Abstract
Purpose
The purpose of this study was to examine the association between glucose variability, diabetes self-management, and cognitive function in participants enrolled in a cognitive rehabilitation intervention for people with type 2 diabetes.
Methods
Baseline data from the Memory, Attention, and Problem-Solving Skills for Diabetes randomized controlled trial (n = 95; mean age 65.6 years, SD 5.99; 59.3% female; 59% non-Hispanic White) were analyzed and included scores from the PROMIS Cognitive Function version 2, a measure of perceived cognitive function; glucose variability measurements from continuous glucose monitors; and scores on the Summary of Diabetes Self-Care Activities Survey.
Results
Participants had higher levels of perceived cognitive dysfunction than the US average. Lower PROMIS scores were associated with higher levels of glucose variability. Better perceived cognitive health was related to better diabetes self-management. Glucose variability, measured by the coefficient of variation, was a significant predictor of perceived cognitive function.
Conclusions
Perceived cognitive function was associated with diabetes self-management and glucose variability. Understanding this association can support the development of interventions to mitigate effects associated with glucose variability and changes in cognitive function. Including measurements of perceived cognitive function in assessments has the potential to alert health care providers about the need for additional support in diabetes management and the possibility of cognitive impairment that may need further objective assessment.
Having diabetes (DM) raises the risk of decline in cognitive function by almost 20% over 20 years compared to those who do not have DM. 1 This is significant because worldwide, over 400 million people have DM. 2 In fact, cognitive changes can begin early in the development of DM, before the onset of overt symptoms, and those who are diagnosed with type 2 DM (T2DM) may have alterations in cognitive function already. 3 Pathologic changes related to T2DM that affect cognitive function have been examined, but this information may be insufficient for people living with T2DM who seek to improve or maintain cognitive function in diabetes self-management (DMSM). Unfortunately, research on the interplay of DMSM and cognitive function is limited. In other chronic illnesses, such as multiple sclerosis and heart failure, interventions that promote beneficial lifestyle practices via comprehensive cognitive rehabilitation to prevent cognitive decline have been effective, sometimes more effective than pharmaceuticals.4-7
In the US, more than 25% of older adults have expressed concerns about the possibility of developing Alzheimer’s disease, 8 and more than 50% worry that such potential cognitive problems will lead to burdens for their family members. 8 Nevertheless, even if some form of cognitive change is expected in older adults, functional impairment related to such changes in middle to late adulthood is usually unanticipated. 9 In people with risk factors for cognitive decline, such as cardiovascular disease and cancer, cognitive changes are complex and multidimensional. 3 However, in those with nonneurologic chronic conditions, such as diabetes and cardiovascular disease, less is known about cognitive changes—risk and prevalence notwithstanding. 10
It is difficult to assess the effect of these cognitive changes with standard neuropsychological tests because such tools do not measure the effect of cognitive problems on individuals’ day-to-day lives. 11 In examinations of cognitive symptoms, it is therefore imperative to consider the perspectives of those with chronic conditions for several reasons. First, perceived cognitive problems may precede the development of mild cognitive impairment and dementia.12,13 Second, assessment of self-reported cognitive function can help us to ascertain the impact of cognitive symptoms on the self-management of chronic conditions.14,15 Finally, perceived cognitive dysfunction may begin as early as midlife, with about 20% of people with chronic conditions reporting perceived cognitive problems by age 45.16,17 This is earlier and more common than in those without chronic conditions. For example, over 25% of middle-age adults with cardiovascular disease have reported perceived cognitive problems, whereas in healthy adults over age 65, the prevalence is 18%. 18
Glucose variability is also associated with cognitive impairment, hypothesized to be related to increased production of reactive oxygen species, leading to oxidative stress.19,20 Both hyperglycemia and hypoglycemic episodes are associated with cognitive changes, and glycemic stability is important for preventing cognitive decline.21,22 Guidelines for the measurement of glucose variability recommend using A1C, but A1C accounts for only about 10% of variance in cognitive function. 23 Because continuous glucose monitoring (CGM) devices are now more widely available, rapid glucose fluctuations can be assessed in real time, making it easier to measure relationships between variability and cognitive function and other clinically important outcomes.24-27 A number of studies evaluating the impact of behavioral interventions on glucose variability in people with type 1 DM (T1DM) have been conducted, but thus far, no studies have explored the effects of glucose variability on cognitive function (perceived or objective) in cognitive rehabilitation interventions for T2DM.28,29
In the present study, baseline data from the Memory, Attention, and Problem-Solving Skills for People With Diabetes (MAPSS-DM) trial was used to examine perceived cognitive function, DMSM, and glucose variability. The research questions were as follows: (1) What is the extent of perceived cognitive dysfunction in adults with T2DM? (2) Is higher glucose variability associated with greater levels of objective and perceived cognitive dysfunction? (3) Does higher glucose variability have a greater effect on perceived cognitive dysfunction in those with better DMSM?
