUT3C + | D94G | 1 | 0.7 | |||
| Inferred resistant | WT, MUT | 536–541 | ||||||
| rrs n(1) | Inferred resistant | 1 | WT1, MUT | region 1400 | 1 | 0.7 | ||
| gyrB + rrs n(1) | Inferred resistant | 1 | WT, MUT | 536–541 | 1 | 0.7 | ||
| True resistant | MUT1 + | a1401g | ||||||
| rpoB + inhA (n8) | gyrA n(7) | True resistant | 4 | MUT1 + | A90V | 2 | 1.4 | |
| MUT3A + | D94A | 1 | 0.7 | |||||
| MUT3D + | D94H | 1 | 0.7 | |||||
| Inferred resistant | 2 | WT2, MUT | 89–93 | 1 | 0.7 | |||
| WT3, MUT | 92–96 | 1 | 0.7 | |||||
| Hetero resistant | 1 | WT + MUT3B + | D94N/D94Y | 1 | 0.7 | |||
| gyrB n(1) | Inferred resistant | 1 | WT, MUT | 536–541 | 1 | 0.7 | ||
| rpoB + katG + inhA n(3) | gyrA n(2) | True resistant | 1 | MUT3D + | D94H | 1 | 0.7 | |
| Hetero resistant | 1 | WT + MUT1 + | A90V | 1 | 0.7 | |||
| gyrA + rrs n(1) | True resistant | 1 | MUT1 + | A90V | 1 | 0.7 | ||
| True resistant | MUT1 + | a1401g |
Mutation patterns in the gyrA and gyrB genes
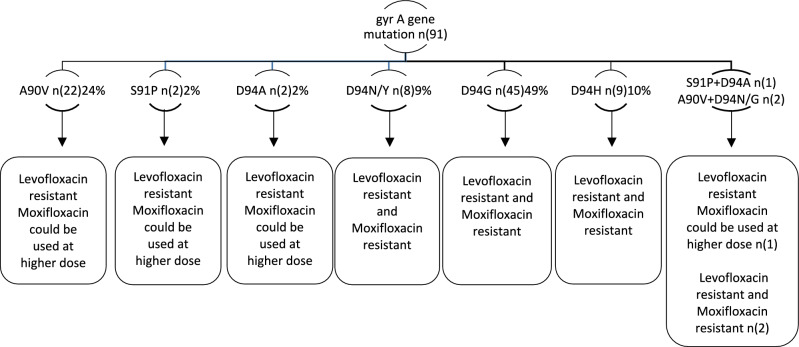
It is known that the primary molecular mechanism of FQ resistance was caused by mutations in the quinolone-resistant determination region (QRDR) of DNA gyrase, which is composed of the gyrA and gyrB subunits encoded by the gyrA and gyrB genes, respectively. Of 144 FQ-resistant isolates, 102 (102/144, 70.8%) carried mutations in the gyrA gene, and 42 (42/144, 29.2%) isolates had mutations in the gyrB gene. Of the 102 gyrA mutants, 52 (57.4%) isolates were truly resistant (one or more WT probes absent and the corresponding MUT probe expressed), and 39 (42.9%) isolates were heteroresistant (expression of the MUT probe in the presence of all WT probes), which includes codons 90, 91 and 94. 11 of 102 (10.8%) were inferred resistant (one or more WT probes missing and without expression of the corresponding MUT probe). The predominant mutation occurred at codon 94, with four different amino acid changes, D94G (45/91, 49.5%), D94A (2/91, 2%), D94Y/N (8/91, 8%), and D94H (9/91, 10%), accounting for 70.3% (64/91) of the FQ-resistant isolates. Of the three FQ-resistant isolates, one had a mutation at D94A/Y and S91A and two had a mutation D94G/N and A90V (Fig. 2). 24% (22.2/91) of the 42 gyrB-mutated isolates were resistant (one or more WT probes were missing, and the corresponding MUT probe was not expressed), and one had a mutation at N538D. For SLID resistance, 6 defined mutations in rrs and 4 undefined mutations in gyrA (3) and gyrB (1) were detected. The most frequently observed mutation (4/10, 40%) for kanamycin and capreomycin resistance was rrs MUT1 (A1401G); 1 of these isolates showed the presence of rrs WT and MUT1. The mutation rrs MUT2 (G1484T) was observed in 2/10 isolates.
