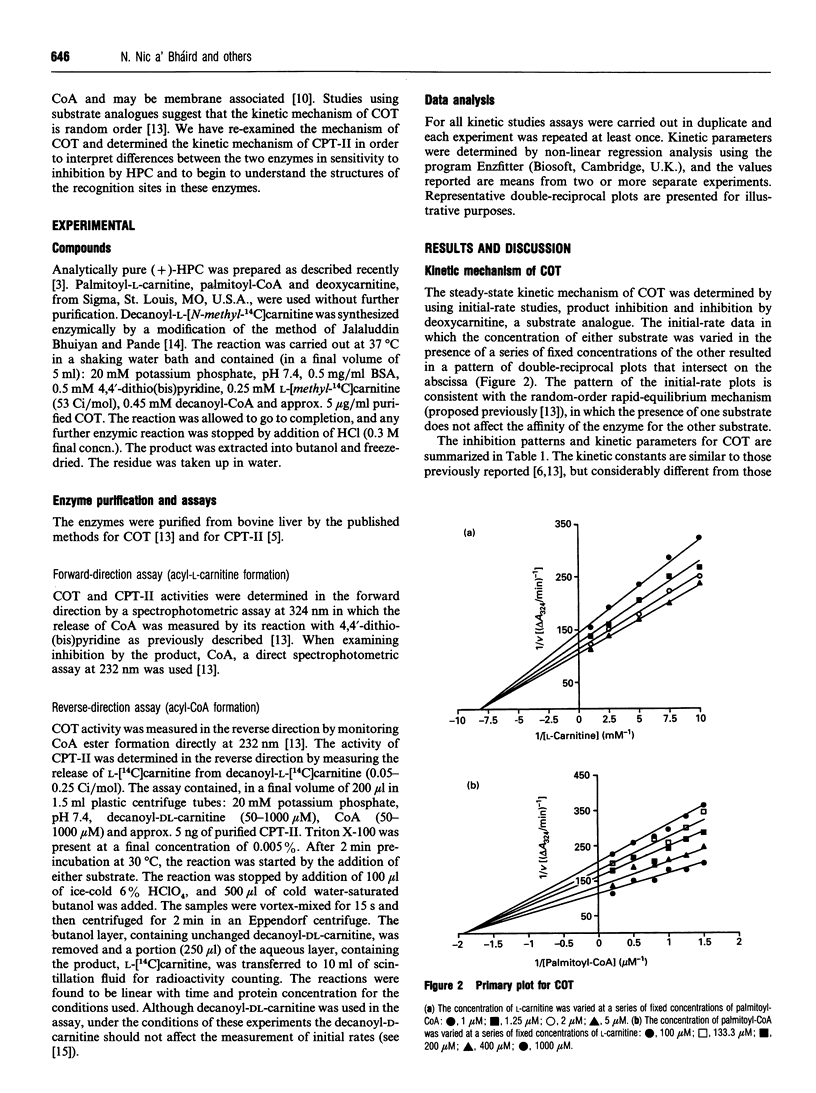
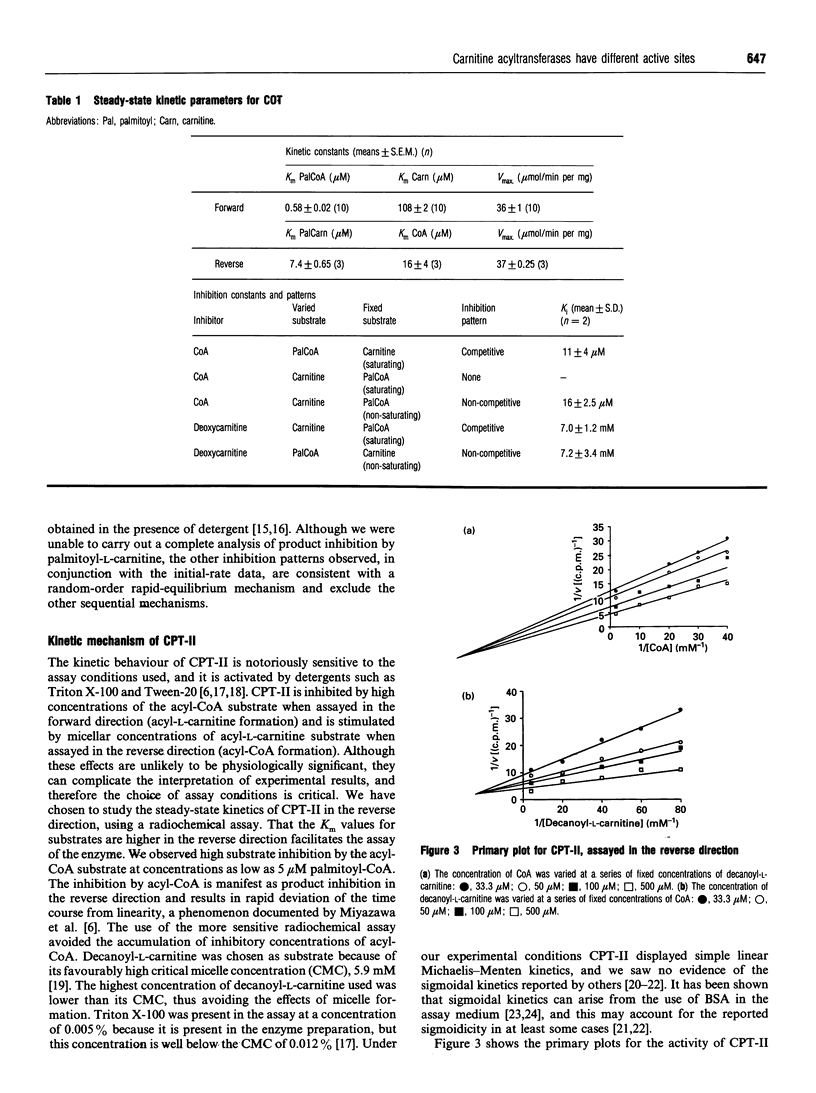
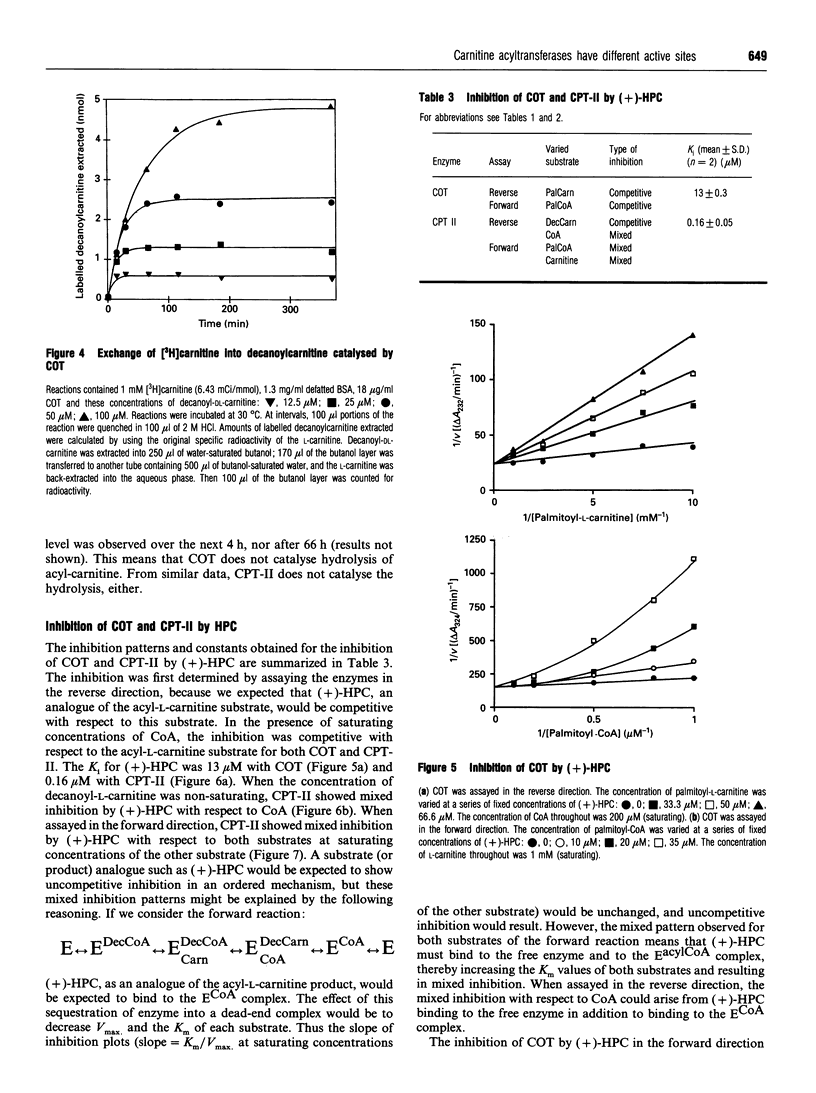
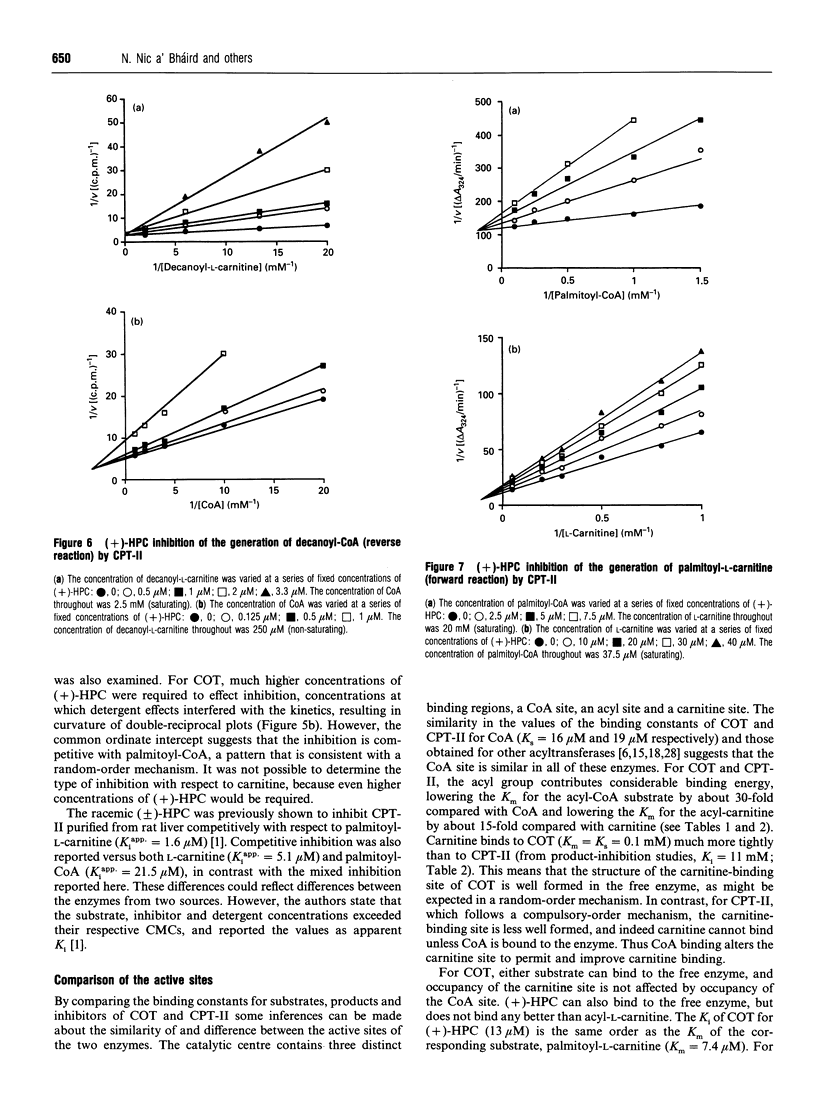
Abstract
The carnitine acyltransferases contribute to the modulation of the acyl-CoA/CoA ratio in various cell compartments with consequent effects on many aspects of fatty acid metabolism. The properties of the enzymes are different in each location. The kinetic mechanisms and kinetic parameters for the carnitine acyltransferases purified from peroxisomes (COT) and from the mitochondrial inner membrane (CPT-II) were determined. Product-inhibition studies established that COT follows a rapid-equilibrium random-order mechanism, but CPT-II follows a strictly ordered mechanism in which acyl-CoA or CoA must bind before the carnitine substrate. Hemipalmitoylcarnitinium [(+)-HPC], a prototype tetrahedral intermediate analogue of the acyltransferase reaction, inhibits CPT-II 100-fold better than COT. (+)-HPC behaves as an