Abstract
Introduction
Reduced thrombin generation is an important component of post cardiopulmonary bypass (CPB) coagulopathy. To replenish coagulation factors and enhance thrombin generation in bleeding surgical patients, frozen plasma (FP) and four-factor prothrombin complex concentrate (4F-PCC) are used. However, the efficacy–safety balance of 4F-PCC relative to FP in cardiac surgery is unconfirmed.
Methods and analysis
LEX-211 (FARES-II) is an active-control, randomised, phase 3 study comparing two coagulation factor replacement therapies in bleeding adult cardiac surgical patients at 12 hospitals in Canada and the USA. The primary objective is to determine whether 4F-PCC (Octaplex/Balfaxar, Octapharma) is clinically non-inferior to FP for haemostatic effectiveness. Inclusion criteria are any index (elective or non-elective) cardiac surgery employing CPB and coagulation factor replacement with 4F-PCC or FP ordered in the operating room for bleeding management. Patients will be randomised to receive 1500 or 2000 international units of 4F-PCC or 3 or 4 units of FP, depending on body weight. The primary endpoint of haemostatic treatment response is ‘effective’ if no additional haemostatic intervention is required from 60 min to 24 hours after the first initiation of 4F-PCC or FP; or ‘ineffective’ if any other haemostatic intervention (including a second dose of study drug) is required. An estimated 410 evaluable patients will be required to demonstrate non-inferiority (one-sided α of 0.025, power ≥90%, non-inferiority margin 0.10). Secondary outcomes include transfusions, bleeding-related clinical endpoints, coagulation parameters and safety.
Ethics and dissemination
The trial has been approved by the institutional review boards of all participating centres. Trial completion is anticipated at the end of 2024, and results will be disseminated via publications in peer-reviewed journals and conference presentations in 2025. The results will advance our understanding of coagulation management in bleeding surgical patients, potentially reducing the need for allogeneic blood products and improving outcomes in surgical patients.
Trial registration number
Keywords: Bleeding disorders & coagulopathies, Cardiac surgery, Randomized Controlled Trial, Blood bank & transfusion medicine
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This is a prospectively randomised controlled trial powered to determine the relative haemostatic effectiveness of four-factor prothrombin complex concentrate and frozen plasma in bleeding cardiac surgery patients.
The study employs a pragmatic design that includes a wide spectrum of cardiac surgery patients, enhancing generalisability.
Clinicians are not blinded to group allocation, but outcomes assessors are blinded.
Outcome measures include several clinically relevant outcomes and a primary outcome that has been accepted by regulatory agencies.
There is a strong focus on safety outcomes, with comprehensive safety data collection up to postoperative day 30.
Introduction
For patients undergoing cardiac surgery requiring cardiopulmonary bypass (CPB), coagulopathic bleeding is a serious complication that can lead to blood product transfusions and poor outcomes.1 2 The aetiology of coagulopathy in these patients is multifactorial and can be related to anticoagulation use, haemorrhage, haemodilution and consumptive losses following tissue injury and during CPB.3 An important component of post-CPB coagulopathy is reduced thrombin generation caused by deficiency of enzymatic coagulation factors (eg, vitamin K-dependent factors II, VII, IX and X).4,7
To replenish coagulation factors and enhance thrombin generation in bleeding patients, frozen plasma (FP) and non-activated prothrombin complex concentrate (PCC) are used during or after surgery.8 FP is the mainstay of therapy for bleeding cardiac surgery patients requiring coagulation factor replacement in many countries; in the USA, approximately 15% of all cardiac surgery patients and one-third of all bleeding patients receive FP.9,11 FP contains the full array of human procoagulant and anticoagulant factors but not in concentrated form, meaning that achieving clinically significant increments can require large volumes.12 Accordingly, FP is associated with adverse reactions such as transfusion-associated circulatory overload and transfusion-related acute lung injury.13
PCC offers a potential alternative to FP for treating bleeding cardiac surgery patients requiring coagulation factor replacement. Non-activated four-factor PCCs (4F-PCCs) contain standardised levels of coagulation factors II (prothrombin), VII, IX and X, as well as the inhibitor proteins C and S and small amounts of heparin.14 4F-PCCs are purified from human pooled plasma and treated to minimise the risk of pathogen transmission and transfusion reactions. They are also administered in a smaller volume than FP, reducing the risk of fluid overload and haemodilution.14 15 However, the efficacy–safety balance of 4F-PCCs relative to FP in cardiac surgery is unconfirmed, with limited studies carried out in this setting. Recent systematic reviews and meta-analyses have suggested that PCC seems to be at least as effective as FP in patients with significant bleeding after cardiac surgery, without additional risk of thromboembolic events (TEEs) or other adverse events.16,18 However, only three relevant randomised studies were identified, two of which were pilot studies designed primarily to assess the feasibility of conducting larger trials,9 19 and the other being a single-centre study in 100 patients.20 Therefore, high-quality evidence from large, multicentre, randomised studies is required.
Following the completion of a phase 2 pilot study (FARES),9 we embarked on a phase 3 study to delineate the risk–benefit profile of 4F-PCC relative to FP and to determine whether 4F-PCC is a suitable substitute for FP to mitigate bleeding in cardiac surgery. Here, we describe the study protocol for this study, LEX-211 (also known as FARES-II), which is a randomised comparison of the efficacy and safety of 4F-PCC with FP as active control for coagulation factor replacement for the management of bleeding in adults undergoing cardiac surgery with CPB.
Methods and analysis
Study design and setting
LEX-211 is a prospective, multicentre, active-control, randomised, phase 3 study comparing 4F-PCC with FP in bleeding adult cardiac surgical patients. The study does not include a placebo arm because delaying coagulation factor replacement in this setting may expose patients to the negative consequences of excessive blood loss.
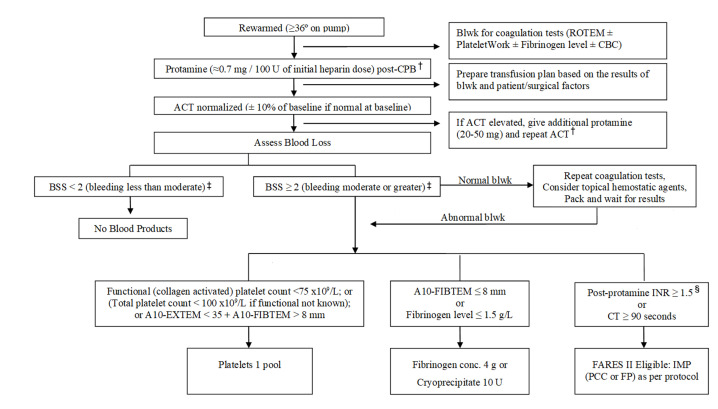
Due to the nature of the intervention, the clinical team and some research personnel are not blinded to treatment allocation, but patients and outcome assessors are blinded. All participating hospitals are required to follow an established transfusion management protocol for the administration of non-interventional blood components and haemostatic agents (figure 1).21 Recognising that the intervention takes place during a very dynamic period (ie, post-CPB bleeding during surgery), however, the study does allow clinicians to use best judgement within the context of the transfusion protocol.