Methods
Methods are described elsewhere, 30 but briefly, MAPSS-DM is a randomized controlled trial to compare a comprehensive cognitive training intervention with an active control. Its primary outcomes were changes in both objective and perceived cognitive function and DMSM. The target population was community-dwelling adults age ≥50 years and with T2DM. Participants were recruited from local endocrinology clinics and by Trialfacts, an online recruiting company. The University of Texas at Austin’s Institutional Review Board approved this project (Study No. 00000464), and the trial was registered with ClinicalTrials.gov (NCT04831775).
Participants had been diagnosed with diabetes for at least 2 years, had access to telephone and the Internet, scored 10 or higher on the Perceived Deficits Questionnaire, and had an A1C of 7% or higher. Exclusion criteria were a diagnosis of dementia/head injury, a score of <3 on the Mini-Cog, an inability to speak English, and a T1DM diagnosis. Those age ≥50 years were included because perceived cognitive complaints trend upward starting in middle age. 17 It is evident that age-associated cognitive decline and influences on it are detectable in midlife, and the research team’s prior work has shown that adults under age 60 with T2DM have concerns about cognition.26,30-32 Interested patients were given the Mini-Cog to assess for dementia. A total score of >3 indicated lower likelihood of dementia. For those who met inclusion criteria and agreed to participate, a meeting was scheduled at a time and private location convenient to each participant. Enrollment began in September 2021 and ended in August 2022 with the recruitment of 171 individuals in central Texas.
The present analysis is based on a subsample (n = 95) of the full MAPSS-DM population who wore continuous glucose monitors at baseline. All data in this analysis were obtained at baseline and do not include any data from follow-up assessments.
Measures
Demographic and survey data were collected via an online link to the questionnaires on REDCap. Clinical variables (eg, medications, A1C, and comorbidities) were gathered via medical record review (see Table 1 for more detail).
Table 1.
Baseline Characteristics of the Study Population (n = 95)
| Characteristic | Value |
|---|---|
| Age (mean ± SD) | 65.6 ± 5.99 |
| Ethnicity (%) | |
| Non-Hispanic White | 44.7 |
| Hispanic | 34.2 |
| Black | 12.2 |
| Asian | 3.5 |
| Multiracial | 5.4 |
| Female (%) | 59.3 |
| Education (%) | |
| Less than high school | 0.6 |
| High school or GED | 22.7 |
| Associate degree | 17.4 |
| Bachelor’s degree | 33.1 |
| Master’s degree | 21.5 |
| Professional or doctoral degree | 4.7 |
| Family with diabetes (%) | 82.4 |
| Comorbidities (%) | |
| Hyperlipidemia | 68.0 |
| Hypertension | 63.0 |
| Retinopathy | 43.8 |
| Peripheral neuropathy | 38.5 |
| Thyroid disease | 20.7 |
| Heart disease and/or stroke | 15.3 |
| PROMIS cognitive function version 2 (mean ± SD) | 34.5 ± 6.8 |
| Glucose variability (mean ± SD) | |
| Mean glucose | 181.8 ± 57.4 |
| Hypoglycemia (% <70 mg/dL) | 14.2 ± 6.8 |
| Hyperglycemia (% >160 mg/dL) | 37.7 ± 6.2 |
| Standard deviation | 34.9 ± 22.1 |
| Mean amplitude of glucose excursions | 112.3 ± 27 |
| Mean of daily differences | 64.1 ± 18 |
| Coefficient of variation | 33.7 ± 11.1 |
| Time in range | 58.1 ± 27.6 |
| Diabetes self-management (mean ± SD) | |
| General diet | 3.9 ± 2.1 |
| Specific diet | 3.1 ± 2.3 |
| General activity | 2.9 ± 2.4 |
| Specific activity | 2.3 ± 2.4 |
| Blood glucose monitoring | 3.2 ± 3.1 |
| Foot health | 3.6 ± 2.82 |
Glucose variability
Participants wore Abbott’s FreeStyle Libre Pro for CGM. 33 These devices consist of a small US-quarter-sized sensor worn on the back of the upper arm. The sensor measures interstitial glucose via a 5-mm long, 0.4-mm wide filament placed in the subcutaneous tissue. Downloaded CGM data are converted to glucose readings on the research project’s computer using LibreView CGM software. International consensus on time in range guidelines were used to choose CGM variables for analysis34-36: (1) the overall mean, (2) the proportion of readings indicating hypoglycemia (% <70 mg/dL), (3) the proportion of readings indicating hyperglycemia (% >160 mg/dL), (4) time in range (% either >70 mg/dL or <160 mg/dL), (5) the standard deviation of CGM glucose readings, (6) the mean amplitude of glucose excursions (MAGE), (7) the coefficient of variation (CV), and (8) the mean of daily differences (MODD). Patients were classified as “in range” if glucose readings were between 70 mg/dL and 180 mg/dL at least 70% of the time or “not in range” if glucose readings were outside those limits. 37
