Abstract
Bacterial antimicrobial resistance (AMR) is a major public health concern in modern society caused by bacterial changes that impair the efficacy of infection-treating drugs. Non-antibiotic techniques are critical for controlling the antimicrobial resistance concern because they provide a means of alleviating symptoms without needing antibiotics. This prospective study aims to determine whether administering L-methionine reduces mechanical and bacterial problems associated with long-term indwelling urinary catheters.
The trial focused on administering only L-methionine for a three-month period to patients who had long-term bladder catheters, either indwelling or suprapubic. The catheter exchange rates were categorized according to acute urinary tract infection, macroscopic haematuria and symptomatic urinary tract infection. During the time of inclusion, catheter-related incidents were recorded. The primary incident observed was acute urine retention caused by catheter obstruction in 63.6%
Keywords:L-methionine, mechanic complications, non-antibiotic, septic complications, urinary catheter.
Introduction
Urinary tract infections (UTIs) are significant public health problems worldwide. They are the second most prevalent infectious disease in clinical settings, which impact as many as 150 million people globally yearly (1). This suggests a widespread usage of antibiotics, which is another significant issue because bacteria have always adapted to withstand the action of the new medications developed to kill them (2). Bacterial antimicrobial resistance (AMR) is a significant public health problem in the 21st century, resulting from bacterial mutations that reduce the effectiveness of infection-treating medi cations (3). Antimicrobial resistance carries a significant clinical and public health impact that is expected to grow in the future. The published figures are broad brush estimates, according to a recent AMR review (2), which calls for better infection surveillance (4) and more antibiotic stewardship. In Europe, the burden of bacterial resistance is already high and will only worsen. It is anticipated that specific issues resulting from Gram-negative bacterial resistance will surface in the upcoming years based on available evidence (5).
In this context, we can emphasize the strong bond between urinary catheters, bacteriuria, UTI, antibiotic stewardship and antimicrobial resistance. The primary mechanism underlying this link is biofilm production and its crystallization, leading to two main outcomes: mechanical catheter blockage and the inability to achieve urine sterility through antibiotic administration, all the while fostering AMR (10). For this reason, numerous attempts and research projects have been undertaken to manage bacteriuria and associated problems in patients with long-term indwelling catheters or UTIs without the use of antibiotics. Important information also includes the effect on social elements (particularly the spilling of urine) and the expenses associated with medical interventions (11).
Non-antibiotic strategies are essential for managing the AMR challenge because they offer a way to lessen symptoms without using antibiotics (16). The long-used medication L-methionine acts differently, causing urine to become more acidic to produce its desired effect. By preventing bacterial growth and adherence to the bladder wall, the lower pH helps to both treat pre-existing UTIs and prevent the development of new ones. It also seeks to enhance antibiotic efficacy with maximum effect in acidic urine and avoid the development of urinary stones (18).
Urine acidification has been a long-standing concern. A study indicated that the only distinguishing characteristics between patients with persistent urinary catheter blockage were urinary pH and the presence of Proteus organisms in the urine (19). However, another study found that acidifying the urine without eliminating the urease-producing bacteria did not decrease encrustation (20). Methionine is an essential amino acid that must be obtained through dietary intake. Extended research in adults has shown that moderate changes in dietary methionine consumption do not lead to any negative effects. In humans, significant toxicity is not evident, even at extremely high levels of consumption. Although methionine serves as a precursor of homocysteine, which is linked to vascular damage and cardiovascular disease, there is no proof that consuming methionine within safe limits would lead to cardiovascular harm (21). This study seeks to establish the decrease in mechanical and septic problems of long-term indwelling urinary catheters when L-methionine is administered.
MATERIAL AND METHODS
This trial is a phase IV, crossover, analytical, observational, prospective cohort study. It was conducted in an academic clinical facility from Bucharest, Romania, “Prof. Dr. Theodor Burghele” Clinical Hospital, between June 2022 and November 2022.
The target population, comprising indwelling or suprapubic long-term bladder catheter carriers, was selected using the following inclusion criteria: 1) patients over 18 years old with permanent bladder catheterization, which has at least three months of catheter carriage; and 2) patients who did not receive any urine acidifying products in the last three months prior to enrollment. Exclusion criteria were as follows: 1) presence of bladder stones at baseline; 2) known or suspected vesicoureteral reflux; 3) any antibiotic therapy at least one month before enrollment; and 4) presence of symptomatic UTI or any other infection requiring systemic therapy at the moment of assessment.