Figure 1. Cardiac surgery blood transfusion algorithm (updated from TACS)*.21 *To determine the need for RBC transfusion, consider patient status and haemoglobin. Transfuse red cells if Hb<70 g/L during CPB; <80 g/L post-CPB and <90 g/L in bleeding or unstable patients. †In general, the initial protamine dose should not exceed 400 mg, irrespective of the amount of heparin given. If an additional protamine dose does not shorten ACT, consider low fibrinogen levels or deficiency of enzymatic coagulation factors as a reason for the prolonged ACT and treat according to the algorithm. ‡BSS<2 (less than moderate)=no therapy; BSS 2–3 (moderate-severe)=institute stepwise treatment; assess bleeding after each product; BSS 4 (life-threatening)=administer therapy as necessary without waiting for laboratory results and combine therapies as appropriate. §POC INR should be performed >10–15 min after protamine or results may be inaccurate. ACT, activated clotting time; Blwk, bloodwork; BSS, bleeding severity scale; CBC, complete blood count; CPB, cardiopulmonary bypass; CT, clotting time; Hb, haemoglobin; IMP, investigational medicinal product; INR, international normalised ratio; POC, point of care.
The study is being conducted at 12 academic hospitals in Canada and the USA. In the USA, prospective, written, informed consent is obtained from patients at a screening visit (performed ≤28 days before surgery) before they are included in the study. In Canada, a delayed consent process is used whereby informed consent is obtained when appropriate after surgery, in accordance with the Tri-Council Policy Statement (TCPS2) on Ethical Conduct for Research Involving Humans.22
Study population
To ensure that the results of the study are clinically relevant and generalisable, the study includes adult patients undergoing any type of cardiac surgery (except highly specialised procedures such as heart transplantation) who require coagulation factor replacement due to haemorrhage in the operating room (OR), post-CPB.
Inclusion criteria
Inclusion criteria are adult patients undergoing any index (elective or non-elective) cardiac surgery employing CPB for whom coagulation factor replacement with 4F-PCC or FP is ordered in the OR for the management of bleeding box 1. This can be either active bleeding or anticipated bleeding in patients deemed to be at high risk for major bleeding post-CPB.
Box 1. Eligibility criteria.
Inclusion criteria
Adult (≥18 years old) patients undergoing any index cardiac surgery employing CPB.
Coagulation factor replacement with PCC or FP was ordered in the operating room for:
Management of bleeding.
Anticipated bleeding in a patient who has been on pump for >2 hours or undergone a complex procedure (eg, aortocoronary bypass plus aortic valve replacement).
Coagulation factor deficiency is either known to exist (eg, as indicated by elevated EXTEM clotting time (CT) or INR) or suspected based on the clinical situation.
Exclusion criteria
Undergoing heart transplantation, insertion or removal of ventricular assist devices (not including intra-aortic balloon pump (IABP)) or repair of thoracoabdominal aneurysm.
Critical state immediately before surgery with a high probability of death within 24 hours of surgery (eg, acute aortic dissection, cardiac arrest 24 hours before surgery).
Severe right heart failure (clinical diagnosis±echocardiography).
Known contraindications to heparin.
PCC required for reversal of warfarin or direct oral anticoagulant (dabigatran, rivaroxaban, apixaban or edoxaban) within 3 days prior to or during surgery.
Known TEE within 3 months prior to surgery.
History of severe allergic reactions to PCC or FP.
Individuals who have IgA deficiency with known antibodies against IgA.
Refusal of allogeneic blood products.
Known pregnancy.
Currently enrolled in any other interventional clinical trials.
CPB, cardiopulmonary bypass; FP, frozen plasma; IgA, immunoglobulin A;INR, international normalised ratio; PCC, prothrombin complex concentrate; TEE, thromboembolic event.
Included patients will receive the intervention post-CPB, but only if they are bleeding severely enough to merit treatment (as determined by the clinical team and confirmed with a validated bleeding severity scale23 and have either an elevated international normalised ratio (INR) (>1.5 as measured at the point of care) postheparin reversal with protamine or their bleeding is severe enough to require immediate therapy, precluding waiting for the INR measurement.
Exclusion criteria
Patients undergoing highly specialised or very high-risk surgeries are excluded. These include heart transplantation, insertion or removal of ventricular assist devices (not including intra-aortic balloon pump), or repair of thoracoabdominal aneurysm. Patients are also excluded if the clinical team deems them to be in a critical state immediately before surgery or at high risk of death within 24 hours of surgery. Examples include patients with acute aortic dissection or cardiac arrest within 24 hours before surgery. Severe right heart failure (clinical diagnosis with or without echocardiography) will also result in exclusion. Additional exclusion criteria are listed in box 1.
Interventions
As described above, patients are included in the study either after consent is obtained before surgery (only in the US sites) or if clinicians order coagulation factor replacement with 4F-PCC or FP in the OR (in the Canadian sites). However, the intervention can be administered only if the patient is experiencing bleeding that is severe enough to necessitate treatment with coagulation factor replenishment. The treatment criteria are the following: adequate reversal of heparin with protamine; at least a grade 2 (moderate) bleed according to the validated bleeding severity scale23 and point-of-care INR (as measured by the Hemochron Signature Elite conducted >10 min after protamine administration) 1.5 or higher. The need for INR measurement is waived if the bleeding is severe enough to require immediate therapy post-CPB.
Grading the severity of bleeding according to the bleeding severity scale23 fulfils the US Food and Drug Administration (FDA) requirement for a validated, clinician-reported scale to standardise bleeding in clinical studies of intraoperative bleeding. The scale was developed to assess the performance of haemostatic agents in clinical studies to generate clinically relevant labelling claims and identify appropriate haemostatic agents for clinical use.