Cognitive function
The dependent variable in the analysis is perceived cognitive dysfunction, measured with PROMIS version 2.0 cognitive function, 38 a 32-item survey that assesses self-reported cognitive deficits such as mental acuity, concentration, verbal and nonverbal memory, and verbal fluency. Example items are “In the past 7 days I have had trouble forming thoughts” and “In the past 7 days my thinking has been slow.” Respondents answer on a scale from 1 (several times a day) to 5 (never). Higher scores reflect better cognitive function. Reliability of the survey ranges from 0.83 to 0.94. 38
Objective cognitive function was assessed with the online cognitive assessment platform BrainCheck 39 using the following tests: (1) Trails A & B, (2) the Stroop Color and Word test, (3) Digit-Symbol Substitution, and (4) Immediate and Delayed Recognition. For Trails A & B (measures of attention), participants are asked to select, in order, 25 random numbered circles as fast as possible and then to select alternating numbers and letters, for example, “A,” “1,” “B,” “2,” and so on. The average duration of each trial is measured. The Stroop Color and Word test measures reaction time required to overcome cognitive interference (executive function). The name of a color is displayed in an incongruent color, and time taken to name the color of the word is measured. The median duration of incongruent trials is measured. In the Digit Symbol Substitution test (processing speed), participants match an arbitrary correspondence of symbols to digits. The number of trials correctly completed in 1 minute is assessed. In the Immediate and Delayed Recognition tests (memory), 10 words are presented, 1 at a time. Next, another 20 words are presented, including the 10 previously shown. Participants are asked to identify whether the word appeared previously as quickly as possible. At the end of the other BrainCheck tests, participants are again shown 20 words and asked to identify if the word was included as part of the 10 originally shown. A composite score was also calculated to assess global cognitive function. The use of a composite score for cognitive function can increase measurement precision and limit the impact of measurement error and confounding biases related to any one test. The composite scores (mean = 100, SD = 15) are standardized for age on the basis of normative data, with higher scores indicating better cognitive performance. 39
Other data used in these analyses were collected as part of baseline assessment. Demographic data included age, sex, years with T2DM, ethnicity/race, socioeconomic status, and years of education. DMSM was assessed with the Summary of Diabetes Self-Care Activities, 40 indicating how many days in the last week participants performed self-management activities, such as monitoring glucose levels.
Data Analysis
Data were analyzed using IBM SPSS 41 (version 22.1), with statistical significance fixed at P < 0.05 for all tests. Means and standard deviations represented continuous variables, and percentages were calculated for categorical variables. Differences between groups (eg, gender, ethnicity) were analyzed with analysis of variance, nonparametric Kruskal–Wallis tests, and chi-square tests for, respectively, normally distributed continuous data, nonparametric continuous data, and categorical data. Associations between variables were analyzed with Spearman correlation coefficients.
Multiple regression was conducted to examine associations between cognitive function and glucose variability, with adjusted odds ratios and 95% confidence intervals for factors associated with cognitive function, such as sex, age, and comorbidities.
Results
Sample Characteristics
Data were gathered from 95 participants (Figure 1); their baseline characteristics are presented in Table 1. Their mean age was 65.6 years (SD 5.99), 59.3% were female, and 59.0% were non-Hispanic White. Sixty-eight percent had hyperlipidemia, 63% had hypertension, and 38.5% had peripheral neuropathy.
Figure 1.
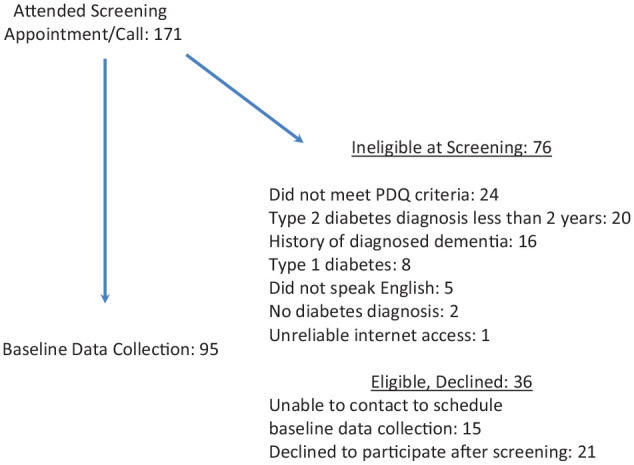
Recruitment flow chart.