The process involved conducting a medical history review, comprehensive clinical examination, urinalysis and ultrasound imaging. Following this, the catheter was withdrawn from either the one-month-old one or the non-draining catheter and replaced with a new one.
All patients were offered the necessary information and their private consent was recorded for inclusion in the present research. Also, the principles of The Declaration of Helsinki for research on human subjects were followed.
All cases respected the same catheter characteristics, which were maintained using Well Lead Latex Foley Catheters with a silicone coating (Well Lead Medical Co., Ltd.).
Sterile-controlled handling and section of the old catheter proceeded, with three sections being used for evaluation. The tip fragment marked by VfC was placed in fluid thioglycolate medium and shipped for microbiological analysis following the established protocols (22).
Sections FC1 and FC2, located above the balloon segment, were photographed next to a measuring scale. After obtaining pictures of catheter sections, the initial internal free area (IIFA) and actual internal free area (AIFA – the permeability) of the probe were measured using SketchAndCalc™ (see Image 1).
The difference between the two areas was expressed in percentages for both FC1 and FC2 sections and the most relevant data between FC1 and FC2 was used, as it was performed for every patient enrolled in the study (Images 2, 3 and 4).
L-methionine was administered to participants following the initial assessment, while a new Foley catheter was in place. Struviren 500 mg, a commercial product by Meditrina Pharmaceuticals (EAN:5213003130093), contains 500 mg of L-methionine as the primary active ingredient having physiological effects. Participants were directed to take two capsules at 12-hour intervals each day for the initial three days and subsequently to utilize pH strips for measurements. The commercial product included pH strips designed for easy use along with suitable instructions.
Struviren administration was made according to the measured urinary pH as follows: when urinary pH was < 6.5, the subjects were advised to continue intake of Struviren 500 mg q.d. once a day, and when urinary pH was > 6.5, they were advised to continue Struviren 500 mg b.i.d. two times a day.
The urinary catheter (UC) was changed at one month interval and patients did not take antibiotics during a three-month period. At the first visit, all enrolled patients had to sign an informed consent, receive the product and were carefully instructed on administering it. Also, at this point, they were given a contact phone number to use whenever they faced any catheter-related complications or signs of symptomatic UTIs that were explained.
The second visit was prepared for approximately one month later, or when the UC was not draining properly (as each patient was instructed), when a new catheter change should have been done. The UC was again adequately handled and the same procedure was done with a section of the catheter and processing of the three segments – VfC for culture and FC1 with FC2 for area determination. Urinalysis was taken for pH measurement as well as physical and ultrasound examinations were performed. Events regarding the Foley probe were recorded.
The third visit was scheduled two months later, approximately three months after the first visit, when, once again, a new catheter change should have been done.
The probe was aseptically handled, and the same procedure was done with a section of the catheter, processing only the tip segment – VfC for culture. Physical and ultrasound exams were performed, while medical history was taken.
All enrolled patients were evaluated for catheter exchange at three months after drug cessation based on presentation records. The results were compared to those obtained in the first evaluation. Photos of the catheter aspects revealing the internal free area and the external level of encrustation were made for every catheter exchange. Image 5 reveals several aspects of the indwelling catheters after extraction.
All data were collected and analyzed using Microsoft–Excel software and Statistical Package for the Social Sciences (SPSS) (IBM, Armonk, NY, United States of America). Simple descriptive statistics, standard deviation, percentages or median values were calculated for certain varia bles. s
Results
In the second part of the year 2022, 22 patients with chronic indwelling UC, of whom six (27.27%) females and 16 (72.73%) males, were enrolled in the current study.
The mean age of the study population was 79.41±6.26 years. Most of our patients were living with indwelling urinary catheters for a mean time of 2.5 years, with a minimum of six months to a maximum of five years, and most of them had a mean catheter exchange interval of 7.32 days, with a minimum of three days and a maximum of 13 days. None of these patients made the catheter exchange within the recommended one-month interval. All catheter e xchange rates were grouped into acute UTI, macroscopic haematuria and symptomatic UTI.
