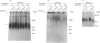Abstract
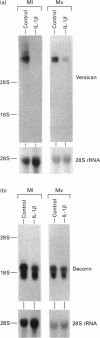
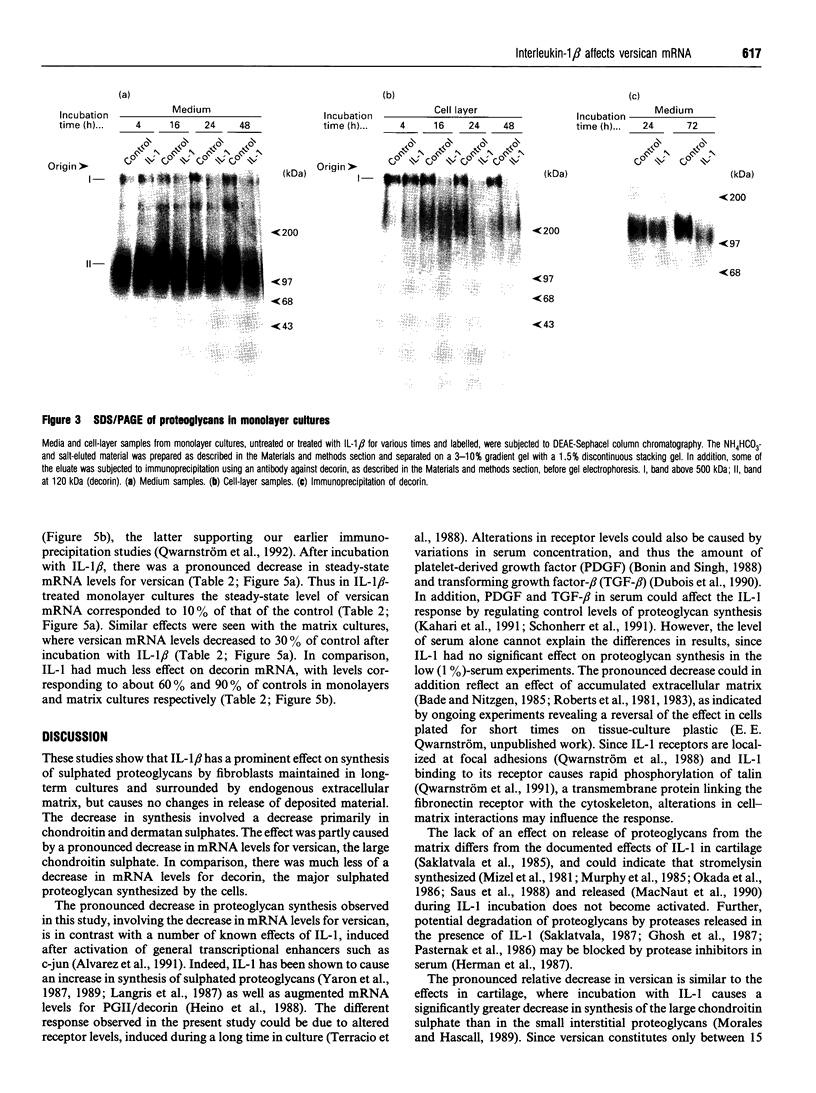
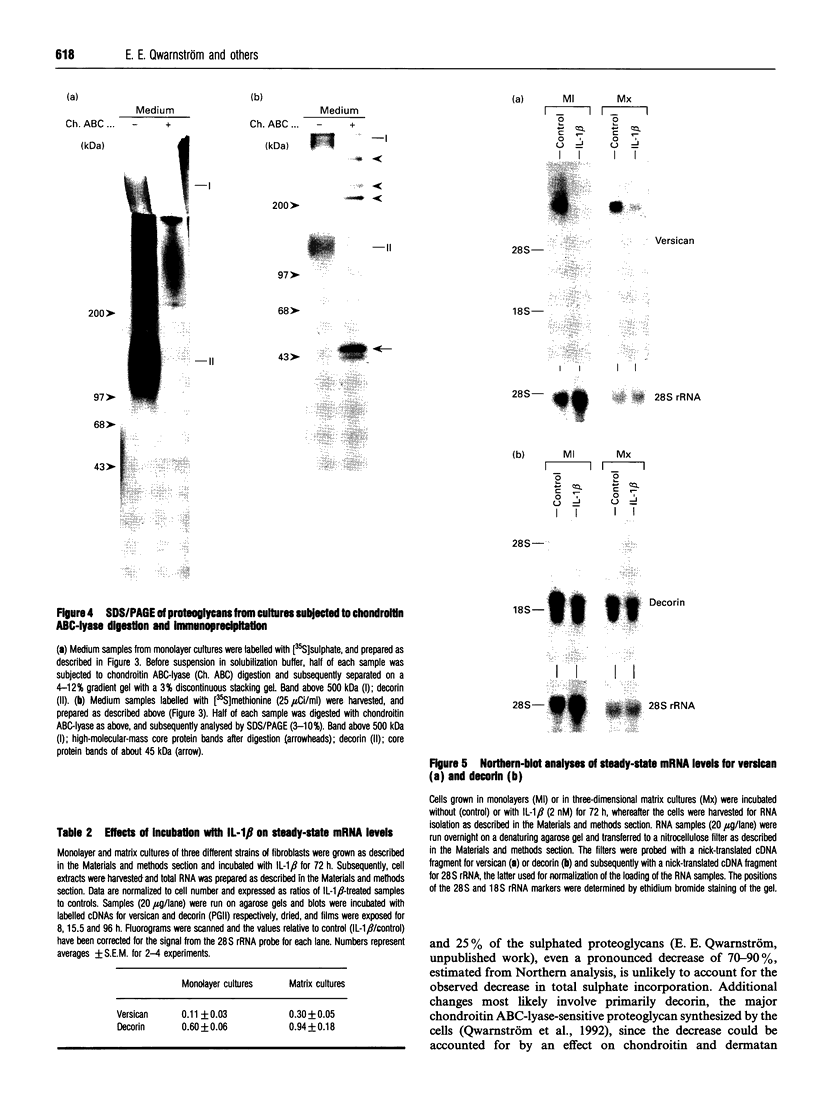
This study investigates the effects of interleukin (IL)-1 beta on proteoglycan metabolism by fibroblasts surrounded by endogenous extracellular matrix. In both three-dimensional matrix cultures and long-term monolayer cultures IL-1 beta caused a significant decrease in synthesis and deposition of sulphated proteoglycans, but had no effect on release of deposited material. The decrease in synthesis became successively more pronounced, and corresponded to 40-60% of the control after 72 h incubation. The reduction was almost totally accounted for by an effect on the chondroitin ABC-lyase-sensitive proteoglycans. Gel electrophoresis showed a significant decrease in a high-molecular-mass chondroitin ABC-lyase-sensitive proteoglycan after incubation with IL-1 beta. Northern-blot analyses of total RNA revealed a pronounced decrease in the steady-state mRNA levels of versican, the large chondroitin sulphate, with levels corresponding to 10-30% of controls. In comparison, the steady-state mRNA level for decorin, the major sulphated proteoglycan synthesized by the cells, was only slightly affected. The prominent decrease in synthesis of sulphated proteoglycans induced in long-term fibroblast cultures, including the pronounced decrease in versican steady-state mRNA levels, is likely to have a significant effect on the structure of the extracellular matrix. Induction of this type of change may constitute a significant mechanism whereby IL-1 beta can affect the properties of connective tissue during inflammation and wound healing.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez E., Northwood I. C., Gonzalez F. A., Latour D. A., Seth A., Abate C., Curran T., Davis R. J. Pro-Leu-Ser/Thr-Pro is a consensus primary sequence for substrate protein phosphorylation. Characterization of the phosphorylation of c-myc and c-jun proteins by an epidermal growth factor receptor threonine 669 protein kinase. J Biol Chem. 1991 Aug 15;266(23):15277–15285. [PubMed] [Google Scholar]
- Arner E. C., Pratta M. A. Independent effects of interleukin-1 on proteoglycan breakdown, proteoglycan synthesis, and prostaglandin E2 release from cartilage in organ culture. Arthritis Rheum. 1989 Mar;32(3):288–297. doi: 10.1002/anr.1780320310. [DOI] [PubMed] [Google Scholar]
- Bade E. G., Nitzgen B. Extracellular matrix (ECM) modulates the EGF-induced migration of liver epithelial cells in serum-free, hormone-supplemented medium. In Vitro Cell Dev Biol. 1985 Apr;21(4):245–248. doi: 10.1007/BF02620936. [DOI] [PubMed] [Google Scholar]
- Benton H. P., Tyler J. A. Inhibition of cartilage proteoglycan synthesis by interleukin I. Biochem Biophys Res Commun. 1988 Jul 15;154(1):421–428. doi: 10.1016/0006-291x(88)90703-6. [DOI] [PubMed] [Google Scholar]
