Abstract
Pleomorphic adenoma is a non-cancerous neoplasm that develops in the salivary glands. Originating from minor salivary glands, it is extremely uncommon and primarily affects females. The peak incidence is observed between 40 and 60 years of age. It usually presents as a slowly growing, painless, solid tumor that does not cause ulcers on the overlying mucosa. Here, a 47-year-old woman experienced repeated swelling on the buccal mucosa following surgical extraction of a pleomorphic adenoma three years ago. The swelling was solid with clearly defined boundaries. An excisional biopsy was conducted under general anesthesia, resulting in total mass removal. The histological evaluation revealed the existence of a recurring pleomorphic adenoma. This instance emphasizes the significance of addressing this entity as a potential etiology for persistent painless and intraoral swellings.
Keywords:salivary gland neoplasms, minor salivary glands, pleomorphic adenoma, recurrent cheek mass, diagnosis, therapy.
Introduction
Salivary gland neoplasms are a diverse collection of tumors that display various clinical, histological and immunohistochemical characteristics (1). These tumors are uncommon, comprising only 3% to 5% of all neoplastic entities that occur in the head and neck area (2). Out of these, around 64.9% to 67.5% are benign tumors (3, 4). Neoplasms originating from minor salivary glands present in the mucosa of the cheek, lip and tongue are exceptionally uncommon (3%-5%). Most minor salivary gland tumors are cancerous.
Pleomorphic adenomas (PAs) are the most prevalent type of benign tumors of the salivary glands, comprising 33.2% to 68.4% of all reported cases (4-6). The yearly occurrence is roughly 2–3.5 instances per 100,000 individuals. It manifests in individuals across all age groups (7). However, it is predominantly prevalent among females, accounting for 60% of cases, particularly in the age range of 40 to 50 years (4, 5, 8, 9). The occurrence of these tumors is observed in both major salivary glands (65%) and minor salivary glands (35%). For the majority of cases (70-80%), this tumour primarily occurs in the superficial lobe of the parotid gland, while in about 10% of cases it is found in the submandibular salivary gland, and only 1% in the sublingual gland (10). Less commonly affected areas include the cheek mucosa, palate, lip and tongue, which are accounting for 5-10% of cases (2, 4, 5, 8). From a clinical perspective, PA manifests as a gradually developing, painless abnormality, with a solid texture and varying sizes (11, 12), but with clear boundaries. The likelihood of PA developing malignancy is approximately 6%. The precise cause of pleomorphic adenoma is still unknown, although long-term exposure to radiation and the presence of simian virus (SV40) may be significant factors. Previous studies have documented clonal chromosomal abnormalities characterized by aberrations affecting the 8q12 and 12q15 regions (13).
The intraoral PA is typically situated in the submucosa and has a stiff or rubbery texture. The mucosal lining remains untouched, but ulcerations are present in certain instances (11, 12). Within these specific areas inside the mouth, such as the cheek mucosa, lip and tongue, which are prone to injury, PA might exhibit similar clinical characteristics to other reactive inflammatory diseases of the salivary glands as well as noncancerous growth processes. In light of this, we provide a case of recurrent PA in the cheek mucosa after surgical removal of pleomorphic adenoma three years ago.
CASE REPORT
A47-year-old female presented to the outpatient section of the Maxillofacial department of our hospital with a painless swelling in her left cheek that has been present for more than six months (Figure 1). Thorough history and evaluation revealed that the swelling initially appeared as a little mass and has progressively grown in the same area, where she had been operated for a pleomorphic adenoma (PA) of minor salivary glands three years ago. No lymph node involvement was observed during the extraoral physical examination. An intraoral examination revealed a sizable, non-painful, movable mass in the mucosa of the left cheek, measuring approximately 5 cm in diameter. The lesion exhibited clear boundaries, displayed a normal color and texture of the overlying mucosa. Furthermore, there was inability to open the mouth and generally there was malfunction to the mobility of oromandibular system. The motor and sensory nerve functioning were undamaged. During bimanual palpation, the mass was detectable in the space between the skin of the cheek and the mucosa. There were not accompanying symptoms. There was no previous medical history of hypertension or diabetes, but she was a heavy smoker. There were no reports of head and neck tumors in her family history.
Based on clinical data, the diagnostic possibilities included fibroma, lipoma or inflammatory fibrous hyperplasia due to the region's susceptibility to trauma. However, the possibility of an inflammatory process can be eliminated, as there was no evidence of infection in this area. The differential diagnosis includes mucoepidermoid carcinoma, adenocystic carcinoma, tumor of the accessory parotid salivary gland, epidermoid cyst and dermoid cyst. Initially, we had to consider the possibility of pleomorphic adenoma recurrence based on the patient's medical background. The patient underwent ultrasound and magnetic resonance imaging (MRI) of the head and neck. In the anatomical position of the masseter muscle on the left side, a clearly defined inhomogeneous (with solid and cystic elements), hypoechoic mass with lobular borders and central vascularization on color Doppler, measuring 4 x 4 x 3.6 cm, was seen (Figure 2). No abnormally enlarged lymph nodes and infiltration of adjacent bony structures were identified. The magnetic resonance imaging revealed a clearly delineated, moderately-enhancing region located in the left cheek, in contact with the masseter muscle.