Cognitive Function
The mean PROMIS cognitive function version 2 score was 34.54 (SD 6.8), indicating higher levels of perceived cognitive dysfunction than the US average of 50. 42 Mean scores on the BrainCheck assessment were within the “average” range: 64% of participants had scores 1 SD or more below the population norm on 1 or more of the cognitive tests. The largest number of participants performed poorly on the Stroop test (42.7%), a measure of executive function. No significant correlations were found between subjective (PROMIS) and objective (BrainCheck) cognitive function (P > 0.05). None of the demographic characteristics were correlated with perceived cognitive function other than number of comorbidities (r = .162, P < 0.05), with higher levels of perceived cognitive problems associated with a greater number of comorbidities.
Cognitive Function and Glucose Variability
No significant associations were found between average glucose and both types of cognitive function (Table 2). Lower PROMIS scores were associated with higher levels of glucose variability as measured by CV (r = –.62, P < 0.05) and less time in range (r = .53, P < 0.01). Additionally, lower PROMIS scores were significantly associated with percentage below range, or hypoglycemia, indicating that more hypoglycemia was strongly related to worse perceived cognitive function (r = –.743, P < 0.01). Scores on Trails A, Trails B, and the Stroop test were positively associated with time in range (rs = .611, .681, .496, respectively; P < 0.01). Stroop test scores were negatively associated with percentage below range, or hypoglycemia, indicating that more hypoglycemia was strongly related to lower performance on the test (r = –.743, P < 0.01). Trails A scores were negatively associated with percentage above target, indicating that poorer performance on Trails A was significantly related to more hyperglycemia (r = –.354, P < 0.01).
Table 2.
Associations Between Cognitive Function and Glucose Variability
| Cognitive function variables | Mean glucose | Time in range | MODD | MAGE | <70 mg/dL | >180 mg/dL | CV | SD |
|---|---|---|---|---|---|---|---|---|
| PROMIS total score | 0.20 | 0.53** | 0.13 | 0.21 | 0.17 | 0.18 | –0.62* | 0.13 |
| Trails A | 0.04 | 0.61** | 0.01 | 0.04 | 0.17 | –0.035** | 0.21 | 0.06 |
| Trails B | 0.05 | 0.68** | 0.00 | 0.23 | 0.03 | 0.05 | 0.11 | 0.09 |
| Stroop test | 0.04 | 0.50** | 0.10 | 0.03 | –0.74** | 0.03 | 0.08 | 0.02 |
| Digit-Symbol Substitution | 0.18 | 0.16 | 0.23 | 0.06 | 0.03 | 0.17 | 0.05 | 0.16 |
| Immediate recognition | 0.05 | 0.01 | 0.07 | 0.15 | 0.09 | 0.02 | 0.17 | 0.14 |
| Delayed recognition | 0.05 | 0.13 | 0.18 | 0.28 | 0.04 | 0.01 | 0.03 | 0.09 |
Abbreviations: CV, coefficient of variation; MAGE, mean amplitude of glucose excursions; MODD, mean of daily differences; SD, standard deviation.
P < 0.05. **P < 0.01.
In the multivariate analysis, glucose variability as measured by the CV was a significant predictor of perceived cognitive function, R 2 = 0.27, F(1, 88) = 2.42, P < 0.01. The interactions between cognitive function and other measures of glucose variability were not significant.
Cognitive Function and Self-Management
Scores on the PROMIS scale were positively associated with general diet, specific diet, general activity, specific activity, and monitoring foot health (rs = .335, .331, .206, .332, 0.296, respectively; P < 0.01), indicating that better perceived cognitive health was related to better DMSM. Trails B was significantly associated with general diet, specific diet, and specific activity (rs = .235, .208, .229, respectively; P < 0.05), indicating that better DMSM was associated with better cognitive flexibility. The Stroop test was also significantly associated with general diet, specific diet, and specific activity (rs = .62, .60, .66, respectively; P < 0.01), indicating that better executive function was related to better DMSM. See Table 3 for full details.
Table 3.
Associations Between Glucose Variability and Diabetes Self-Management
| DMSM variables | Mean glucose | Time in range | MODD | MAGE | <70 mg/dL | >180 mg/dL | CV | SD |
|---|---|---|---|---|---|---|---|---|
| General diet | 0.05 | 0.02 | 0.08 | 0.05 | –0.01 | 0.08 | 0.13 | 0.12 |
| Specific diet | 0.07 | 0.19 | 0.14 | 0.01 | –0.01 | 0.02 | –0.50** | .00 |
| General activity | –0.00 | 0.40** | 0.05 | 0.33 | 0.05 | 0.07 | –0.57** | 0.04 |
| Specific activity | 0.01 | 0.43** | 0.00 | 0.03 | –0.05 | 0.09 | –0.30** | 0.18 |
| Foot health | –0.30* | 0.33 | 0.34 | 0.08 | –0.02 | 0.01 | 0.03 | 0.18 |
| Glucose monitoring | –0.05 | 0.02 | 0.17 | 0.11 | –0.12 | –0.23 | –0.40** | 0.27 |
Abbreviations: CV, coefficient of variation; MAGE, mean amplitude of glucose excursion; MODD, mean of daily differences; SD, standard deviation.