Fig. 2.
The association of gyrA mutation with levofloxacin and moxifloxacin resistance.
Transmission of FQs resistance between MDR and non-MDR TB strains
Here is the revised text: Table 2 presents the multivariable logistic regression analysis results regarding the factors associated with the transmission of FQ-resistant patients. In this analysis, the relative risk (RR) indicates the ratio of risk (probability), with RR > 1 suggesting that exposure increases risk, while RR < 1 shows that exposure is protective against risk. After conducting the multivariable logistic regression analysis, it was found that MDR/RR patients in previously treated cases have a higher risk (RR = 1.81; 95%CI: 1.21–2.70, p < 0.037) of developing FQ resistance. Additionally, unfavourable treatment outcomes of H-resistant cases (RR = 1.15; 95%CI: 0.66–2.02) are associated with the development of FQ resistance. Likewise, MDR/RR-resistant cases (RR = 1.14; 95%CI: 0.76–1.70) are also associated with FQ resistance for developing unfavourable treatment outcomes. Furthermore, the multivariable logistic regression analysis revealed that MDR-TB patients have a higher risk (Relative risk = 5.02; 95%CI: 3.70–6.82, p < 0.001) of developing fluoroquinolone resistance compared to patients with isoniazid mono-resistant tuberculosis (RR = 0.22; 95% CI: 0.16–0.29, p < 0.001). It was also found that rifampicin monoresistant patients have a higher risk (Relative risk = 1.99; 95%CI: 1.31–3.00, p < 0.011) of developing FQ resistance. Moreover, rifampicin-resistant strains with a mutation in codon S450L have a higher risk (RR = 12.9697; 95% CI: 8.34–20.13, p < 0.001) for developing FQ resistance than other codons in the rpoB gene. Similarly, isoniazid-resistant strains with a mutation at codon S315T in the katG gene have a higher risk (RR = 2.09; 95% CI: 1.25–3.50, p < 0.049) for developing FQ resistance than the c-15t region of the inhA gene. Of 144 FQ-resistant cases, 45.8% (66/144) transferred H resistance to FQ resistance, and 54.2% (78/144) transferred MDR/RR tuberculosis to FQ resistance. 34.8% of H-resistant TB patients were fully resistant to levofloxacin and moxifloxacin, and the remaining 65.2% can be treated with high-dose moxifloxacin. Additionally, 41% of MDR/RR-TB patients were completely resistant to both levofloxacin and moxifloxacin, and the remaining 59% can be treated with high-dose moxifloxacin.
Table 2.
Multivariable logistic regression on the risk factors of FQ-R patients (N = 138).
| Characteristics | FQ-S n(1381)% | FQ-R n(138)% | Total n(1519)% | RR 95%Cl | p-value |
|---|---|---|---|---|---|
| Demographic factors | |||||
| Gender | |||||
| Female | 298(89.2) | 36(10.8) | 334(22.0) | Ref | |
| Male | 1083(91.4) | 102(8.4) | 1185(78.0) | 0.80(0.55–1.14) | 0.2208 |
| Age | |||||
| < 45 | 578(89.2) | 70(10.8) | 648(42.7) | Ref | |
| ≥ 45 | 803(92.2) | 68(7.8) | 871(57.3) | 0.72(0.53–0.99) | 0.0452 |
| TB treatment history | |||||
| H-resistant cases | |||||
| New cases | 558(93.6) | 38(6.4) | 596(39.2) | Ref | |
| Previously treated cases | 606(95.6) | 28(4.4) | 634(41.7) | 0.69(0.43–1.11) | 0.1299 |
| MDR/RR cases | |||||
| New cases | 139(81.3) | 32(18.7) | 171(11.3) | Ref | |
| Previously treated cases | 78(66.1) | 40(33.9) | 118(7.8) | 1.81(1.21–2.70) | 0.0037 |
| First Diagnostic Unit | |||||
| District-level Hospitals | 401(87.7) | 56(12.3) | 457(30.1) | Ref | |
| Tertiary hospitals | 980(92.3) | 82(7.7) | 1062(69.9) | 0.63(0.45–0.87) | 0.0049 |
| Treatment outcomes | |||||