Intervention
Patients randomised to the intervention group who meet the treatment criteria will receive 4F-PCC (Octaplex/Balfaxar, Octapharma), administered intravenously. The 4F-PCC is supplied as a powder for solution for injection together with a solvent (sterile water for injection), which will be used for the reconstitution per the manufacturer’s instructions. The dose for 4F-PCC will be 1500 international units (IU) for patients whose body weight is ≤60 kg and 2000 IU for patients whose body weight is >60 kg.
Active control
Patients assigned to the comparator arm who meet the treatment criteria will receive an intravenous infusion of FP as active control. The dose of FP will be three units (U) (approximately 750 mL) for patients whose body weight is ≤60 kg and 4 U (approximately 1000 mL) for patients whose body weight is >60 kg.
Repeat dosing
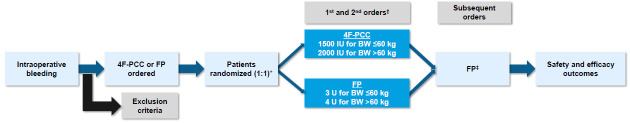
If a second order for coagulation factors is received during the treatment period of 24 hours after initiation of the first dose, the blood bank will release a second dose of the intervention to which the patient was originally allocated (figure 2). In both groups, if further doses of coagulation factors are required, non-interventional FP will be administered in 1–4 U increments at the discretion of the ordering physician; these additional FP units will not be counted as part of the investigational medicinal product (IMP). Thus, the maximum allowable cumulative dose of 4F-PCC will be 3000 IU if body weight is ≤60 kg and 4000 IU if >60 kg.
Figure 2. Study design. *OR personnel will remain blinded to treatment until treatment decision; patients will be blinded to treatment allocation. †A second dose of 4F-PCC or FP (as per original randomised allocation) can be given within the 24-hour treatment period (ie, within 24 hours after the first dose of IMP) if the patient continues to have at least a grade 2 bleed and an INR≥1.5 after the first dose; for subsequent doses, the patient will receive FP. ‡FP in 1–4 U increments at the discretion of the ordering physician. 4F-PCC, four-factor prothrombin complex concentrate; BW, body weight; FP, frozen plasma; IMP, investigational medicinal product; IU, international units; OR, operating room; U, units.
Dosing determination
The doses of PCC and FP selected for the study are the same as those used in the pilot FARES study, which showed high effectiveness rates without any indication of increasing TEEs.9 There is currently an absence of consensus regarding PCC dosing in acquired coagulation factor deficiency; however, the study dosing of PCC represents current dosing as used for bleeding indications in other (including cardiac) settings.2124,26 Moreover, the maximum dose is equal to or lower than doses of PCC that have demonstrated a good safety and tolerability profile in other clinical settings, for example, factor Xa inhibitor reversal in major bleeding27,29 and trauma.30 Neither PCC nor FP will be administered to patients unless they are deemed to be clinically indicated by the ordering physician based on objective clinical criteria that will ensure adherence to best blood management practices.
Outcomes and study duration
Primary outcome
A dichotomous classification will be used to assess the primary endpoint of comparison of haemostatic treatment response to 4F-PCC versus FP in bleeding cardiac surgical patients (table 1). Treatment will be considered ‘effective’ if no additional haemostatic intervention is required in the time window from 60 min to 24 hours after initiation of the first IMP dose. ‘Haemostatic intervention’ comprises the administration of any systemic haemostatic agents (including platelets, cryoprecipitate, fibrinogen concentrate, activated factor VII, other coagulation factor products or a second dose of IMP) or any other haemostatic interventions (including surgical reopening for bleeding). Because of the complexity and dynamic nature of cardiac surgery, and the multifactorial nature of haemorrhage during cardiac surgery, it is inherently not feasible to assess the specific haemostatic response to individual therapeutic agents. Patients categorised as having an ‘ineffective’ haemostatic treatment response to IMP, due to requiring administration of any haemostatic intervention (including a second dose of IMP) in the time window from 60 min to 24 hours after initiation of the first IMP dose, will be considered as treatment failures. The 60 min time period will allow for the administration of the IMP and establishment of treatment effect and for correction of any other identified coagulation defects (eg, thrombocytopaenia, platelet dysfunction, hypofibrinogenaemia and hyperfibrinolysis).3
Table 1. Objective criteria used for determination of the primary endpoint of haemostatic treatment response to investigational medicinal product.
| Haemostatic response | Haemostatic intervention |
| Effective | No additional haemostatic interventions* administered between 60 min and 24 hours after initiation of infusion† |
| Ineffective | Additional haemostatic interventions* administered between 60 min and 24 hours after initiation of infusion† |
Administration of any systemic haemostatic agents (including platelets, cryoprecipitate, fibrinogen concentrate, activated recombinant factor VII, other coagulation factor products or a second dose of IMP) or any haemostatic interventions (including surgical re-opening for bleeding).
The 60-minute min period allows for the administration of the IMP and establishment of treatment effect.
IMP, investigational medicinal product; .
The primary endpoint was selected for its clinical relevance, was discussed with regulatory authorities and was demonstrated by the FARES pilot study to be feasible.9
Secondary outcomes
The secondary endpoints and their assessment timings are summarised in table 2. The following secondary endpoints are included to provide supportive evidence of haemostatic efficacy: global haemostatic response, as measured by a composite of the need for post-therapy haemostatic interventions (per the primary endpoint) and drop in haemoglobin level (table 3); chest tube drainage; the incidence of severe to massive bleeding; the incidence and amount of allogeneic blood products transfused; the incidence of administration of other coagulation factor products; the incidence of additional bleeding-related clinical endpoints (ie, intracerebral haemorrhage, gastrointestinal haemorrhage and surgical re-exploration); changes in INR and other coagulation parameters from before to after IMP administration; and the time from initiation of the first IMP dose to the patient’s arrival into the intensive care unit (ICU). The assessment of global haemostatic response will be based on the haemostatic efficacy scale used successfully in a phase 3b randomised clinical trial by Sarode et al31 (in discussion with the US FDA), modified to reflect the special considerations of haemorrhage during cardiac surgery. Severe to massive bleeding will be assessed using a modification of the universal definition of perioperative bleeding in cardiac surgery.32 Safety endpoints are the incidence of serious treatment-emergent adverse events, the duration of mechanical ventilation, ICU stay and hospitalisation, the incidence of death, and the number of days alive and out of hospital up to postoperative day 30.