P < 0.05. **P < 0.01.
Glucose Variability and Diabetes Self-Management
Greater glucose variability as measured by the CV was associated with poorer glucose monitoring (r = –.40, P < 0.01), less general physical activity and specific exercise (rs = –.30, –.57; P < 0.01), and lower intake of fruit and vegetables (r = –.50, P < 0.01). Time in range was positively associated with general physical activity (r = .40, P < 0.01) and specific exercise (r = .432, P < 0.01). Better foot monitoring was associated with lower mean glucose (r = –.30, P < 0.05). None of the other glucose variability measures were significantly correlated with DMSM activities. See Table 3.
Discussion
In this study, levels of perceived cognitive problems were higher than the US average. 42 Studies examining perceived cognitive problems in T2DM are sparse. Reviews of the qualitative literature on T2DM and perceived cognitive function show that people with diabetes frequently experience cognitive symptoms and do not realize that they have diagnosable cognitive decline.43,44 In response to these symptoms, various strategies to cope with cognitive decline have been tried, even strategies unsupported by research.43,45 Indeed, awareness of cognitive decline in people with diabetes is much lower than in those with other comorbidities. For example, Lin et al 46 found that in adults age 60 and over, proportions of disease awareness for hyperlipidemia and hypertension were 38.0% and 58.1%, respectively, but 45% of those who felt confident in their memory had mild to severe cognitive impairment.
Other studies have shown that people with diabetes and poorer perceptions of cognitive problems also struggle with creating approaches to solving self-management issues. 26 In the present study, glucose variability was associated with several areas of DMSM, and greater variability was associated with worse DMSM. Considering that glucose variability negatively affects cognitive function, poor cognitive function may affect DMSM. In prior studies of variability and DMSM, participants have stated that CGM helped with self-management—even when scores on self-management measures were low.47,48 It is possible that the use of CGM helps those who are not actively involved in DMSM in ways that tools to measure DMSM do not.
Several studies have shown that glucose variability affects cognitive function.21,29,47 A greater degree of glucose variability is negatively associated with cognitive function in persons with DM after adjustment for A1C because A1C accounts for less than 10% of the change in cognition.21,29 These previous studies align with these current findings that maintaining time in range (70-180 mg/dL) was associated with better perceived cognitive function. 47 This suggests that consistent glucoses within this range throughout the day may help prevent cognitive decline in individuals with T2DM. However, most studies of cognition and glucose variability use a 7-point self-monitoring of blood glucose profile to assess fluctuations, but up to 80% of hypoglycemia goes unassessed at set time points. 37 CGM can capture glucose excursions more completely and precisely than self-monitoring of blood glucose, and it is the gold standard for assessing glucose variability.
Additionally, the findings demonstrated that a greater number of comorbidities and being Latino were associated with lower cognitive function scores, highlighting the crucial need for health care providers to conduct routine screenings for cognitive impairment in people with T2DM, particularly those with multiple comorbidities and within the Latino community. With approximately 30% of people with T2DM presenting 3 or more additional health conditions at diagnosis, 48 they are already at a higher risk of complications. Uncontrolled glucose variability and cognitive decline may exacerbate this risk by lowering DMSM, leading to poorer health outcomes. 49 Also, Latinos with T2DM consistently reported that their perceived cognitive problems affected their ability to manage their conditions. 32 Indeed, perceived cognitive function was associated with all self-management activities measured in this study. This underscores the importance of addressing high glucose variability through CGM and cognitive impairment in diabetes care to enhance DMSM, ultimately improving health outcomes and quality of life for people with T2DM.
Limitations
Every effort was made to follow rigorous protocols in data collection, but some limitations should be noted. The sample size was small, and subgroup comparisons may lack statistical power. Additionally, the external validity of the results may not be robust because the recruited sample may not be generalizable to the broader population with T2DM. More detailed information on diabetes history and cognitive function was not available at baseline but could have been useful for clarifying concerns about how cognitive function can potentially impact DMSM. Concern about cognitive function was addressed because it is a factor in how people choose to self-manage diabetes, but adults with diabetes do have other concerns about self-management that affect their decisions. Given these limitations, results should be considered preliminary findings.