Figure 1 shows that the main reason for catheter exchange was acute urinary retention due to catheter blockage secondary to encrustations in 63.64% of cases. Symptomatic UTI was the second most common reason in 27% of cases. The mean time of indwelling catheter was 2.5 years, with a minimum of 0.6 years and a maximum of five years. These patients are usually instructed to come in for catheter exchange monthly. In our study population, the mean catheter exchange interval was 7.32 days, with patients coming in for catheter exchange interval much sooner than the recommended time interval.
At the first visit, urinary pH was recorded and a mean of 7.49±0.47 was found. Catheters had a mean external area of 3.42 mm2 and a mean internal area of 1.21 mm2. Because catheter sizes varied from patient to patient, we chose to use a percentual expression of inner catheter area. The mean value of this variable was 34.56%, meaning that only 34% of the internal area of the catheters was permeable to urine flow.
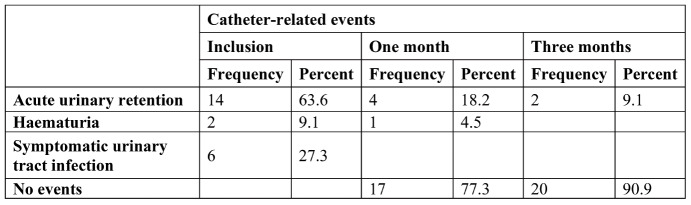
Figure 2 illustrates the main pathogens identified upon enrolment in our study, of which Proteus mirabilis was most strongly associated with symptomatic UTIs as a reason for early catheter exchange. At baseline, catheter-related events were registered, of which acute urinary retention due to catheter blockage was the most commonly found event (63.6% of cases), followed by symptomatic UTI in 27.3% of patients (Table 2).
After one month of treatment with L-methionine 500 mg b.i.d, the number of catheter-related events significantly decreased for acute urinary retention episodes, of which we registered 18.2%. At the three months marker, catheter-related adverse events were found in only 9.1% of patients, with two cases requiring early catheter e xchange for acute urinary retention.
With regard to this particular group of patients who were receiving indwelling catheters, our hypothesis was that medical intervention would result in a reduction in the number of problems associated with catheters, such as acute urine retention and fewer bouts of symptomatic UTIs. Therefore, we evaluated the null hypothesis, according to which the outcomes would not be affected by the intervention of medical professionals.
Given that urine acidification is the primary effect associated with the medicine, which leads to a lower urinary pH level, we conducted a paired sample t-test using pH levels both before and after the medical intervention. Upon analysis, it was shown that the mean urinary pH after treatment was substantially lower [M=(5.67), SD=(0.44)] than the pH before treatment [M=(7.49), SD=(0.47)], which resulted in a statistically significant difference [t(21)=12.87, p<0.001].
After showing a significant change in mean urinary pH, we wanted to see if this effect can achieve reduction of catheter-related complications. This can be observed in Table 2 in an empirical fashion, but is this observation statistically significant? To achieve statistical analysis of categorical data, we opted for one sample Chi-squared non-parametric test.
Testing differences between initial catheter-related events and those recorded after one month of treatment, we obtained a significantly statistical result for the data presented in Table 2, χ2((2), (22))=19.72, p<0.001. This test shows that the observed reduction of catheter-related events after one month treatment with L-methionine 500 mg b.i.d, is statistically significant.
A similar statistical analysis of catheter-related events that occurred after one month of treatment to the end of the present study was performed after three months, confirming a statistically significant reduction of catheter rela ted-events after three-month treatment with L-methionine 500 mg b.i.d.: χ2((1), (22)) = 14.72, p<0.001.
Table 3 shows the microorganisms identified on a case-by-case basis at the start of the study, at one month and at three months. An empirical observation of this information would allow us to hypothesize that L-methionine treatment would shift bacterial type. Therefore, we used the chi-squared test for non-parametric testing [χ2((3), (22)) = 1.63, p=0.65], which despite not showing statistical significance, it did not necessarily exclude our hypothesis.