| H-resistant cases | |||||
| Successful outcome | 930(94.8) | 51(5.2) | 981(64.6) | Ref | |
| Unsuccessful outcome | 234(94.0) | 15(6.0) | 249(16.4) | 1.15(0.66–2.02) | 0.6052 |
| MDR/RR cases | |||||
| Successful outcome | 127(76.5) | 39(23.5) | 166(10.9) | Ref | |
| Unsuccessful outcome | 90(73.2) | 33(26.8) | 123(8.1) | 1.14(0.76–1.70) | 0.5161 |
| Bacteriological factors | |||||
| Isoniazid mono-resistant n(1230) | |||||
| Sensitive | 217(75.1) | 72(24.9) | 289(19.0) | Ref | |
| Resistant | 1164(94.6) | 66(5.4) | 1230(81.0) | 0.22(0.16–0.29) | 0.0001 |
| Rifampicin mono-resistant n(139) | |||||
| Sensitive | 1265(91.7) | 115(8.3) | 1380(90.8) | Ref | |
| Resistant | 116(83.5) | 23(16.5) | 139(9.2) | 1.99(1.31–3.00) | 0.0011 |
| Multidrug resistant n(150) | |||||
| No | 1280(93.5) | 89(6.5) | 1369(90.1) | Ref | |
| Yes | 101(67.3) | 49(32.7) | 150(9.9) | 5.02(3.70–6.82) | 0.0001 |
| Mutation site–MDR/RR-TB | |||||
| rpoB S450L (n = 156) | |||||
| No | 1334(97.9) | 29(2.1) | 1363(89.7) | Ref | |
| Yes | 113(72.4) | 43(27.6) | 156(10.3) | 12.96(8.34–20.13) | 0.0001 |
| Non-MDR/RR-TB | |||||
| katG S315T (n = 795) | |||||
| No | 704(97.2) | 20(2.8%) | 724(47.7) | Ref | |
| Yes | 749(94.2) | 46(5.8%) | 795(52.3) | 2.09(1.25–3.50) | 0.0049 |
H Isoniazid, RR Rifampicin resistant, MDR Multidrug-resistant, FQ Fluoroquinolone.
Discussion
Fluoroquinolone has been widely used to treat various bacterial infectious diseases. However, the misuse of FQ without proper prescription has led to a significant increase in FQ resistance. FQ are essential for treating multidrug-resistant tuberculosis (MDR-TB), and resistance to them is linked to poor treatment outcomes14.Global prevalence data on FQ resistance is limited due to inadequate FQ testing facilities in many tuberculosis-endemic areas. Therefore, localized data is urgently needed to understand FQ resistance in MDR and non-MDR-TB, which is crucial for determining the feasibility of introducing a standardized shorter MDR-TB regimen. In our study, the overall genotypic resistance rate of fluoroquinolones among MDR-TB was 33%, 5.4% in non-MDR, and 16.5% in rifampicin mono-resistant tuberculosis patients. Sethi et al. (2020)15 reported 38.6% in RR isolates in India, and Li et al. (2024)16 reported 34.7% FQ resistance among MDR-TB patients in China. In an Indian study, Sharma et al. (2019)17 and Mamatha et al. (2018)18 reported Fluoroquinolone resistance rates of 27.3% and 32% respectively, which are lower than the rates reported in our study. Our findings indicate a lower resistance rate than previous reports, but the 33% FQ resistance among MDR-TB in our study was significantly higher than the global rate (20.0%) of WHO (2020)19 report. The high rate of FQ resistance in MDR-TB patients in Southern India suggests that including FQ in treatment could lead to ineffective treatments and worsen treatment outcomes. These findings emphasize the urgent need for FQ resistance testing before initiating MDR-TB treatment. In our study, the detection rate for extensively drug-resistant tuberculosis (XDR-TB) was 1.33% (2 isolates), which is relatively lower than the reported global prevalence of XDR-TB and the 8.6% reported in India by Sethi et al., 202015. The resistance rate to any FQs in our cohort among non-MDR-TB was 5.4%, which is relatively higher than the 0.8% reported by Kim et al., 201814, indicating a trend that warrants comparison with the recent prevalence of FQ resistance in TB-endemic countries like India.