Conclusion
Self-reported cognitive dysfunction can be considered a risk factor for dementia, and it may present before diagnosable impairments are found on neuropsychological evaluations. 50 Self-reported cognitive dysfunction can also affect day-to-day self-management of chronic conditions and quality of life.31,32 Although self-reported cognitive problems have been studied extensively in the general population, less is known about how people with T2DM perceive their cognition or how it may be related to self-management and self-management outcomes, such as glucose variability. Developing a better understanding of these relationships can lead to better functioning in life and better DMSM.47,51 Given a mechanistic understanding of cognitive function through use of CGM, perceived cognitive function is strongly associated with glucose variability.
Footnotes
The authors declare that there is no conflict of interest.
Funding: This work was funded by National Institutes of Health’s Institute of Nursing Research R21 Grant NR019266.
ORCID iDs: Heather Cuevas  https://orcid.org/0000-0003-4314-6686
https://orcid.org/0000-0003-4314-6686
Jeeyeon Kim  https://orcid.org/0000-0001-7913-4463
https://orcid.org/0000-0001-7913-4463
Contributor Information
Heather Cuevas, The University of Texas at Austin, Austin, Texas.
Alexa K. Stuifbergen, The University of Texas at Austin, Austin, Texas.
Robin Hilsabeck, The University of Texas Health Science Center at San Antonio, Texas.
Jeeyeon Kim, The University of Texas at Austin, Austin, Texas.
Shenell Wood, The University of Texas at Austin, Austin, Texas.
References
- 1. Dove A, Shang Y, Xu W, et al. The impact of diabetes on cognitive impairment and its progression to dementia. Alzheimers Dement. 2021;17(11):1769-1778. doi: 10.1002/alz.12482 [DOI] [PubMed] [Google Scholar]
- 2. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biessels GJ, Whitmer RA. Cognitive dysfunction in diabetes: how to implement emerging guidelines. Diabetologia. 2020;63(1):3-9. doi: 10.1007/s00125-019-04977-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Becker H, Henneghan AM, Volker DL, Mikan SQ. A pilot study of a cognitive behavioral intervention for breast cancer survivors. Oncol Nurs Forum. 2017;44(2):255-264. [DOI] [PubMed] [Google Scholar]
- 5. Pressler SJ, Titler M, Koelling TM, et al. Nurse-enhanced computerized cognitive training increases serum brain-derived neurotropic factor levels and improves working memory in heart failure. J Card Fail. 2015;21(8):630-641. doi: 10.1016/j.cardfail.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 6. Sigmundsdottir L, Longley WA, Tate RL. Computerised cognitive training in acquired brain injury: a systematic review of outcomes using the International Classification of Functioning (ICF). Neuropsychol Rehabil. 2016;26(5-6):673-741. doi: 10.1080/09602011.2016.1140657 [DOI] [PubMed] [Google Scholar]
- 7. Stuifbergen A, Becker H, Perez F, et al. Computer-assisted cognitive rehabilitation in persons with multiple sclerosis: results of a multi-site randomized controlled trial with six-month follow-up. Disabil Health. 2018;11(3):427-434. doi: 10.1016/j.dhjo.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Werner P, AboJabel H, Maxfield M. Conceptualization, measurement and correlates of dementia worry: a scoping review. Arch Gerontol Geratr. 2021;92:104246. doi: 10.1016/j.archger.2020.104246 [DOI] [PubMed] [Google Scholar]
- 9. García C, Moreno L, Alacreu M, Muñoz FJ, Martinez LA. Addressing psychosocial factors in cognitive impairment screening from a holistic perspective: the DeCo-Booklet methodology design and pilot study. Int J Environ Res Public Health. 2022;19(19):12911. doi: 10.3390/ijerph191912911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hadley G, Zhang J, Harris-Skillman E, Alexopulou Z, DeLuca GC, Pendlebury ST. Cognitive decline and diabetes: a systematic review of the neuropathological correlates accounting for cognition at death. J Neurol Neurosurg Psychiatry. 2022;93(3):246-253. doi: 10.1136/jnnp-2021-328158 [DOI] [PubMed] [Google Scholar]
- 11. Kiselica AM, Karr JE, Mikula CM, et al. Recent advances in neuropsychological test interpretation for clinical practice. Neuropsychol Rev. 2024;34(2):637-667. doi: 10.1007/s11065-023-09596-1 [DOI] [PubMed] [Google Scholar]
- 12. Chapman S, Sunderaraman P, Joyce JL, et al. Optimizing subjective cognitive decline to detect early cognitive dysfunction. J Alzheimers Dis. 2021;80(3):1185-1196. doi: 10.3233/JAD-201322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Markova H, Nikolai T, Mazancova AF, et al. Differences in subjective cognitive complaints between non-demented older adults from a memory clinic and the community. J Alzheimers Dis. 2019;70(1):61-73. doi: 10.3233/JAD-180630 [DOI] [PubMed] [Google Scholar]