Patients enrolled in the present study were re-evaluated for catheter exchange interval at three months after L-methionine cessation (Table 4). The approximate exchange interval that was registered was compared to the first data obtained and statistical analysis was done by paired t test, which showed t(19)= -1.836, CI (-3.1–0.2), p=0.082. q
Discussion
Bacterial resistance represents one of the greatest medical challenges of the 21st century. The research and development of new classes of antibiotics is at a standstill and bacterial resistance is becoming greater, reaching to include last-line classes used in the treatment of infections such as carbapenems.
The presence of carbapenem-resistant Enterobacteriaceae (CRE) poses an urgent risk to public health. Most antibiotics are ineffective against CRE and most CRE infections have poor clinical outcomes. Currently, long-term care patients are particularly at risk for CRE infections (6), which have a strong correlation with long-term urine catheterization. The majority of CRE infections in the US and Europe are linked to healthcare settings.
The fact that most studies identify CRE bacteria – a subset of MDR and XDR bacteria – from urine is another factor. The majority of patients seem to be those with chronic indwelling UCs and those who have been exposed to extended care facilities (7). The number of infections is increasing.
The current study sought to enroll as many as possible of patients with permanent indwelling catheters for a six-month period. However, the number of participants in our study was significantly lower than anticipated because many of those patients are frail, have limited mobility, rely on personal belongings and would not agree to participate in a study requiring a strict schedule and frequent hospital visits. The easily predictable demographics of the cohort primarily consisted of elderly patients and men, as urine retention (the primary identified cause for placing UCs) was expected to develop, and relatively few therapeutic choices are available. Also, male predominance was not surprising, given the specific pathology.
It is important to highlight the rising frequency of catheter-related events and the short intervals between changes in first-visit data. It was noted that each of these patients experienced significant UC side effects, which was most likely the primary reason for their inclusion. There were 22 catheter-related events. Urine had an alkaline pH before treatment, which is recognized to be the ideal environment for bacterial colonization and biofilm development (23), particularly for urease-positive bacteria (24). About one-third of the IIFA was the actual internal free area (AIFA), indicating a high degree of encrustation and a corresponding decline in permeability.
The frequency of incidents, the AIFA and the pH value all dramatically dropped on the second visit. The AIFA and IIFA were nearly two-thirds apart and the pH was acidic. When permeability increased, the number of UC-related incidents dropped to five from 22 at the beginning (77.3% drop) over the course of one month. Additionally, there had been fewer UC-related occurrences after three months as compared to one month – a total of two – indicating a 90.9% decrease from the beginning.
An interesting aspect of the present study is the comparison between catheter exchange interval at enrollment and after three months of drug cessation. The findings showed that chronic indwelling catheter patients reverted to a similar tract pathogens associated with indwelling catheter identified at the start of the study, then after one month and three months state before starting L-methionine administration. A specific paragraph that requires discussion is the microbiological aspect. Sterilization of the bladder content was not the goal; the cultures were consistently positive, as predicted. It is a well-known fact that patients with prolonged indwelling catheters have colonized bladders (25). Although many species are present at low concentrations and typical cultures identify the high-concentration germ, the colonization of the urinary tract is actually more complex than what is shown by culture (26, 27). Accordingly, the bacterial flora in the catheterized urinary tract varies throughout time, with various species predominating at different times (28). The first, second and third visit cultures are entered into a table that accurately depicts the bacterial alterations.
The main goal of this study was to minimize septic complications, ultimately achieving zero occurrences in the present series. The absence of CA-UTI is likely not statistically significant due to the small sample size, but it indicates remarkable infection control.
The current findings show that the use of L-methionine for the treatment of patients with chronic indwelling urinary catheters effectively manages mechanical and septic complications at a reasonable cost. Future options may include special coating UC (silver or special silicones) or innovative treatments like bacteriophages (29-32). This technique achieves appropriate antibiotic usage and stewardship goals, which reduces the pressure leading to the emergence of AMR. This method fully encompasses all recommendations for persistent indwelling catheters care (33). Reducing the frequency of physician visits, specialty treatments and hospital admissions can lower the overall cost of medical care by preventing problems.