- Bocquet J., Langris M., Daireaux M., Jouis V., Pujol J. P., Beliard R., Loyau G. Mononuclear cell-mediated modulation of synovial cell metabolism. II. Increased hyaluronic acid synthesis by a monocyte cell factor (MCF). Exp Cell Res. 1985 Sep;160(1):9–18. doi: 10.1016/0014-4827(85)90231-9. [DOI] [PubMed] [Google Scholar]
- Bonin P. D., Singh J. P. Modulation of interleukin-1 receptor expression and interleukin-1 response in fibroblasts by platelet-derived growth factor. J Biol Chem. 1988 Aug 15;263(23):11052–11055. [PubMed] [Google Scholar]
- Brennan M. J., Oldberg A., Hayman E. G., Ruoslahti E. Effect of a proteoglycan produced by rat tumor cells on their adhesion to fibronectin-collagen substrata. Cancer Res. 1983 Sep;43(9):4302–4307. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Day A. A., McQuillan C. I., Termine J. D., Young M. R. Molecular cloning and sequence analysis of the cDNA for small proteoglycan II of bovine bone. Biochem J. 1987 Dec 15;248(3):801–805. doi: 10.1042/bj2480801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower S. K., Dwek R. A. Phosphorus-31 nuclear magnetic resonance probes for the combining site of the myeloma protein M315. Biochemistry. 1979 Aug 21;18(17):3668–3674. doi: 10.1021/bi00584a005. [DOI] [PubMed] [Google Scholar]
- Dower S. K., Kronheim S. R., March C. J., Conlon P. J., Hopp T. P., Gillis S., Urdal D. L. Detection and characterization of high affinity plasma membrane receptors for human interleukin 1. J Exp Med. 1985 Aug 1;162(2):501–515. doi: 10.1084/jem.162.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois C. M., Ruscetti F. W., Palaszynski E. W., Falk L. A., Oppenheim J. J., Keller J. R. Transforming growth factor beta is a potent inhibitor of interleukin 1 (IL-1) receptor expression: proposed mechanism of inhibition of IL-1 action. J Exp Med. 1990 Sep 1;172(3):737–744. doi: 10.1084/jem.172.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P., Collier S., Andrews J. Synovial membrane-cartilage interactions--the role of serine proteinase inhibitors in interleukin-1 mediated degradation of articular damage. J Rheumatol. 1987 May;14(Spec No):122–124. [PubMed] [Google Scholar]
- Glössl J., Beck M., Kresse H. Biosynthesis of proteodermatan sulfate in cultured human fibroblasts. J Biol Chem. 1984 Nov 25;259(22):14144–14150. [PubMed] [Google Scholar]
- Goldring M. B., Birkhead J., Sandell L. J., Kimura T., Krane S. M. Interleukin 1 suppresses expression of cartilage-specific types II and IX collagens and increases types I and III collagens in human chondrocytes. J Clin Invest. 