Table 2. Secondary efficacy and safety endpoints.
| Outcome | Timing of assessment |
| Efficacy parameters | |
| Global haemostatic response, based on requirement for additional haemostatic intervention (as per the primary endpoint) and haemoglobin level decrease | 60 min to 24 hours after initiation of the first IMP dose |
| Total amount of chest tube drainage | 12 and 24 hours after chest closure |
| Incidence of severe to massive bleeding, using a modification of the UDPB in cardiac surgery32 and its individual components | First 24 hours after surgery commencement, after the end of CPB and after IMP initiation |
| Mean number of total allogeneic blood products administered, including red cells, platelets and all (interventional and non-interventional) FP | First 24 hours after the end of CPB |
| Mean number of total non-interventional allogeneic blood products administered, including red cells, platelets and non-interventional FP | First 24 hours after the end of CPB |
| Mean number of total non-interventional allogeneic blood products administered, including red cells, platelets, cryoprecipitate and non-interventional FP | First 24 hours and 7 days after IMP initiation |
| Mean number and incidence of transfusion of individual allogeneic blood products (including red cells, platelets, cryoprecipitate and non-interventional FP), and incidence of administration of non-interventional coagulation factor products (including fibrinogen concentrate and rFVIIa) | First 24 hours and 7 days after surgery commencement, after the end of CPB and after IMP initiation |
| Incidences of ICH, GI haemorrhage and surgical re-exploration | First 24 hours after surgery commencement, after the end of CPB and after IMP initiation |
| Change in INR* | Within 30 min before to within 60 min after IMP initiation |
| Changes in coagulation parameters, including PT, aPTT, fibrinogen activity, ROTEM EXTEM CT and MCF, ROTEM FIBTEM MCF and platelets | Within 75 min before to within 75 min after IMP initiation |
| Time elapsed from initiation of first IMP dose to the patient’s arrival into the ICU | To be measured |
| Safety parameters | |
| Incidence of serious treatment-emergent adverse events, individually and as a composite where appropriate (eg, TEEs, MACE) | All from the beginning of surgery up to postoperative day 30 |
| Duration of mechanical ventilation, ICU stay and hospitalisation | |
| Incidence of death | |
| Number of days alive and out of hospital | |
INR reduction will be considered successful if the magnitude of the reduction is >1.0 or the post-treatment level falls to below 1.5.
aPTT, activated partial thromboplastin time; CPB, cardiopulmonary bypass; CT, clotting time; GI, gastrointestinal; ICH, intracerebral haemorrhage; ICU, intensive care unit; IMP, investigational medicinal product; INR, international normalised ratio; MACE, major adverse cardiac events; MCF, maximum clot firmness; PT, prothrombin time; rFVIIa, activated recombinant factor VII; ROTEM, rotational thromboelastometry; TEEs, thromboembolic events; UDPB, universal definition of perioperative bleeding
Table 3. Objective criteria used for determination of the secondary endpoint of global haemostatic response, adapted from Sarode et al31.
| Global haemostatic response | Haemostatic intervention | Drop in haemoglobin | |
| Excellent (positive) | No additional haemostatic interventions* administered between 60 min and 24 hours after initiation of infusion† | AND | <15% decrease in haemoglobin between 60 min and 24 hours after initiation of infusion†‡ |
| Good (positive) | No additional haemostatic interventions* administered between 60 min and 24 hours after initiation of infusion† | AND | 15% to <30% decrease in haemoglobin between 60 min and 24 hours after initiation of infusion†‡ |
| Poor (negative) | Additional haemostatic interventions* administered between 60 min and 24 hours after initiation of infusion† | OR | ≥30% decrease in haemoglobin between 60 min and 24 hours after initiation of infusion†‡ |
Administration of any systemic haemostatic agents (including platelets, cryoprecipitate, fibrinogen concentrate, activated recombinant factor VII, other coagulation factor products or a second dose of IMP) or any haemostatic interventions (including surgical re-opening for bleeding).
The 60-minute min period allows for the administration of the IMP and establishment of treatment effect.
Each unit of RBC transfused during this time period will be counted as a drop of 1.0 g/dL g/dL in haemoglobin.
IMPinvestigational medicinal productRBCred blood cell
Study duration
The duration of the treatment period is 24 hours from IMP initiation or until the maximum dose of IMP has been administered, whichever occurs first. The duration of the study for an individual patient is 30 days from the time of randomisation (ie, day of surgery). The study visits are described In table 4. The study will be considered completed when at least 410 patients have been randomised, treated and consented and have finalised data through day 30.
Table 4. Flow chart of study procedures and information collected at each study visit*.
| Assessments | Visit 1POD 0Prerandomisation visit(blood bank) | Visit 2POD 0–1First visit after IMP initiation(0–24 hours after IMP initiation)† | Visit 3POD 2–7 after IMP initiation (or at discharge if earlier) | Visit 4POD 30 after IMP initiation (in person if in hospital or by phone) |
| Blood bank receives PCC or FP order‡ | x | |||
| Inclusion and exclusion criteria§ | x | |||
| Body weight | x | |||
| Randomisation | x | |||
| IMP (PCC or FP) administration¶ | x | |||
| Patient (or legally authorised representative) debriefing and consent** | x | (x) | (x) | |
| Baseline data | ||||
| Demographics | x | |||
| Medical history | x | |||
| Preoperative medications | x | |||
| Laboratory assessments | X | |||
| Surgical data | ||||
| Bleeding score | x | |||
| Intraoperative medications | x | |||
| CPB time | x | |||
| Cross-clamp time | x | |||
| Circulatory arrest | x | |||
| Fluid intake and output monitoring | x | |||
| Inotropes and vasopressors | x | |||
| Start and end time of IMP administration | X | |||
| OR length of stay | x | |||
| Laboratory assessments | ||||
| Clinical chemistry†† | x | x | ||
| Haematology (CBC)†† | x‡‡ | x§§ | ||
| Coagulation measures††¶¶ | x*** | x | ||
| Safety laboratory analyses†† | x | x | ||
| INR | x††† | |||
| Transfusions and haemostatic therapies and timings | ||||
| Second dose of PCC (IMP), if needed‡‡‡ | x | |||
| Second dose of FP (IMP), if needed‡‡‡ | x | |||
| FP (non-interventional) | x | x | ||
| RBCs | x | x | ||
| Pooled and apheresis platelets | x | x | ||
| Cryoprecipitate | x | x | ||
| Fibrinogen concentrate | X | X | ||
| Activated recombinant factor VII | x | x | ||
| Other haemostatic products | x | x | ||
| Blood loss determination | ||||
| Total chest tube drainage at 1, 6, 12 and 24 hours after chest closure | X | X | ||
| Bleeding-related clinical endpoints | ||||
| Occurrence of intracerebral haemorrhage§§§ | x | X | ||
| Occurrence of gastrointestinal haemorrhage§§§ | x | X | ||
| Occurrence of surgical re-exploration§§§ | x | X | ||
| Extubation time | x | (x) | (x) | |
| ICU length of stay | (x) | (x) | ||
| Hospital length of stay | (x) | (x) | ||
| Hospital readmissions | X | |||
| AEs and SAEs | x | x | x | |
| Concomitant medications | x | x | x | |
| Patient survival | X |
() If needed.