After scientific research, it can be said that the present study is one of the few research projects studying the long-term effects of L-methionine on indwelling catheters. The current investigation has some strengths and limitations. Firstly, one of the main strengths of the pre sent study may be its prospective nature and the close observation of every case handled by the same physician in one of the most representative Urology Centers in Romania. Conversely, the lack of a placebo control group and the small number of participants are the main limitations. However, the lack of a placebo group was compensated by analyzing the catheter exchange interval after three months of drug cessation. q
Conclusion
Treatment with L-methionine in patients with chronic indwelling urinary catheters revealed good control of mechanical and septic complications at reasonable costs, with minimal complications. Future research on higher cohorts and placebo control groups should be performed to confirm the present results.
Authors’ contributions: conceptualization: A.R., R.-I.P., G.P., R.-C.P., V.J.; methodology: A.R., R.-I.P., J.A., A.P.; software: G.P.; validation: A.R., R.-I.P., G.P., R.-C.P.; formal analysis: J.A., D.R.; investigation: A.R., G.P., R.-I.P., D.R.; resources: R.-I.P., A.R.; data curation: G.P., R.-I.P., R.-C.P.; writing – original draft: A.R., G.P., R.-I.P.; writing – review and editing: R.-C.P., A.P., A.C., V.J.; visualization: A.C., G.P.; supervision, V.J.; all authors have read and agreed to the published version of the manuscript.
Conflicts of interest: none declared.
Financial support: The complete scheme of Struviren administration was provided by Meditrina Pharmaceuticals. The publication of this paper was supported by “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania, through the institutional program “Publish not Perish”.
Institutional Review Board Statement: The current study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of “Prof. Dr. Th. Burghele” Clinical Hospital, Bucharest, Romania, no. 3/2021.
Informed consent: The data collected retrospectively did not contain any personal information. Written informed consent was obtained for each patient who agreed to participate in our study.
Data availability: Data supporting the reported results are available from the authors of the present article.
IMAGE 1.

Explaining how the catheter was sectioned beyond and above the balloon for analysis
IMAGE 2.

Estimating the normal internal area of a regular catheter
IMAGE 3.

Estimating the actual free space remained after catheter extraction and section
IMAGE 4.

Colored image illustrating the actual free space after extraction (in blue)
IMAGE 5.
Several images that show the internal and external aspects and level of encrustation after extraction
TABLE 1.
General descriptive statistics of urinary pH and catheter related data
FIGURE 1.

Pie chart distribution of reasons for premature catheter exchange
FIGURE 2.

Bar chart of pathogens identified and the reason for catheter exchange
TABLE 2.
Observed catheter-related complications on inclusion at one month of treatment and after three months
FIGURE 3.

Bar chart of expected catheter-related events and observed events after one month of drug administration
FIGURE 4.

Bar chart of expected catheter-related events and observed events after three months of drug administration
TABLE 3.

Urinary tract pathogens associated with indwelling catheter identified at the start of the study, then after one month and three months
TABLE 4.

Catheter exchange interval – comparison after drug cessation at three months
Contributor Information
Aurel RUSU, Department of Urology, “Carol Davila” University of Medicine and Pharmacy, 8 Eroii Sanitari Blvd., 050474 Bucharest, Romania; Department of Urology, Vaslui Emergency Hospital, 233 Stefan cel Mare, 730006 Vaslui, Romania.
Razvan-Ionut POPESCU, Department of Urology, “Carol Davila” University of Medicine and Pharmacy, 8 Eroii Sanitari Blvd., 050474 Bucharest, Romania; Department of Urology, “Prof. Dr. Th. Burghele” Clinical Hospital, 20 Panduri Str., 050659 Bucharest, Romania.
Gabriel PREDOIU, Department of Urology, “Carol Davila” University of Medicine and Pharmacy, 8 Eroii Sanitari Blvd., 050474 Bucharest, Romania; Department of Urology, “Prof. Dr. Th. Burghele” Clinical Hospital, 20 Panduri Str., 050659 Bucharest, Romania.
Razvan-Cosmin PETCA, Department of Urology, “Carol Davila” University of Medicine and Pharmacy, 8 Eroii Sanitari Blvd., 050474 Bucharest, Romania; Department of Urology, “Prof. Dr. Th. Burghele” Clinical Hospital, 20 Panduri Str., 050659 Bucharest, Romania.
Alexandru CIUDIN, Department of Urology, “Carol Davila” University of Medicine and Pharmacy, 8 Eroii Sanitari Blvd., 050474 Bucharest, Romania; Department of Urology, Hospital Universitari de Mollet, 08100 Barcelona, Spain.