1988 Dec;82(6):2026–2037. doi: 10.1172/JCI113823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Heino J., Kähäri V. M., Mauviel A., Krusius T. Human recombinant interleukin-1 regulates cellular mRNA levels of dermatan sulphate proteoglycan core protein. Biochem J. 1988 May 15;252(1):309–312. doi: 10.1042/bj2520309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J. H., Appel A. M., Hess E. V. Modulation of cartilage destruction by select nonsteroidal antiinflammatory drugs. In vitro effect on the synthesis and activity of catabolism-inducing cytokines produced by osteoarthritic and rheumatoid synovial tissue. Arthritis Rheum. 1987 Mar;30(3):257–265. doi: 10.1002/art.1780300303. [DOI] [PubMed] [Google Scholar]
- Ikebe T., Hirata M., Koga T. Effects of human recombinant tumor necrosis factor-alpha and interleukin 1 on the synthesis of glycosaminoglycan and DNA in cultured rat costal chondrocytes. J Immunol. 1988 Feb 1;140(3):827–831. [PubMed] [Google Scholar]
- Ikebe T., Hirata M., Koga T. Human recombinant interleukin 1-mediated suppression of glycosaminoglycan synthesis in cultured rat costal chondrocytes. Biochem Biophys Res Commun. 1986 Oct 15;140(1):386–391. doi: 10.1016/0006-291x(86)91102-2. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe M. L., Hasselaar P., Sage H. Differential expression of extracellular proteins is correlated with angiogenesis in vitro. Lab Invest. 1991 Feb;64(2):174–186. [PubMed] [Google Scholar]
- Kolibas L. M., Goldberg R. L. Effect of cytokines and anti-arthritic drugs on glycosaminoglycan synthesis by bovine articular chondrocytes. Agents Actions. 1989 Jun;27(3-4):245–249. doi: 10.1007/BF01972787. [DOI] [PubMed] [Google Scholar]
- Krusius T., Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähäri V. M., Larjava H., Uitto J. Differential regulation of extracellular matrix proteoglycan (PG) gene expression. Transforming growth factor-beta 1 up-regulates biglycan (PGI), and versican (large fibroblast PG) but down-regulates decorin (PGII) mRNA levels in human fibroblasts in culture. J Biol Chem. 1991 Jun 5;266(16):10608–10615. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langris M., Daireaux M., Jouis V., Bocquet J., Loyau G. Interleukin-1-like factor (mononuclear cell factor) modulates proteoglycan synthesis in cultured human synovial cells. Biochim Biophys Acta. 1987 Jan 19;927(1):34–42. doi: 10.1016/0167-4889(87)90063-2. [DOI] [PubMed] [Google Scholar]
- LeBaron R. G., Zimmermann D. R., Ruoslahti E. Hyaluronate binding properties of versican. J Biol Chem. 1992 May 15;267(14):10003–10010. [PubMed] [Google Scholar]
- Lewandowska K., Choi H. U., Rosenberg L. C., Zardi L., Culp L. A. Fibronectin-mediated adhesion of fibroblasts: inhibition by dermatan sulfate proteoglycan and evidence for a cryptic glycosaminoglycan-binding domain. J Cell Biol. 1987 Sep;105(3):1443–1454. doi: 10.1083/jcb.105.3.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNaul K. L., Chartrain N., Lark M., Tocci M. J., Hutchinson N. I. Discoordinate expression of stromelysin, collagenase, and tissue inhibitor of metalloproteinases-1 in rheumatoid human synovial fibroblasts. Synergistic effects of interleukin-1 and tumor necrosis factor-alpha on stromelysin expression. J Biol Chem. 1990 Oct 5;265(28):17238–17245. [PubMed] [Google Scholar]