All data are collected by trained research personnel. All outcome data collectors are blinded research personnel. In-hospital data are obtained from patients or their medical records and recorded into electronic case report forms (eCRFs) with validity checks (see online supplemental appendixAppendix). Post--discharge data are collected from the patients by blinded research personnel. Independent monitors review collected data against patients’ records to ensure completeness and accuracy. Missing data or loss to follow-up should be minimal as the vast majority of data is are in-hospital. The investigator will ensure that the patient’s confidentiality is preserved. On CRFs or any other documents submitted to the sponsor, the patients are not identified by their names, but by a unique patient identifier. Documents not intended for submission to the sponsor, that is, the confidential patient identification code list, original consent forms, and source records, are maintained by the investigator in strict confidence.
For any specified activity that cannot be completed during the first visit after IMP initiation, additional visits are made on postoperative day 1 until all study data are obtained.
After the start of surgery.
In the USA, a screening visit is performed ≤28 days before surgery to apply the study inclusion criteria requiring that the patient is aged ≥18 years, is to undergo index cardiac surgery employing CPB and provides written informed consent, as well as to apply all exclusion criteria. If screening is performed before the day of surgery, the exclusion criterion of severe right heart failure (clinical diagnosis±echocardiography) is rechecked on the day of surgery.
IMP is administered during surgery based on objective clinical criteria of bleeding status and point-of-care INR, as assessed by the surgical staff.
At study centres in Canada, due to the emergency nature of the condition being studied, informed consent is obtained from the patient or legally authorised representative as soon as possible after surgery. If neither the patient nor the legally authorised representative is reachable for consent during the follow-up period, a family member who is not a legally authorised representative is provided with an opportunity to object to the patient’s participation in the study. In the USA, prospective, voluntarily given, written (signed and dated), informed consent must be obtained from the patient at a screening visit performed ≤28 days before surgery. Model informed consent forms are provided in Appendix.
As per standard practice.
Measure haemoglobin within 30 min before and at 60 min after IMP initiation.
Measure haemoglobin at 24 hours after IMP initiation and document results.
For example, PT, aPTT, INR, plasma fibrinogen level, ROTEM EXTEM CT and MCF, FIBTEM MCF, platelet count and function (PlateletWorks; Helena Laboratories, Texas, USA). Either ROTEM or TEG can be used.
Measure INR within 30 min before and at 60 min after IMP initiation and document results.
If the patient continues to have at least moderate bleeding and a suspected coagulation deficiency (eg, INR ≥1.5) after completion of the first dose.
If the patient continues to have at least moderate bleeding and a suspected coagulation deficiency (eg, INR ≥1.5) after completion of the first dose.
Data collected during visit 2 (0–24 hours after IMP initiation).
AE, adverse event; aPTT, activated partial thromboplastin time; CBC, complete blood count; CPB, cardiopulmonary bypass; CT, clotting time; DC, discharge; FP, frozen plasma; ICU, intensive care unit; IMP, investigational medicinal product; INR, international normalised ratio; MCF, maximum clot firmness; OR, operating room; PCC, prothrombin complex concentrate; POD, postoperative day; PT, prothrombin time; RBC, red blood cell; ROTEM, rotational thromboelastometry; SAE, serious adverse event; TEG, thrombelastography
Randomisation and blinding
Eligible patients will be randomly assigned to receive 4F-PCC or FP. Randomisation lists using a permuted-block randomisation scheme (stratified by site) will be prepared by the biostatistician, and sealed randomisation envelopes based on these randomisation lists will then be provided to the blood banks of the participating centres who will be responsible for providing the IMP. Patients will be identified using a sequential numbering system within the centre, and randomisation performed in sequential order of the patient identification numbers.
This is a partially blinded study, with patients and outcome assessors blinded to treatment allocation. Given the physical differences in the products and the emergency nature of the intervention, attending clinicians and research personnel in the OR cannot be blinded to the treatment. Thus, breaking the study blind is not an issue in this study. The biostatistician who prepares the random allocation schedule is not involved in the conduct of the study. To minimise bias, neither the individual performing the randomisation nor any of the healthcare providers will know which treatment will be assigned to a given patient when coagulation factor replacement is ordered. Blood products will be transported to the OR in weighted tamper-proof boxes to maintain the blind until IMP administration criteria are met post-CPB and the clinical decision is made to administer the IMP. The type of IMP administered will be recorded in a manner that will not unblind the outcome assessor.
Sample size determination
In the FARES pilot study, approximately 75% of patients in the PCC group and 65% in the FP group demonstrated haemostatic treatment response from 60 min to 24 hours after initiation of the first IMP dose.9 Using a more conservative estimate of 70% vs 65%, it is estimated that 410 evaluable patients will be required to demonstrate non-inferiority with a one-sided α of 0.025, power of ≥90% and non-inferiority margin of 0.10 when using a Farrington-Manning score test.
It is anticipated that up to 20% of randomised patients may not meet IMP administration criteria (based on objective bleeding severity scale and INR) between randomisation and delivery of IMP to the OR and termination of CPB, and therefore, will not receive the therapy. Thus, it is anticipated that the study will include approximately 500 randomised patients, which, accounting for randomised but untreated patients in both arms, as well as evaluable patients who do not provide informed consent (anticipated to occur in <5% of cases), is expected to provide at least 410 evaluable patients, with a minimum of 205 patients in each group.
Interim analysis
The study design included a preplanned administrative unblinded interim analysis (conducted by an independent statistician) after 200 patients were enrolled to re-estimate the sample size or stop the study for futility (non-binding), without pausing patient recruitment during the conduct of the interim analysis.