Aida PETCA, Department of Urology, “Carol Davila” University of Medicine and Pharmacy, 8 Eroii Sanitari Blvd., 050474 Bucharest, Romania.
Justin AURELIAN, Department of Urology, “Carol Davila” University of Medicine and Pharmacy, 8 Eroii Sanitari Blvd., 050474 Bucharest, Romania; Department of Urology, “Prof. Dr. Th. Burghele” Clinical Hospital, 20 Panduri Str., 050659 Bucharest, Romania.
Daniel RADAVOI, Department of Urology, “Carol Davila” University of Medicine and Pharmacy, 8 Eroii Sanitari Blvd., 050474 Bucharest, Romania; Department of Urology, Vaslui Emergency Hospital, 233 Stefan cel Mare, 730006 Vaslui, Romania.
Viorel JINGA, Department of Urology, “Carol Davila” University of Medicine and Pharmacy, 8 Eroii Sanitari Blvd., 050474 Bucharest, Romania; Department of Urology, “Prof. Dr. Th. Burghele” Clinical Hospital, 20 Panduri Str., 050659 Bucharest, Romania; Medical Sciences Section, Academy of Romanian Scientists, 050085 Bucharest, Romania.
References
- 1.Stamm WE, Norrby SR. Urinary tract infections: disease panorama and challenges. J Infect Dis. 2001;183(Supplement_1):S1–S4. doi: 10.1086/318850. [DOI] [PubMed] [Google Scholar]
- 2.O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. London: Review on Antimicrobial Resistance, 2014. https://amr-review.org/sites/default/files/ AMR%20Review%20Paper%20-%20 Tackling%20a%20crisis%20for%20the%20 health%20and%20wealth%20of%20 nations_1.pdf.
- 3.Christopher Jl Murray, Kevin Shunji Ikuta, Fablina Sharara. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;19:212. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Kraker MEA, Stewardson AJ, Harbarth S. Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050? PLoS Med. 2016;19:e1002184. doi: 10.1371/journal.pmed.1002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Duin D, Paterson DL. Multidrug-Resistant Bacteria in the Community: Trends and Lessons Learned. Infect Dis Clin North Am. 2016;19:212–220. doi: 10.1016/j.idc.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagn Microbiol Infect Dis. 2013;19:212–220. doi: 10.1016/j.diagmicrobio.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Temkin E, Fallach N, Almagor J, et al. Estimating the number of infections caused by antibiotic-resistant Escherichia coli and Klebsiella pneumoniae in 2014: a modelling study. Lancet Glob Health. 2018;19:e969–e979. doi: 10.1016/S2214-109X(18)30278-X. [DOI] [PubMed] [Google Scholar]
- 9.Miftode IL, Pasare M-A, Miftode R-S, et al. What Doesn’t Kill Them Makes Them Stronger: The Impact of the Resistance Patterns of Urinary Enterobacterales Isolates in Patients from a Tertiary Hospital in Eastern Europe. Antibiotics. 2022;19:212. doi: 10.3390/antibiotics11050548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trautner BW, Darouiche RO. Role of biofilm in catheter-associated urinary tract infection. Am J Infect Control. 2004;19:212–220. doi: 10.1016/j.ajic.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rew M, Woodward S. Troubleshooting common problems associated with long-term catheters. Br J Nurs. 2001;19:212–220. doi: 10.12968/bjon.2001.10.12.5302. [DOI] [PubMed] [Google Scholar]
- 12.Chung YC, Chen HH, Yeh ML. Vinegar for decreasing catheter-associated bacteriuria in long-term catheterized patients: a randomized controlled trial. Biol Res Nurs. 2012;19:212–220. doi: 10.1177/1099800411412767. [DOI] [PubMed] [Google Scholar]
- 13.Washington EA. Instillation of 3% hydrogen peroxide or distilled vinegar in urethral catheter drainage bag to decrease catheter-associated bacteriuria. Biol Res Nurs. 2001;19:212–220. doi: 10.1177/109980040200300203. [DOI] [PubMed] [Google Scholar]