- Mizel S. B., Dayer J. M., Krane S. M., Mergenhagen S. E. Stimulation of rheumatoid synovial cell collagenase and prostaglandin production by partially purified lymphocyte-activating factor (interleukin 1). Proc Natl Acad Sci U S A. 1981 Apr;78(4):2474–2477. doi: 10.1073/pnas.78.4.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales T. I., Hascall V. C. Effects of interleukin-1 and lipopolysaccharides on protein and carbohydrate metabolism in bovine articular cartilage organ cultures. Connect Tissue Res. 1989;19(2-4):255–275. doi: 10.3109/03008208909043900. [DOI] [PubMed] [Google Scholar]
- Murphy G., Reynolds J. J., Werb Z. Biosynthesis of tissue inhibitor of metalloproteinases by human fibroblasts in culture. Stimulation by 12-O-tetradecanoylphorbol 13-acetate and interleukin 1 in parallel with collagenase. J Biol Chem. 1985 Mar 10;260(5):3079–3083. [PubMed] [Google Scholar]
- Narayanan A. S., Page R. C. Biochemical characterization of collagens synthesized by fibroblasts derived from normal and diseased human gingiva. J Biol Chem. 1976 Sep 25;251(18):5464–5471. [PubMed] [Google Scholar]
- Nietfeld J. J., Wilbrink B., Den Otter W., Huber J., Huber-Bruning O. The effect of human interleukin 1 on proteoglycan metabolism in human and porcine cartilage explants. J Rheumatol. 1990 Jun;17(6):818–826. [PubMed] [Google Scholar]
- Okada Y., Nagase H., Harris E. D., Jr A metalloproteinase from human rheumatoid synovial fibroblasts that digests connective tissue matrix components. Purification and characterization. J Biol Chem. 1986 Oct 25;261(30):14245–14255. [PubMed] [Google Scholar]
- Pasternak R. D., Hubbs S. J., Caccese R. G., Marks R. L., Conaty J. M., DiPasquale G. Interleukin-1 stimulates the secretion of proteoglycan- and collagen-degrading proteases by rabbit articular chondrocytes. Clin Immunol Immunopathol. 1986 Dec;41(3):351–367. doi: 10.1016/0090-1229(86)90006-1. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Lachman L. B., Mainardi C. L., Kang A. H. Interleukin 1 stimulation of collagenase production by cultured fibroblasts. J Exp Med. 1983 Feb 1;157(2):801–806. doi: 10.1084/jem.157.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Raghow R., Stricklin G. P., Poppleton H., Seyer J. M., Kang A. H. Modulation of fibroblast functions by interleukin 1: increased steady-state accumulation of type I procollagen messenger RNAs and stimulation of other functions but not chemotaxis by human recombinant interleukin 1 alpha and beta. J Cell Biol. 1988 Feb;106(2):311–318. doi: 10.1083/jcb.106.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Smith G. N., Jr, Lachman L. B., Endres R. O., Poppleton H. M., Hasty K. A., Seyer J. M., Kang A. H. Stimulation of glycosaminoglycan synthesis in cultured human dermal fibroblasts by interleukin 1. Induction of hyaluronic acid synthesis by natural and recombinant interleukin 1s and synthetic interleukin 1 beta peptide 163-171. J Clin Invest. 1989 Feb;83(2):629–636. doi: 10.1172/JCI113927. [DOI] [PMC free article] [PubMed] [Google Scholar]