- 14. Cuevas H, Danesh V, Henneghan A. Self-reported cognitive function in persons with nonneurological chronic diseases: a systematic review. J Aging Res. 2022:5803337. doi: 10.1155/2022/5803337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Whitfield T, Demnitz-King H, Schlosser M, et al. Effects of a mindfulness-based versus a health self-management intervention on objective cognitive performance in older adults with subjective cognitive decline (SCD): a secondary analysis of the SCD-Well randomized controlled trial. Alzheimers Res Ther. 2022;14:125. doi: 10.1186/s13195-022-01057-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jessen F, Amariglio RE, Buckley RF, et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19(3):271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu Y, Jiang D. Multimorbidity patterns in US adults with subjective cognitive decline and their relationship with functional difficulties. J Aging Health. 2022;34(6-8):929-938. doi: 10.1177/08982643221080287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Nieuwkerk AC, Delewi R, Wolters FJ, et al. Cognitive impairment in patients with cardiac disease: implications for clinical practice. Stroke. 2023;54(8):2181-2191. doi: 10.1161/STROKEAHA.123.040499 [DOI] [PubMed] [Google Scholar]
- 19. Jung HS. Clinical implications of glucose variability: chronic complications of diabetes. Endocrinol Metab. 2015;30(2):167-174. 10.3803/EnM.2015.30.2.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith-Palmer J, Brändle M, Trevisan R, Federici MO, Liabat S, Valentine W. Assessment of the association between glycemic variability and diabetes-related complications in type 1 and type 2 diabetes. Diab Res Clin Pract. 2014;105(3):273-284. doi: 10.1016/j.diabres.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 21. Kim C, Sohn J-H, Jang MU, et al. Association between visit-to-visit glucose variability and cognitive function in aged type 2 diabetic patients: a cross-sectional study. PLoS One. 2015;10(7):e0132118. doi: 10.1371/journal.pone.0132118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ceriello A, Monnier L, Owens D. Glycaemic variability in diabetes: clinical and therapeutic implications. Lancet Diabetes Endocrinol. 2019;7(3):221-230. doi: 10.1016/S2213-8587(18)30136-0 [DOI] [PubMed] [Google Scholar]
- 23. Rutter MK. Devoting attention to glucose variability and hypoglycemia in type 2 diabetes. Diabetologia. 2018;61(1):43-47. doi: 10.1007/s00125-017-4421-1 [DOI] [PubMed] [Google Scholar]
- 24. Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5(1):64-74. doi: 10.1016/S1474-4422(05)70284-2 [DOI] [PubMed] [Google Scholar]
- 25. Cukierman-Yaffe T. Diabetes, dysglycemia, and cognitive dysfunction. Diabetes Metab Res Rev. 2014;30(5):341-345. doi: 10.1002/dmrr.2507 [DOI] [PubMed] [Google Scholar]
- 26. Cuevas H, Stuifbergen A. Perceived cognitive deficits are associated with diabetes self-management in a multi-ethnic sample. J Diabetes Metabol Disorders. 2017;16:7. doi: 10.1186/s40200-017-0289-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gatlin PK, Insel KC. Severity of type 2 diabetes, cognitive function, and self-care. Biol Res Nurs. 2015;17(5):540-548. doi: 10.1177/1099800414557565 [DOI] [PubMed] [Google Scholar]
- 28. Wilmot EG, Choudhary P, Leelarathna L, Baxter M. Glycaemic variability: the under-recognized therapeutic target in type 1 diabetes care. Diabetes Obes Metab. 2019;21(12):2599-2608. doi: 10.1111/dom.13842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iceta S, Sohier L, Bégin C, Brazeau A-S, Rabasa-Lhoret R, Gagnon C. Impact of glycemic variability on cognitive impairment, disordered eating behaviors and self-management skills in patients with type 1 diabetes: study protocol for a cross-sectional online study, the Sugar Swing study. BMC Endocr Disord. 2022;22:283. doi: 10.1186/s12902-022-01191-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cuevas H, Stuifbergen AK, Hilsabeck RC, Sales A, Wood S, Kim J. The role of cognitive rehabilitation in people with type 2 diabetes: a study protocol for a randomized controlled trial. PLoS One. 2023;18(5):e0285553. doi: 10.1371/journal.pone.0285553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cuevas HE, Stuifbergen AK, Brown SA, Rock JL. Thinking about cognitive function: perceptions of cognitive changes in people with type 2 diabetes. Diabetes Educ. 2017;43(5):486-494. doi: 10.1177/0145721717729806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cuevas H, Zuñiga J. (2021). Latinx with type 2 diabetes: perceptions of cognitive health. J Immigr Minor Health. 2021;23(2):337-343. doi: 10.1007/s10903-020-00995-7 [DOI] [PubMed] [Google Scholar]