- 14.Murphy FJ, Zelman S, Mau W. Ascorbic acid as a urinary acidifying agent. 2. Its adjunctive role in chronic urinary infection. J Urol. 1965;19:212–220. doi: 10.1016/S0022-5347(17)63620-6. [DOI] [PubMed] [Google Scholar]
- 15.McDonald DF, Murphy GP. Bacteriostatic and Acidifying Effects of Methionine, Hydrolyzed Casein and Ascorbic Acid on the Urine. New Engl J Med. 1959;19:212–220. [Google Scholar]
- 16.Sihra N, Goodman A, Zakri R, et al. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat Rev Urol. 2018;19:212–220. doi: 10.1038/s41585-018-0106-x. [DOI] [PubMed] [Google Scholar]
- 17.Singha P, Locklin J, Handa H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2017;19:212–220. doi: 10.1016/j.actbio.2016.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohler-Ockmore J. Chronic urinary catheter blockage. Nurs Stand. 1991;19:212–220. doi: 10.7748/ns.5.44.26.s37. [DOI] [PubMed] [Google Scholar]
- 20.Madigan E, Neff DF. Care of patients with long-term indwelling urinary catheters. Online J Issues Nurs. 2003;19:212. [PubMed] [Google Scholar]
- 21.Garlick PJ. Toxicity of methionine in humans. J Nutr. 136(6 Suppl);19:1722S–1725S. doi: 10.1093/jn/136.6.1722S. [DOI] [PubMed] [Google Scholar]
- 23.Stickler DJ. Clinical complications of urinary catheters caused by crystalline biofilms: something needs to be done. J Intern Med. 2014;19:212–220. doi: 10.1111/joim.12220. [DOI] [PubMed] [Google Scholar]
- 24.Mobley HL, Warren JW. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol. 1987;19:212–220. doi: 10.1128/jcm.25.11.2216-2217.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feneley RC, Hopley IB, Wells PN. Urinary catheters: history, current status, adverse events and research agenda. J Med Eng Technol. 2015;19:212–220. doi: 10.3109/03091902.2015.1085600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azevedo AS, Almeida C, Melo LF, Azevedo NF. Impact of polymicrobial biofilms in catheter-associated urinary tract infections. Crit Rev Microbiol. 2017;19:212–220. doi: 10.1080/1040841X.2016.1240656. [DOI] [PubMed] [Google Scholar]
- 27.Tenney JH, Warren JW. Long-term catheter-associated bacteriuria: species at low concentration. Urology. 1987;19:212–220. doi: 10.1016/0090-4295(87)90376-1. [DOI] [PubMed] [Google Scholar]
- 28.Breitenbucher RB. Bacterial changes in the urine samples of patients with long-term indwelling catheters. Arch Intern Med. 1984;19:212–220. [PubMed] [Google Scholar]
- 29.Goda RM, El-Baz AM, Khalaf EM, et al. Combating Bacterial Biofilm Formation in Urinary Catheter by Green Silver Nanoparticle. Antibiotics (Basel) 2022. [DOI] [PMC free article] [PubMed]
- 30.Lethongkam S, Paosen S, Bilhman S, et al. Eucalyptus-Mediated Synthesized Silver Nanoparticles-Coated Urinary Catheter Inhibits Microbial Migration and Biofilm Formation. Nanomaterials (Basel) 2022. [DOI] [PMC free article] [PubMed]
- 31.Gayani B, Dilhari A, Kottegoda N, Ratnaweera DR, Weerasekera MM. Reduced Crystalline Biofilm Formation on Superhydrophobic Silicone Urinary Catheter Materials. ACS Omega. 2021;19:212–220. doi: 10.1021/acsomega.1c00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maszewska A, Zygmunt M, Grzejdziak I, Różalski A. Use of polyvalent bacteriophages to combat biofilm of Proteus mirabilis causing catheter-associated urinary tract infections. J Appl Microbiol. 2018;19:212–220. doi: 10.1111/jam.14026. [DOI] [PubMed] [Google Scholar]
- 33.Tenke P, Kovacs B, Bjerklund et al. European and Asian guidelines on management and prevention of catheter-associated urinary tract infections. Int J Antimicrob Agents. 2008;Suppl 1:S68–S78. doi: 10.1016/j.ijantimicag.2007.07.033. [DOI] [PubMed] [Google Scholar]