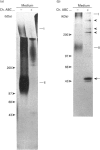
- Qwarnström E. E., Kinsella M. G., MacFarlane S. A., Page R. C., Wight T. N. Modulation of proteoglycan metabolism by human fibroblasts maintained in an endogenous three-dimensional matrix. Eur J Cell Biol. 1992 Feb;57(1):101–108. [PubMed] [Google Scholar]
- Qwarnström E. E., MacFarlane S. A., Page R. C., Dower S. K. Interleukin 1 beta induces rapid phosphorylation and redistribution of talin: a possible mechanism for modulation of fibroblast focal adhesion. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1232–1236. doi: 10.1073/pnas.88.4.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qwarnström E. E., MacFarlane S. A., Page R. C. Effects of interleukin-1 on fibroblast extracellular matrix, using a 3-dimensional culture system. J Cell Physiol. 1989 Jun;139(3):501–508. doi: 10.1002/jcp.1041390308. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Dower S. K., Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. 1989 Jan 20;243(4889):393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- Ratcliffe A., Tyler J. A., Hardingham T. E. Articular cartilage cultured with interleukin 1. Increased release of link protein, hyaluronate-binding region and other proteoglycan fragments. Biochem J. 1986 Sep 1;238(2):571–580. doi: 10.1042/bj2380571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Meyers C. A., Wideman J., Blacher R., Pan Y. C., Stein S., Lehrman S. R., Smith J. M., Lamb L. C. Purification and properties of a type beta transforming growth factor from bovine kidney. Biochemistry. 1983 Dec 6;22(25):5692–5698. doi: 10.1021/bi00294a002. [DOI] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Saklatvala J. Interleukin 1: purification and biochemical aspects of its action on cartilage. J Rheumatol. 1987 May;14(Spec No):52–54. [PubMed] [Google Scholar]
- Saklatvala J., Sarsfield S. J., Townsend Y. Pig interleukin 1. Purification of two immunologically different leukocyte proteins that cause cartilage resorption, lymphocyte activation, and fever. J Exp Med. 1985 Oct 1;162(4):1208–1222. doi: 10.1084/jem.162.4.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saus J., Quinones S., Otani Y., Nagase H., Harris E. D., Jr, Kurkinen M. The complete primary structure of human matrix metalloproteinase-3. Identity with stromelysin. J Biol Chem. 1988 May 15;263(14):6742–6745. [PubMed] [Google Scholar]