The sample size re-estimation was based on evaluation of the conditional power, calculated as described previously,33 making use of the observed response rates at the time of the interim analysis and inverse normal combination test statistic with equal weights given by (Φ−1(1–p1)+Φ−1(1–p2))/√2, where p1 and p2 denote the p values for testing the non-inferiority null hypothesis for the first and the second stage of the trial, respectively. The aspired conditional power used for the new intended sample size was 90%. A 25% drop-out rate was added to the re-estimated sample size of evaluable patients to obtain the number of patients to be enrolled in the second stage. The minimum number of patients for the second stage was specified at 210 evaluable subjects (as per the original sample size estimate, even if the re-estimated sample size was below this number) or 263 patients, including the possible dropouts (+25%). The maximum allowed sample size for the second stage was specified at 1000 patients (dropouts included). If the drop-out-adjusted re-estimated sample size exceeded this threshold, the independent data and safety monitoring committee (IDSMC) would recommend stopping the trial for futility or enrolling the maximum overall number of 1250 patients in the study.
Statistical methods
The non-inferiority of the primary endpoint of haemostatic response will be tested between the treatment groups by means of a Farrington-Manning score test with a non-inferiority margin of 0.10. At the end of the trial (as at the interim analysis), the inverse normal test statistic with equal weights given by (Φ−1(1–p1) Φ−1(1–p2))/√2 will be calculated. If the test statistic exceeds the value of 1.96, non-inferiority is demonstrated. Only in the case that non-inferiority is demonstrated, that is, the null hypothesis is rejected at the one-sided 2.5% level of significance, the superiority of 4F-PCC with regard to the primary endpoint will be investigated.
Descriptive statistics or frequency tables will be presented for all efficacy and safety data in addition to the inferences performed. The summary tables and exploratory inferences will be chosen according to the scaling level of the measurements, for example, frequency tables for categorical responses and sampling statistics for continuous data. Safety endpoints will be analysed analogously to the primary endpoint, presenting point estimates and two-sided 95% CIs in addition to descriptive statistics. A p<0.05 will be considered significant without adjustment for multiplicity. A statistical analysis plan has been prepared that details all analyses to be undertaken; this plan will be finalised before data lock.
The full analysis set will consist of all consented and randomised patients who receive any amount of the intervention, which will serve as the primary analysis set for efficacy and safety. Consented and randomised but untreated patients will not be included in the efficacy or safety analyses but will be followed for 30 days to determine between-group comparability in baseline characteristics and outcomes. A secondary efficacy analysis will be performed for the per-protocol set, which will exclude all patients with major protocol deviations. To ensure that the safety reporting is complete, all haemostatic therapy and serious adverse event data will be collected in cases where consent for remaining in the study cannot be obtained due to logistical issues (eg, the patient died and a legally authorised representative could not be reached) and research ethics board (REB) approval is obtained to collect the information. For patients in Canada who refuse consent, only treatment allocation data will be collected and patients will not be included in any analyses.
Study management
Study oversight is provided by the steering committee and the IDSMC (see online supplemental appendix 1 for members). Study conduct is organised and managed by Ozmosis Research, Toronto, and the Anesthesia Clinical Trials Unit (ACTU) at the University Health Network, Toronto (the coordinating centre).
The IDSMC is composed of recognised experts in the fields of statistics, perioperative medicine and haematology who are not actively recruiting patients. The IDSMC conducts a review of the accumulating safety, endpoint and other study data (recruitment, retention and compliance, data quality and timeliness, risk vs benefit and summary statistics of outcomes) every time 100 patients complete the study. The IDSMC provides recommendations about continuing, modifying and/or stopping the study based on considerations of treatment efficacy, patient safety and trial futility, as appropriate. A written study-specific charter defines in detail the composition, responsibilities and procedures of the IDSMC.
Patient and public involvement
Patients and the public were not involved in the design of the study.
Ethics and dissemination
The study is being conducted in accordance with the ethical principles laid down in the Declaration of Helsinki and in compliance with the protocol as approved by Health Canada (V.8.0, 17 April 2023), the US FDA (V.9.0, 25 August 2023) and the institutional review boards of all participating sites. The study also complies with Good Clinical Practice guidelines and all applicable regulatory requirements governing the participating study sites.
The study, protocol and all other study documents have been approved by the REB of the coordinating centre (University Health Network Research Ethics Board (UHN REB), Toronto, Ontario, Canada; CTO no. 3996; initial approval date: 3 August 2022), as well as the local REB of all participating sites (see online supplemental appendix 1 for specific REB names and approval numbers). Model informed consent forms are provided in online supplemental appendix 1.
In Canada, the study meets the criteria stated in Article 3.7A on Alterations to Consent Requirements in the TCPS2 on Ethical Conduct for Research Involving Humans for identifying situations in which exceptions may be sought for the requirement to seek prior consent.22 Thus, at participating centres in Canada, all patients who meet the inclusion criteria are randomised in the OR, and delayed consent is sought from patients (or from their legally authorised representative where appropriate) at the earliest possible opportunity after surgery.
In the USA, an exception from informed consent was not granted by the FDA, and so prospective, voluntarily given, written (signed and dated), informed consent is obtained from the patient at the screening visit performed ≤28 days before surgery. Patients for whom this is not possible are excluded. At participating centres in the USA, the study is being conducted under the FDA’s requirements for investigational new drug applications, protection of human subjects and institutional review boards.34,36
Study results will be disseminated via publications in peer-reviewed journals and conference presentations. It is currently not planned to share individual participant data.
Trial status and anticipated impact
The study was initiated in 2022 in Canada and in 2023 in the USA and is currently in progress. An interim analysis was conducted after 202 patients were enrolled, with the IDSMC recommending that the study continue with no protocol modifications and a maintained total sample size of 410 evaluable patients. We expect the trial to be completed in late 2024 and the final report to be presented and published in 2025.
The study is anticipated to have an important impact on clinical practice, potentially altering the long-established practice of administering FP for coagulation factor replacement in bleeding surgical patients. Depending on the findings of the study, it could lead to the adoption of PCC over FP as first-line therapy in this setting, or illustrate that the current prevailing practice of using FP as first-line therapy is more appropriate, or show that both interventions can be used interchangeably. In any case, the study will advance our understanding of coagulation management in bleeding surgical patients and has the potential to reduce the need for allogeneic blood product transfusions and improve outcomes in surgical patients.
supplementary material
Acknowledgements
Editorial assistance was provided by Portland Medical Communications, funded by Octapharma.
Footnotes
Funding: KK and JB are in part supported by merit awards from the Department of Anesthesiology and Pain Medicine, University of Toronto. KK and JB are in part supported by merit awards from the Department of Anesthesiology and Pain Medicine, University of Toronto.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-091381).