Among 91 fluoroquinolone (FQ) true resistant isolates, the frequency of gyrA mutations was higher than gyrB, which aligns with the findings of Kabir et al9. The most common mutations in the gyrA gene associated with FQ resistance in M.tuberculosis are S91P, A90V, and D94A/N/Y/G/H. Most FQ-resistant isolates exhibited a mutation at codon D94G, with A90V being the most prevalent. Our study found that 49% of the FQ-resistant isolates carried the D94G mutation, a notably higher figure compared to Tania Matsui et al.'s (2020)20 report of 44% in Brazil. Our findings are further supported by a recent study in Ethiopia, which detected a gyrA/D94A gene mutation (2%) in FQ-resistant TB isolates21.Moreover, our study identified a rare gyrA mutation at codon D94H in nine isolates, a mutation not commonly reported in other studies14. Among the 91 FQ-true resistant isolates, 26 exhibited resistance to levofloxacin and low-level resistance to moxifloxacin, while 62 displayed resistance to levofloxacin and high-level resistance to moxifloxacin. Additionally, alanine, asparagine, and serine are nonessential amino acids that promote brain functions, remove toxins, and synthesize blood cells. Any functional changes in these nonessential amino acids resulting from mutations can lead to difficulty in producing proteins necessary for cell growth, maintenance, and repair mechanisms22. In our study, we observed a heteroresistance mutation pattern in the gyrA gene exhibited by 39 (42.9%) isolates, characterized by the expression of MUT probe and all WT probes, including at codons 90, 91, and 94. This aligns with a recent report by Dixit et al. (2023)23, who documented a 39.3% heteroresistance mutation pattern in the gyrA gene in India.
Heteroresistance (HR) is common in M. tuberculosis and is considered one of the major steps in the development of drug resistance in bacterial isolates (Ye et al., 2021)24. It may arise from a mixed infection when both resistant and susceptible strains infect a person simultaneously, or when a single clone changes from a susceptible strain to a resistant one due to genetic mutation under antibiotic pressure. It’s worth noting that heteroresistance has been linked to limited treatment options and an increasing rate of unfavorable treatment outcomes, as reported by Rigouts et al25.
The prevalence rate of fluoroquinolone (FQ) resistance among non-MDR-TB in this study is 5.4%, higher than the global estimate of 0.8%. This suggests a need to be cautious about the widespread use of FQ in the community. In this study, FQ resistance among MDR-TB and RR-TB were 32.7% and 16.5%, respectively, which is also higher than the global estimates (Dixit et al., 2023)23. The FQ resistance in this study among the MDR/RR-TB is 24.9%, higher than the global estimates of 18.0%26. Among newly diagnosed H-resistance cases, the FQ resistance was 6.9%, lower than the 9.8% reported in a recent study in Pakistan27. Similarly, the FQ resistance in the previously treated cases was 3.81% compared to the previous study report of 44.6% by Sethi et al.14 Among newly diagnosed MDR/RR-TB cases, the FQ resistance was 23.4%, which was higher than the 21.82% reported in the recent study carried out in China15 and 14.2% in an Indian research28.Similarly, the FQ resistance in the previously treated cases was 74.24% compared to the previous study report of 44.6% by Sethi et al.15 Another study in India reported 72.8% FQ resistance10. The high FQ resistance was noted in previously newly diagnosed MDR/RR TB cases, which might be due to the high transmission of the drug-resistant strains. The high rate of FQ resistance in MDR and non-MDR-TB (H-resistance) patients could lead to ineffective treatment of H mono-resistant and unfavorable outcomes.