Fine needle aspiration (FNA) with local anesthetic indicated the presence of pleomorphic adenoma. Following the essential preoperative assessments, a horizontal incision was performed on the mucous membrane of the cheek, parallel to the occlusal plane, while the patient was under general anesthesia. The parotid duct was observed and circumvented. The lesion was located within the space between the mucosal and the buccinator muscle while diligent hemostasis was performed. It was readily separated from the adjacent tissues during dissection. The mucosa was stitched and the sample was sent for histological examination. The tumour size was 4 × 4 cm (Figures 3 and 4).
The patient's healing and recovery proceeded smoothly, with no indications of recurrence during the one-year follow-up period (Figures 5 and 6). The authors acquired the patient’s explicit written approval to publish this material, adhering to the ethical requirements.
From a histopathological perspective, the tumor mass was clearly surrounded by a well-defined capsule. The epithelial cells exhibited a configuration characterized by a duct-like pattern, cystic formations and anastomosing cords. The specimen exhibited a fragile collagenous framework with regions displaying a gelatinous and cartilaginous composition. Every characteristic indicated the presence of pleomorphic adenoma.
DISCUSSION
The buccal area is highly intricate and encompasses a wide array of components. The differential diagnosis of a buccal space lesion encompasses the possibility of a tumor arising from several tissues, such as glandular, vascular, lymphatic, connective, muscular, ductal and neural structures (14).
Pleomorphic adenoma primarily affects the large salivary glands (4, 9, 12, 15) and is seldom in the minor salivary glands (2, 9, 12, 15, 16). Therefore, the current instance of PA in the minor salivary glands of the cheek mucosa validates the existing literature that indicates a relatively low occurrence in this location (2, 16) compared to other sites like the palate (64%) and less frequently afflicted areas such as the upper lip (13%) (8). The epidemiologic data of PA aligns with the literature, indicating that the gender and age of the current case are consistent with the majority of cases. It is commonly observed that PA in the minor salivary glands predominantly affects women in their forties and fifties, with an average age range of 45-50 years (4, 5, 8).
Fine-needle aspiration cytology (FNAC) is considered the most accurate method for diagnosing PA. Nevertheless, PAs may erroneously be identified as low-grade cancers. This discrepancy is uncommon in the literature. Hence, if there is any doubt, it is advisable to have a guided FNAC procedure, ideally with confirmation from two distinct pathologists. Some locations, such as tiny tumors near the jaw, are challenging to evaluate with FNAC. In such cases, an incisional biopsy may be necessary due to the diverse nature of salivary gland tumors (17).
Pleomorphic adenomas originating from the minor salivary glands exhibit a low likelihood of recurring, with recurrence rates ranging from 2% to 44%. The recurrence of surgical issues is mostly attributed to pseudopodia, capsular penetration, tumour rupture and inadequate surgical technique. It is imperative to perform dissection of salivary gland tumors due to their inherent tendency to develop into malignancies. The initial therapeutic approach should be wide local excision with negative margins (7) and preservation of the capsule (11, 12, 16, 18, 19). It is imperative to choose the appropriate surgical procedure in order to prevent recurrence. Early detection of PA is crucial due to the potential for secondary malignant change into carcinoma ex pleomorphic adenoma, despite the low incidence of the tumour (9, 15, 18, 20).
CONCLUSION
The current findings underscore the need of accurately diagnosing and treating the abnormalities that may impact the oral and maxillofacial areas as well as the need for continual evaluation of cases, especially those identified as PA. Early diagnosis is crucial as it allows appropriate treatment of the lesion and improves the prognosis. Regarding this matter, PA necessitates extended observation to examine potential recurrences and/or progression of the case.q
Conflicts of interest: none declared.
Financial support: none declared.
FIGURE 1.

Painless swelling in the left cheek
FIGURE 2.
A clearly defined hypoechoic, inhomogeneous mass with lobular borders and central vascularization on color Doppler
FIGURE 3.