- Schmidt G., Robenek H., Harrach B., Glössl J., Nolte V., Hörmann H., Richter H., Kresse H. Interaction of small dermatan sulfate proteoglycan from fibroblasts with fibronectin. J Cell Biol. 1987 Jun;104(6):1683–1691. doi: 10.1083/jcb.104.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönherr E., Järveläinen H. T., Sandell L. J., Wight T. N. Effects of platelet-derived growth factor and transforming growth factor-beta 1 on the synthesis of a large versican-like chondroitin sulfate proteoglycan by arterial smooth muscle cells. J Biol Chem. 1991 Sep 15;266(26):17640–17647. [PubMed] [Google Scholar]
- Seckinger P., Yaron I., Meyer F. A., Yaron M., Dayer J. M. Modulation of the effects of interleukin-1 on glycosaminoglycan synthesis by the urine-derived interleukin-1 inhibitor, but not by interleukin-6. Arthritis Rheum. 1990 Dec;33(12):1807–1814. doi: 10.1002/art.1780331208. [DOI] [PubMed] [Google Scholar]
- Terracio L., Rönnstrand L., Tingström A., Rubin K., Claesson-Welsh L., Funa K., Heldin C. H. Induction of platelet-derived growth factor receptor expression in smooth muscle cells and fibroblasts upon tissue culturing. J Cell Biol. 1988 Nov;107(5):1947–1957. doi: 10.1083/jcb.107.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. A. Articular cartilage cultured with catabolin (pig interleukin 1) synthesizes a decreased number of normal proteoglycan molecules. Biochem J. 1985 May 1;227(3):869–878. doi: 10.1042/bj2270869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. A. Chondrocyte-mediated depletion of articular cartilage proteoglycans in vitro. Biochem J. 1985 Jan 15;225(2):493–507. doi: 10.1042/bj2250493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel K. G., Paulsson M., Heinegård D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984 Nov 1;223(3):587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson A., Uthne K., Westermark B. A novel assay for the biosynthesis of sulphated polysaccharide and its application to studies on the effects of somatomedin on cultured cells. Biochem J. 1973 Dec;136(4):1069–1074. doi: 10.1042/bj1361069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M., Yamada K. M., Yoneda M., Suzuki S., Kimata K. Chondroitin sulfate proteoglycan (PG-M-like proteoglycan) is involved in the binding of hyaluronic acid to cellular fibronectin. J Biol Chem. 1986 Oct 15;261(29):13526–13535. [PubMed] [Google Scholar]
- Yaron I., Meyer F. A., Dayer J. M., Bleiberg I., Yaron M. Some recombinant human cytokines stimulate glycosaminoglycan synthesis in human synovial fibroblast cultures and inhibit it in human articular cartilage cultures. Arthritis Rheum. 1989 Feb;32(2):173–180. doi: 10.1002/anr.1780320210. [DOI] [PubMed] [Google Scholar]
- Yaron I., Meyer F. A., Dayer J. M., Yaron M. Human recombinant interleukin-1 beta stimulates glycosaminoglycan production in human synovial fibroblast cultures. Arthritis Rheum. 1987 Apr;30(4):424–430. doi: 10.1002/art.1780300410. [DOI] [PubMed] [Google Scholar]
- Zimmermann D. R., Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989 Oct;8(10):2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]