Of the 289 MDR/RR –TB isolates, 53% exhibited a mutation at codon S450L of the rpoB gene, which is lower than the previously reported rate of 77% by Tania Matsui et al20. Among 1230 H-mono-resistant isolates, 65% showed a mutation at codon S315T of the katG gene, resulting in high-level isoniazid resistance—a rate lower than the previously reported 72%. Notably, out of the 53% with a mutation at the S450L codon of the rpoB gene and the 65% with a mutation at the S315T codon of the katG gene, 27.9% and 5.9% were at an increased risk for FQ-resistant, a previously unreported finding. This information will be valuable for policymakers and decision-makers, providing timely evidence. Additionally, this study offers crucial insights for physicians in their daily treatment practices and serves as essential baseline information for researchers.
The study found that the rates of unfavorable outcomes were 42.9% for MDR/RR tuberculosis patients and 20.4% for non-MDR-TB patients. Our study showed a higher rate of unfavorable outcomes (42.9%) for MDR/RR tuberculosis patients compared to a previous study in Ethiopia by Bogale et al.(2023)29, which reported a rate of 23.68%. Nair et al. (2017)30 reported a 40% unfavorable outcome rate in India. Aaina et al. (2022)22 reported rates of 29.4% for MDR-TB and 14.5% for non-MDR-TB cases in India. The unfavorable outcome for non-MDR (H-resistance) patients in our study was 20%, lower than the 29.4% reported by Aaina et al. (2021)22 in India. These unsuccessful treatment outcomes were significantly associated with FQ resistance. The increasing percentage of unfavorable outcomes could pose a risk of transmitting tuberculosis-resistant forms and negatively impact the country's GDP.
The study has significant strengths, such as recruiting a large sample size and using various diagnostic methods. However, our study has several limitations to our study. We relied on secondary data for patient characteristics, and only patients with a laboratory diagnosis of tuberculosis were included. Our study used a cross-sectional design and only identified associated factors, not risk factors, for FQ-resistant transmission. We were unable to determine if FQ resistance resulted from previous treatment exposure because data on previous FQ use before DR-TB diagnosis were not available. Additionally, drug susceptibility tests for Drug-Sensitive tuberculosis are not routinely conducted, although FQ resistance among DS-TB could lead to unfavorable treatment outcomes. FQ resistance is higher in regions where these drugs are widely prescribed and sometimes misused as fluoroquinolones.
This text highlights the concerning rise of FQ resistance in India due to the unregulated prescription of these drugs. The study found a higher proportion of MDR/RR-TB cases with FQ-resistant genotypes, even in isolates with resistance to a single drug. The high FQ resistance rate identified in the study is alarming for the National Tuberculosis Elimination Programme and underscores the need for reasonable use of fluoroquinolone drugs. This report also describes specific mutations related to high FQ resistance in TB patients from India, which could inform the development of a new algorithm for rapid drug-resistant TB diagnosis, leading to better treatment outcomes. Around one-third of FQ-resistant cases were presumed to be transmitted, indicating the urgent need for policymakers to address the higher rate of FQ resistance in India among DR-TB patients. The findings suggest implementing the diagnosis of FQ resistance, preferably at the initial diagnosis stage, to identify all resistance-promoting mutations and ensure effective treatment and resistance control.
Acknowledgements
The authors would like to acknowledge the staff of the Intermediary Reference Laboratory and State TB cell (NTEP) for their skilful technical assistance
Author contributions
Author contributions VJN and MJV–prepared manuscript UB and VR–prepared figures GP and BRM–prepared tables AD and MM–prepared manuscript, MM–statistical analysis. All authors reviewed the manuscript.