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Patient consent for publication: Not applicable.
Collaborators: FARES II Study Group: Keyvan Karkouti, Jeannie Callum, Miki Peer, Lusine Abrahamyan, Blaine Achen, Justyna Bartoszko, Sukhpal Brar, Matthew Coley, Etienne Couture, Philippe Demers, Dana Devine, Alana Flexman, Hilary Grocott, Christopher Harle, Yoan Lamarche, Michael Law, Camila Machado de Souza, David Mazer, Katerina Pavenski, Darrin Payne, Mark Peterson, Vivek Rao, Antoine Rochon, Maria Rosal Martins, Fraser Rubens, Tarit Saha, Damon Scales, Andrew Shih, Terri Sun, Summer Syed, Rob Tanzola, Alan Tinmouth, George Tomlinson, Daniel Wong, Michelle Wong, Sophia Wong, Mathew Yan, Raffael Zamper, Michelle Zeller, Stuart McCluskey, Deep Grewal, Jo Carrol, Nishanthi Liyanage, Humara Poonawala, Simryn Selby, Reezanoor Kabir, Shelley Oliver, Alioska Escorcia, Susana Medic, Michelle Mozel, Ramanjot Kaur, Rebecca Randall, Kelly Bizovie, Sarah Buchko, Raven Arly Gonzales, Korey Sutherland, Jenna Van Roekel, Alveena Babul, Ken Tanaka, Kofi Vandyck, Amir Butt, Penny Johnson, Hisako Okada, Deborah DuMerton, Robert Tanzola, Reegan Tod, Bethany Smethurst, Shagun Jain, Aiden Scholey, Angela Sirosky-Yanyk, Andrew Stevens, Wafaa Haider, Ester Cisneros-Aguilera, Alexandre Bergeron, Genevieve Belanger, Maria Rosal Martins, Darren Mullane, Bevan Hughes, Sakara Hutspardol, Shirley Lim, Jian Mi, Debbie Kalar, Matthew Eang, Kamrouz Ghadimi, Erick Lorenzana-Saldivar, Edward P. Chen, Yulia Lin, Fuad Moussa, Akash Gupta, Angela Jerath, Philip Lau, Pablo Perez D’Empaire, Chantal Armali, Lilia Kaustov, Amie Malkin, Connie Colavecchia, Harley Meirovich, Jane Yang, Annie Bergeron, Francois Laforge, Kim Paradis, Nathalie Gagne, Marie Soleil Saillant, Marie-Claude Vézina, Olivier Royer, Eric Dumont, and Marie-Ève Charest, Raffael Zamper, Mackenzie Quantz, Robert Mayer, Jeff Kinney, Lee-Anne Fochesato, Yuxin Bai, Iqbal Jaffar, Nour Alhomsi, Janine Guevarra, Erin Jamula, Wan Chien (Betty) Hsu, Abbey Drew, Diem Tran, Hakan Buyukdere, Drashtee Patel, Melanie Tokessy, Elizabeth Watt, Hadia Arabi Katbi, Kim Luciano, Amy Moorehead, Gianluigi Bisleri, Kamola Kasimova.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Keyvan Karkouti, Email: keyvan.karkouti@uhn.ca.
Jeannie Callum, Email: jeannie.callum@kingstonhsc.ca.
Justyna Bartoszko, Email: justyna.bartoszko@uhn.ca.
Cristina Solomon, Email: cristina.solomon@octapharma.com.
Sigurd Knaub, Email: sigurd.knaub@octapharma.com.
Jerrold H Levy, Email: jerrold.levy@duke.edu.
Kenichi A Tanaka, Email: kenichi-tanaka@ouhsc.edu.
References
- 1.Karkouti K, Callum J, Rao V, et al. Protocol for a phase III, non-inferiority, randomised comparison of a new fibrinogen concentrate versus cryoprecipitate for treating acquired hypofibrinogenaemia in bleeding cardiac surgical patients: the FIBRES trial. BMJ Open. 2018;8:e020741. doi: 10.1136/bmjopen-2017-020741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karkouti K, Wijeysundera DN, Yau TM, et al. The independent association of massive blood loss with mortality in cardiac surgery. Transfusion. 2004;44:1453–62. doi: 10.1111/j.1537-2995.2004.04144.x. [DOI] [PubMed] [Google Scholar]
- 3.Bartoszko J, Karkouti K. Managing the coagulopathy associated with cardiopulmonary bypass. J Thromb Haemost. 2021;19:617–32. doi: 10.1111/jth.15195. [DOI] [PubMed] [Google Scholar]
- 4.Brummel KE, Paradis SG, Butenas S, et al. Thrombin functions during tissue factor-induced blood coagulation. Blood. 2002;100:148–52. doi: 10.1182/blood.v100.1.148. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald J, McMonnies R, Sharkey A, et al. Thrombin generation and bleeding in cardiac surgery: a clinical narrative review. Can J Anaesth. 2020;67:746–53. doi: 10.1007/s12630-020-01609-4. [DOI] [PubMed] [Google Scholar]
- 6.Kremers RMW, Bosch YPJ, Bloemen S, et al. A reduction of prothrombin conversion by cardiac surgery with cardiopulmonary bypass shifts the haemostatic balance towards bleeding. Thromb Haemost. 2016;116:442–51. doi: 10.1160/TH16-02-0094. [DOI] [PubMed] [Google Scholar]
- 7.Mann KG, Brummel K, Butenas S. What is all that thrombin for? J Thromb Haemost. 2003;1:1504–14. doi: 10.1046/j.1538-7836.2003.00298.x. [DOI] [PubMed] [Google Scholar]
- 8.Ferraris VA, Brown JR, Despotis GJ, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944–82. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 9.Karkouti K, Bartoszko J, Grewal D, et al. Comparison of 4-Factor Prothrombin Complex Concentrate With Frozen Plasma for Management of Hemorrhage During and After Cardiac Surgery: A Randomized Pilot Trial. JAMA Netw Open. 2021;4:e213936. doi: 10.1001/jamanetworkopen.2021.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanworth SJ, Grant-Casey J, Lowe D, et al. The use of fresh-frozen plasma in England: high levels of inappropriate use in adults and children. Transfusion. 2011;51:62–70. doi: 10.1111/j.1537-2995.2010.02798.x. [DOI] [PubMed] [Google Scholar]