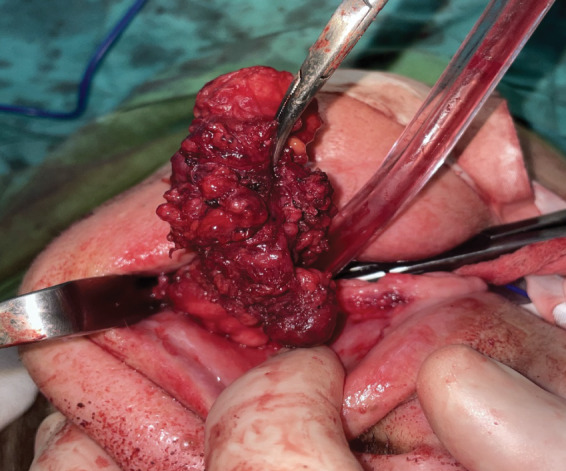
Ιntraoral removal of the tumor en block
FIGURE 4.
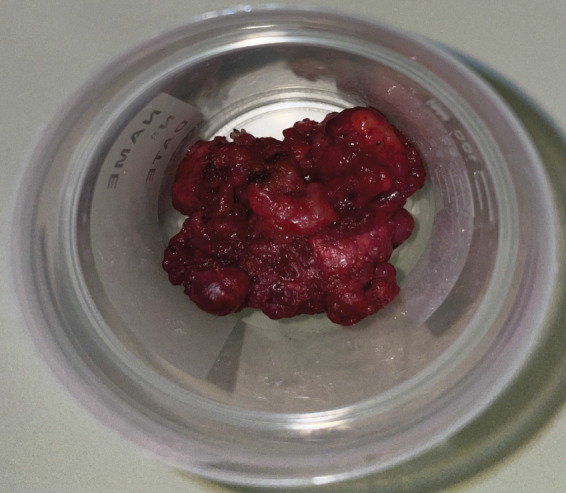
Surgical specimen
FIGURE 5 and 6.
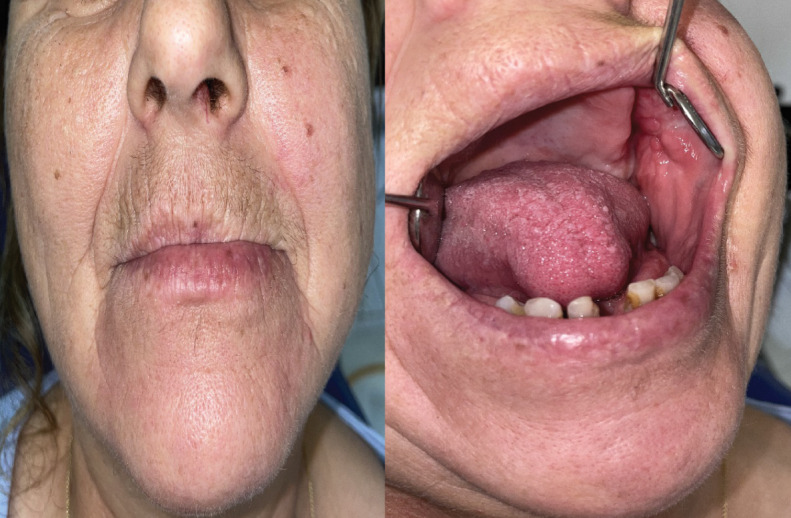
Smooth wound healing and no recurrence one year postoperatively
Contributor Information
Aikaterini D. LIANOU, Department of Otorhinolaryngology, Primary National Health Network, Ioannina Health Unit, Ioannina, Greece
Athina ZARACHI, Department of Otorhinolaryngology, Head and Neck Surgery, Faculty of Medicine, School of Medical Sciences, University of Ioannina, Ioannina, Greece.
Spyridoula DERKA, Maxillofacial Surgeon, Attikon Hospital, Greece.
Chrissa SIOKA, Associate Professor in Nuclear Medicine at University Hospital of Ioannina, Greece.
Napoleon-Georgios RAGKOS, School of Medicine, European University Cyprus, Nicosia, Cyprus.
Angelos LIONTOS, Department of Pathology, Faculty of Medicine, School of Health Sciences, Ioannina, Greece.
Evangelos TSIAMBAS, Department of Cytology, 417 Veterans Army Hospital (NIMTS), Athens, Greece.
Asimakis ASIMAKOPOULOS, Otorhinolaryngology Department, Centre Hospitalier du Valais Romand, Switzerland.
Vasileios RAGOS, Department of Maxillofacial Surgery, Medical School, University of Ioannina, Ioannina, Greece.