- 33. Blum A. Freestyle Libre glucose monitoring system. Clin Diabetes. 2018;36(2):203-204. doi: 10.2337/cd17-0130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593-1603. doi: 10.2337/dci19-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnson ML, Martens TW, Criego AB, Carlson AL, Simonson GD, Bergenstal RM. Utilizing the ambulatory glucose profile to standardize and implement continuous glucose monitoring in clinical practice. Diabetes Technol Ther. 2019;21(S2):S217-S225. doi: 10.1089/dia.2019.0034 [DOI] [PubMed] [Google Scholar]
- 36. Kovatchev B, Cobelli C. Glucose variability: timing, risk analysis, and relationship to hypoglycemia in diabetes. Diabetes Care. 2016;39(4):502-510. doi: 10.2337/dc15-2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blevins T, Lane W, Rodbard D, et al. Glucose variability and time in range in type 2 diabetes treated with U-500R by pump or injection: CGM findings from the VIVID study. Diabetes Technol Ther. 2021;23(1):51-58. doi: 10.1089/dia.2020.0030 [DOI] [PubMed] [Google Scholar]
- 38. Edelen MO, Harrison JM, Rodriguez A, et al. Evaluation of PROMIS cognitive function scores and correlates in a clinical sample of older adults. Gerontol Geriatr Med. 2022;8. doi: 10.1177/23337214221119057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sun KH, Huang B, Ghomi RH. Early detection of dementia and mild cognitive impairment with BrainCheck. Alzheimers Dement. 2021;17(suppl 6):e053429. doi: 10.1002/alz.053429 [DOI] [Google Scholar]
- 40. Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943-950. doi: 10.2337/diacare.23.7.943 [DOI] [PubMed] [Google Scholar]
- 41. IBM Corp. IBM SPSS Statistics for Windows, version 22.0. 2013. [Google Scholar]
- 42. Jensen RE, Potosky AL, Moinpour CM, et al. United States population-based estimates of Patient-Reported Outcomes Measurement Information System symptom and functional status reference values for individuals with cancer. J Clin Oncol. 2017;35(17):1913-1920. doi: 10.1200/JCO.2016.71.4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang M, Guan X, Yan J, et al. Perceptions and responses to cognitive decline in people with diabetes: a systematic review of qualitative studies. Front Public Health. 2023;11:1076030. doi: 10.3389/fpubh.2023.1076030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hill NL, Bhargava S, Brown MJ, et al. Cognitive complaints in age-related chronic conditions: a systematic review. PLoS One. 2021;16:e0253795. doi: 10.1371/journal.pone.0253795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Buckley RF, Saling MM, Frommann I, Wolfsgruber S, Wagner M. Subjective cognitive decline from a phenomenological perspective: a review of the qualitative literature. J Alzheimers Dis. 2015;48(suppl 1):S125-S140. doi: 10.3233/JAD-150095 [DOI] [PubMed] [Google Scholar]
- 46. Lin Z, Fu M, Chen X. Self-perceived memory is negatively associated with chronic disease awareness: evidence from blood biomarker data. SSM Popul Health. 2023;22:101361. doi: 10.1016/j.ssmph.2023.101361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cuevas H, Heitkemper E, Haque B. Relationships among perception of cognitive function, diabetes self-management, and glucose variability in older adults: a mixed methods study. Res Gerontol Nurs. 2022;15(4):203-212. doi: 10.3928/19404921-20220609-02 [DOI] [PubMed] [Google Scholar]
- 48. Pearson-Stuttard J, Holloway S, Polya R, et al. Variations in comorbidity burden in people with type 2 diabetes over disease duration: a population-based analysis of real-world evidence. EClinicalMedicine. 2022;52:101584. doi: 10.1016/j.eclinm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yen HY, Lee SC, Lin CF, Lee TI, Yamaguchi Y, Lee PH. Complications and comorbidities as influencing factors of health outcomes in older adults with type 2 diabetes mellitus. Collegian. 2023;30(2):230-235. doi: 10.1016/j.colegn.2022.08.010 [DOI] [Google Scholar]
- 50. Heitkemper EM, Wilcox GB, Zuñiga J, Kim MT, Cuevas H. A text-mining analysis to examine dominant sources of online information and content on continuous glucose monitors. Sci Diabetes Self Manag Care. 2023;49(2):101-111. doi: 10.1177/26350106231158828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hess C, Levy B, Hashmi AZ, et al. Subjective versus objective assessment of cognitive functioning in primary care. J Am Board Fam Med. 2020;33(3):417-425. doi: 10.3122/jabfm.2020.03.190265 [DOI] [PubMed] [Google Scholar]