Data availability
All primary and secondary data are available with the corresponding author and in the Nikshay portal, Government of India. Permission is granted to the corresponding author to access the data through login credentials. The datasets generated and analyzed during the current study are part of the first author's Ph.D. thesis and are not publicly available. The datasets are available from the corresponding author upon reasonable request. Contact no: + 91 9,944,737,597 Email.ID:drmuthurajm@gmail.com.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singh, N., Singh, P. K., Singh, U., Garg, R. & Jain, A. Fluroquinolone drug resistance among MDR-TB patients increases the risk of unfavourable interim microbiological treatment outcome: An observational study. J. Glob. Antimicrob. Resist.24, 40–44 (2021). 10.1016/j.jgar.2020.11.011 [DOI] [PubMed] [Google Scholar]
- 2.Ho, J., Jelfs, P. & Sintchenko, V. Fluoroquinolone resistance in non-multidrug-resistant tuberculosis—a surveillance study in New South Wales, Australia, and a review of global resistance rates. Int. J. Infect. Dis.26, 149–153 (2014). 10.1016/j.ijid.2014.03.1388 [DOI] [PubMed] [Google Scholar]
- 3.Lee, H.-W. & Yim, J.-J. Fluoroquinolone resistance in multidrug-resistant tuberculosis patients. Korean J. Intern. Med.34, 286–287 (2019). 10.3904/kjim.2019.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma, R., Singh, B. K., Kumar, P., Ramachandran, R. & Jorwal, P. Presence of Fluoroquinolone mono-resistance among drug-sensitive Mycobacterium tuberculosis isolates: An alarming trend and implications. CEGH7, 363–366 (2019). [Google Scholar]
- 5.Prasad, J., Kumar, P. & Ramachandran, R. Report of the First National Anti-Tuberculosis Drug Resistance Survey India: 2014–2016: Revised National TB Control Programme (2018). https://tbcindia.mohfw.gov.in.
- 6.Dutt, R., Singh, R., Majhi, J. & Basu, G. Status of drug resistant tuberculosis among patients attending a tuberculosis unit of West Bengal: A record based cross-sectional study. J. Family Med. Prim. Care.11, 84 (2022). 10.4103/jfmpc.jfmpc_576_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatt, S. & Chatterjee, S. Fluoroquinolone antibiotics: Occurrence, mode of action, resistance, environmental detection, and remediation – A comprehensive review. Environ. Pollut.315, 120440 (2022). 10.1016/j.envpol.2022.120440 [DOI] [PubMed] [Google Scholar]
- 8.Kumar, A., Harakuni, S., Paranjape, R., Korabu, A. S. & Prasad, J. B. Factors determining successful treatment outcome among notified tuberculosis patients in Belagavi district of North Karnataka. India. CEGH.25, 101505 (2024). [Google Scholar]
- 9.Kabir, S. et al. Fluoroquinolone resistance and mutational profile of gyrA in pulmonary MDR tuberculosis patients. BMC Pulm. Med.10.1186/s12890-020-1172-4 (2020). 10.1186/s12890-020-1172-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gopalaswamy, R. et al. Resistance Profiles to Second-Line Anti-Tuberculosis Drugs and Their Treatment Outcomes: A Three-Year Retrospective Analysis from South India. Medicina59, 1005 (2023). 10.3390/medicina59061005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman, S. M. M. et al. Performance of GenoType MTBDRsl assay for detection of second-line drugs and ethambutol resistance directly from sputum specimens of MDR-TB patients in Bangladesh. PLoS One16, e0261329 (2021). 10.1371/journal.pone.0261329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidelines for Programmatic Management of Drug-resistant TB in India 2021, National TB Elimination Programme, Central TB Division, Ministry of Health and Family Welfare, Government of India, New Delhi, India. https://tbcindia.mohfw.gov.in.
- 13.MedCalc: MedCalc’s Relative risk calculator. MedCalc Software Ltd, https://www.medcalc.org/calc/relative _ risk.php. (2024).