- 11.Triulzi D, Gottschall J, Murphy E, et al. A multicenter study of plasma use in the United States. Transfusion. 2015;55:1313–9. doi: 10.1111/trf.12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins PW, Solomon C, Sutor K, et al. Theoretical modelling of fibrinogen supplementation with therapeutic plasma, cryoprecipitate, or fibrinogen concentrate. Br J Anaesth. 2014;113:585–95. doi: 10.1093/bja/aeu086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012;52 Suppl 1:65S–79S. doi: 10.1111/j.1537-2995.2012.03663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghadimi K, Levy JH, Welsby IJ. Prothrombin Complex Concentrates for Bleeding in the Perioperative Setting. Anesth Analg. 2016;122:1287–300. doi: 10.1213/ANE.0000000000001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy JH, Douketis J, Steiner T, et al. Prothrombin Complex Concentrates for Perioperative Vitamin K Antagonist and Non-vitamin K Anticoagulant Reversal. Anesthesiology. 2018;129:1171–84. doi: 10.1097/ALN.0000000000002399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J-P, Li Y, Li B, et al. Hemostasis Using Prothrombin Complex Concentrate in Patients Undergoing Cardiac Surgery: Systematic Review with Meta-Analysis. Braz J Cardiovasc Surg. 2024;39:e20230076. doi: 10.21470/1678-9741-2023-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roman M, Biancari F, Ahmed AB, et al. Prothrombin Complex Concentrate in Cardiac Surgery: A Systematic Review and Meta-Analysis. Ann Thorac Surg. 2019;107:1275–83. doi: 10.1016/j.athoracsur.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Viana P, Relvas JH, Persson M, et al. Prothrombin Complex Concentrate versus Fresh Frozen Plasma in Adult Patients Undergoing Cardiac Surgery: A Systematic Review and Meta-Analysis. J Chest Surg. 2024;57:25–35. doi: 10.5090/jcs.23.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green L, Roberts N, Cooper J, et al. Prothrombin complex concentrate vs. fresh frozen plasma in adult patients undergoing heart surgery - a pilot randomised controlled trial (PROPHESY trial) Anaesthesia. 2021;76:892–901. doi: 10.1111/anae.15327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith MM, Schroeder DR, Nelson JA, et al. Prothrombin Complex Concentrate vs Plasma for Post-Cardiopulmonary Bypass Coagulopathy and Bleeding: A Randomized Clinical Trial. JAMA Surg. 2022;157:757–64. doi: 10.1001/jamasurg.2022.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karkouti K, Callum J, Wijeysundera DN, et al. Point-of-Care Hemostatic Testing in Cardiac Surgery: A Stepped-Wedge Clustered Randomized Controlled Trial. Circulation. 2016;134:1152–62. doi: 10.1161/CIRCULATIONAHA.116.023956. [DOI] [PubMed] [Google Scholar]
- 22.Government of Canada.Panel on Research Ethics Tri-council policy statement: ethical conduct for research involving humans – TCPS 2 2022. 2022. [1-Jul-2024]. https://ethics.gc.ca/eng/policy-politique_tcps2-eptc2_2022.html Available. Accessed.
- 23.Lewis KM, Li Q, Jones DS, et al. Development and validation of an intraoperative bleeding severity scale for use in clinical studies of hemostatic agents. Surgery. 2017;161:771–81. doi: 10.1016/j.surg.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Chowdary P, Tang A, Watson D, et al. Retrospective Review of a Prothrombin Complex Concentrate (Beriplex P/N) for the Management of Perioperative Bleeding Unrelated to Oral Anticoagulation. Clin Appl Thromb Hemost. 2018;24:1159–69. doi: 10.1177/1076029617753537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitzgerald J, Lenihan M, Callum J, et al. Use of prothrombin complex concentrate for management of coagulopathy after cardiac surgery: a propensity score matched comparison to plasma. Br J Anaesth. 2018;120:928–34. doi: 10.1016/j.bja.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Weber CF, Görlinger K, Meininger D, et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012;117:531–47. doi: 10.1097/ALN.0b013e318264c644. [DOI] [PubMed] [Google Scholar]
- 27.Majeed A, Ågren A, Holmström M, et al. Management of rivaroxaban- or apixaban-associated major bleeding with prothrombin complex concentrates: a cohort study. Blood. 2017;130:1706–12. doi: 10.1182/blood-2017-05-782060. [DOI] [PubMed] [Google Scholar]
- 28.Schulman S, Gross PL, Ritchie B, et al. Prothrombin Complex Concentrate for Major Bleeding on Factor Xa Inhibitors: A Prospective Cohort Study. Thromb Haemost. 2018;118:842–51. doi: 10.1055/s-0038-1636541. [DOI] [PubMed] [Google Scholar]
- 29.Tao J, Bukanova EN, Akhtar S. Safety of 4-factor prothrombin complex concentrate (4F-PCC) for emergent reversal of factor Xa inhibitors. J Intensive Care. 2018;6:34. doi: 10.1186/s40560-018-0303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grassetto A, De Nardin M, Ganzerla B, et al. ROTEM®-guided coagulation factor concentrate therapy in trauma: 2-year experience in Venice, Italy. Crit Care. 2012;16:428. doi: 10.1186/cc11322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarode R, Milling TJ, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation. 2013;128:1234–43. doi: 10.1161/CIRCULATIONAHA.113.002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dyke C, Aronson S, Dietrich W, et al. Universal definition of perioperative bleeding in adult cardiac surgery. J Thorac Cardiovasc Surg. 2014;147:1458–63. doi: 10.1016/j.jtcvs.2013.10.070. [DOI] [PubMed] [Google Scholar]
- 33.Wassmer G, Brannath W. Springer Series in Pharmaceutical Statistics. 1st. Springer; 2016. Group sequential and confirmatory adaptive designs in clinical trials. edn. [Google Scholar]
- 34.U.S. Food and Drug Administration CFR - code of federal regulations title 21, chapter I, subchapter D, part 312. 2024. [1-Jul-2024]. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-D/part-312?toc=1 Available. Accessed.
- 35.U.S. Food and Drug Administration CFR - code of federal regulations title 21, chapter I, subchapter A, part 50-protection of human subjects. 2024. [1-Jul-2024]. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-50?toc=1 Available. Accessed.
- 36.U.S. Food and Drug Administration CFR - code of federal regulations title 21, chapter I, subchapter A, part 56-institutional review boards. 2024. [1-Jul-2024]. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-56?toc=1 Available. Accessed.