References
- 1.Cavalcante RB, Nonaka CFW, Rabenhorst SHB, et al. Pleomorphic adenoma and adenoid cystic carcinoma of salivary glands: E-cadherin immunoexpression and analysis of the CDH1 -160C/A polymorphism. Arch Oral Biol. 2017;19:434–438. doi: 10.1016/j.archoralbio.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Khandekar VS, Dive A, Munde P, Wankhede ND. Pleomorphic adenoma of the buccal salivary gland. J Oral Maxillofac Pathol. 2015;19:434–438. doi: 10.4103/0973-029X.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito FA, Ito K, Vargas PA, et al. Salivary gland tumors in a Brazilian population: a retrospective study of 496 cases. Int J Oral Maxillofac Surg. 2005;19:434–438. doi: 10.1016/j.ijom.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Jones AV, Craig GT, Speight PM, Franklin CD. The range and demographics of salivary gland tumors diagnosed in a UK population. Oral Oncol. 2008;19:434–438. doi: 10.1016/j.oraloncology.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Pires FR, Pringle GA, Paes de Almeida O, Chen S. Intra-oral minor salivary gland tumors:A clinicopathological study of 546 cases. Oral Oncol. 2007;19:434–438. doi: 10.1016/j.oraloncology.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Júnior ECF, Dias IJ, Alves PM, et al. Pleomorphic adenoma of buccal mucosa: a case report and review of the literature. Stomatologija. 2020;19:434–438. [PubMed] [Google Scholar]
- 7.Khan MN, Raza SS, Zaidi SAH, et al. Case report. Pleomorphic adenoma of minor salivary glands. J Ayub Med Coll Abbottabad. 2016;19:434–438. [PubMed] [Google Scholar]
- 8.Abrahão AC, Santos Netto JN, Pires FR, et al. Clinicopathological characteristics of tumours of the intraoral minor salivary glands in 170 Brazilian patients. Br J Oral Maxillofac Surg. 2016;19:434–438. doi: 10.1016/j.bjoms.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 9.Kiciński K, Mikaszewski B, Stankiewicz C. Risk factors for recurrence of pleomorphic adenoma. Otolaryngol Pol. 2016;19:434–438. doi: 10.5604/00306657.1193691. [DOI] [PubMed] [Google Scholar]
- 10.Alves FA, Perez DE, Almeida OP, et al. Pleomorphic adenoma of the submandibular gland: clinicopathological and immunohistochemical features of 60 cases in Brazil. Arch Otolaryngol Head Neck Surg. 2002;19:434–438. doi: 10.1001/archotol.128.12.1400. [DOI] [PubMed] [Google Scholar]
- 11.Rahnama M, Orzędała-Koszel U, Czupkałło L, Lobacz M. Pleomorphic adenoma of the palate: a case report and review of the literature. Contemp Oncol (Pozn) 2013. [DOI] [PMC free article] [PubMed]
- 12.Singh AK, Kumar N, Sharma P, Singh S. Pleomorphic adenoma involving minor salivary glands of upper lip: A rare phenomenon. J Cancer Res Ther. 2015;19:434. doi: 10.4103/0973-1482.148682. [DOI] [PubMed] [Google Scholar]
- 13.Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;19:434–438. doi: 10.1002/hed.2890080309. [DOI] [PubMed] [Google Scholar]
- 14.Gallia L, Rood SR, Myers EN. Management of buccal space masses. Otolaryngol Head Neck Surg. 1981;19:434–438. doi: 10.1177/019459988108900215. [DOI] [PubMed] [Google Scholar]
- 15.Vento SI, Numminen J, Kinnunen I, et al. Pleomorphic adenoma in the nasal cavity: a clinicopathological study of ten cases in Finland. Eur Arch Otorhinolaryngol. 2016;19:434–438. doi: 10.1007/s00405-016-4023-4. [DOI] [PubMed] [Google Scholar]
- 16.Velpula N, Annam SR, Pallepati SR, et al. Pleomorphic adenoma of cheek masquerading as fi brolipoma – Case report with review. J Clin Diagn Res 2015. [DOI] [PMC free article] [PubMed]
- 17.Jose D, Mohiyuddin S, Mohammadi K, et al. Extra-Parotid Pleomorphic Adenoma and Low-Grade Salivary Malignancy in the Head and Neck Region. Cureus. 2023;19:e39463. doi: 10.7759/cureus.39463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goyal P, Sehgal S, Ghosh S, et al. Rare carcinoma ex pleomorphic adenoma of buccal mucosa:case report and review of literature. Rare Tumors. 2016;19:434–438. doi: 10.4081/rt.2016.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laturiya R, Kasim JS, Jankar AS, Mohiuddin SA. Pleomorphic adenoma of minor salivary gland arising de novo in the parapharyngeal space – a rare case report. J Clin Diagn Res 2016. [DOI] [PMC free article] [PubMed]
- 20.Mariano FV, Egal ES, Pramio D, et al. Evaluation of a subset of tumor suppressor gene for copy number and epigenetic changes in pleomorphic adenoma and carcinoma ex-pleomorphic adenoma carcinogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;19:434–438. doi: 10.1016/j.oooo.2016.05.002. [DOI] [PubMed] [Google Scholar]