- 14.Kim, H. et al. Trend of multidrug and fluoroquinolone resistance in Mycobacterium tuberculosis isolates from 2010 to 2014 in Korea: a multicenter study. Korean J. Intern. Med.34, 344–352 (2019). 10.3904/kjim.2018.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sethi, S. et al. Second-line Drug Resistance Characterization in Mycobacterium tuberculosis by Genotype MTBDRsl Assay. J. Epidemiol. Glob. Health10, 42 (2020). 10.2991/jegh.k.191215.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, M. et al. Transmission of Fluoroquinolones Resistance among Multidrug-Resistant Tuberculosis in Shanghai, China: A Retrospective population-based Genomic Epidemiology Study. Emerg Microb. Infect.10.1080/22221751.2024.2302837 (2024). 10.1080/22221751.2024.2302837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma, R., Sharma, S. K., Singh, B. K., Mittal, A. & Kumar, P. High degree of fluoroquinolone resistance among pulmonary tuberculosis patients in New Delhi India. Indian J. Med. Res.149, 62–66 (2019). 10.4103/ijmr.IJMR_1220_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mamatha, H. G. & Shanthi, V. Baseline resistance and cross-resistance among fluoroquinolones in multidrug-resistant Mycobacterium tuberculosis isolates at a national reference laboratory in India. J. Glob. Antimicrob. Resist.12, 5–10 (2018). 10.1016/j.jgar.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Global Tuberculosis Report 2020 (WHO, 2020). [Google Scholar]
- 20.Matsui, T. et al. Frequency of first and second-line drug resistance-associated mutations among resistant Mycobacterium tuberculosis clinical isolates from São Paulo. Brazil. Mem Inst Oswaldo Cruz.10.1590/0074-02760200055 (2020). 10.1590/0074-02760200055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reta, M. A., Maningi, N. E. & Fourie, P. B. Patterns and profiles of drug resistance-conferring mutations in Mycobacterium tuberculosis genotypes isolated from tuberculosis-suspected attendees of spiritual holy water sites in Northwest Ethiopia. Front Public Health10.3389/fpubh.2024.1356826 (2024). 10.3389/fpubh.2024.1356826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aaina, M. et al. Risk Factors and Treatment Outcome Analysis Associated with Second-Line Drug-Resistant Tuberculosis. J. Respir.2, 1–12 (2021). 10.3390/jor2010001 [DOI] [Google Scholar]
- 23.Dixit, R. et al. Fluoroquinolone resistance mutations among Mycobacterium tuberculosis and their interconnection with treatment outcome. Int. J. Mycobacteriol.12, 294–298 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Ye, M. et al. Antibiotic heteroresistance in Mycobacterium tuberculosis isolates: A systematic review and meta-analysis. Ann. Clin. Microbiol. Antimicrob.20, 73 (2021). 10.1186/s12941-021-00478-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rigouts, L. et al. Specific gyrA gene mutations predict poor treatment outcome in MDR-TB. J. Antimicrob. Chemother.71, 314–323 (2015). 10.1093/jac/dkv360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Global Tuberculosis Report 2023 (WHO, 2023). [Google Scholar]
- 27.Tahseen, S. et al. Isoniazid resistance profile and associated levofloxacin and pyrazinamide resistance in rifampicin resistant and sensitive isolates from pulmonary and extrapulmonary tuberculosis patients in Pakistan: A laboratory based surveillance study 2015–19. PLoS One15, e0239328 (2020). 10.1371/journal.pone.0239328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suresh, K., Vimala, Y., Mohan, N. & Padmaja, I. J. Additional Resistance to any Fluoroquinolones among Multidrug-resistant Mycobacterium tuberculosis Isolates from North Coastal Andhra Pradesh India. JPAM15, 68–74 (2021). [Google Scholar]
- 29.Bogale, L., Tsegaye, T., Abdulkadir, M. & Akalu, T. Y. Unfavorable Treatment Outcome and Its Predictors Among Patients with Multidrug-Resistance Tuberculosis in Southern Ethiopia in 2014 to 2019: A multi-center retrospective follow-up study. InfectDrug Resist.14, 1343–1355 (2021). 10.2147/IDR.S300814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nair, D. et al. Predictors of unfavourable treatment outcome in patients with multidrug-resistant tuberculosis in India. PHA7, 32–38 (2017). 10.5588/pha.16.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All primary and secondary data are available with the corresponding author and in the Nikshay portal, Government of India. Permission is granted to the corresponding author to access the data through login credentials. The datasets generated and analyzed during the current study are part of the first author's Ph.D. thesis and are not publicly available. The datasets are available from the corresponding author upon reasonable request. Contact no: + 91 9,944,737,597 Email.ID:drmuthurajm@gmail.com.