analogue of palmitoyl-L-carnitine with COT. In contrast, with CPT-II(+)-HPC binds more tightly to the enzyme than do substrates or products, suggesting that it is a good model for the transition state and, unlike palmitoyl-L-carnitine, (+)-HPC can bind to the free enzyme. The data support the concept of three binding domains for the acyltransferases, a CoA site, an acyl site and a carnitine site. The CoA site is similar in COT and CPT-II, but there are distinct differences between the carnitine-binding site which may dictate the kinetic mechanism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A'Bháird N. N., Ramsay R. R. Malonyl-CoA inhibition of peroxisomal carnitine octanoyltransferase. Biochem J. 1992 Sep 1;286(Pt 2):637–640. doi: 10.1042/bj2860637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom J. D., Reitz R. C. Studies on carnitine palmitoyl transferase: the similar nature of CPTi (inner form) and CPTo (outer form). Arch Biochem Biophys. 1980 Oct 1;204(1):71–79. doi: 10.1016/0003-9861(80)90008-9. [DOI] [PubMed] [Google Scholar]
- Bhuiyan A. K., Pande S. V. One-step synthesis of radioactive acyl-CoA and acylcarnitines using rat liver mitochondrial outer membrane as enzyme source. Lipids. 1992 May;27(5):392–395. doi: 10.1007/BF02536155. [DOI] [PubMed] [Google Scholar]
- Bieber L. L. Carnitine. Annu Rev Biochem. 1988;57:261–283. doi: 10.1146/annurev.bi.57.070188.001401. [DOI] [PubMed] [Google Scholar]
- Bieber L. L., Emaus R., Valkner K., Farrell S. Possible functions of short-chain and medium-chain carnitine acyltransferases. Fed Proc. 1982 Oct;41(12):2858–2862. [PubMed] [Google Scholar]
- Bremer J., Norum K. R. The effects of detergents of palmityl coenzyme A:carnitine palmityltransferase. J Biol Chem. 1967 Apr 25;242(8):1749–1755. [PubMed] [Google Scholar]
- Bremer J., Norum K. R. The mechanism of substrate inhibition of palmityl coenzyme A:carnitine palmityltransferase by palmityl coenzyme A. J Biol Chem. 1967 Apr 25;242(8):1744–1748. [PubMed] [Google Scholar]
- Chase J. F., Tubbs P. K. Some kinetic studies on the mechanism of action of carnitine acetyltransferase. Biochem J. 1966 Apr;99(1):32–40. doi: 10.1042/bj0990032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P. R., Bieber L. L. Effect of micelles on the kinetics of purified beef heart mitochondrial carnitine palmitoyltransferase. J Biol Chem. 1981 Oct 10;256(19):9869–9873. [PubMed] [Google Scholar]
- Clarke P. R., Bieber L. L. Isolation and purification of mitochondrial carnitine octanoyltransferase activities from beef heart. J Biol Chem. 1981 Oct 10;256(19):9861–9868. [PubMed] [Google Scholar]
- Derrick J. P., Ramsay R. R. L-carnitine acyltransferase in intact peroxisomes is inhibited by malonyl-CoA. Biochem J. 1989 Sep 15;262(3):801–806. doi: 10.1042/bj2620801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiol C. J., Bieber L. L. Sigmoid kinetics of purified beef heart mitochondrial carnitine palmitoyltransferase. Effect of pH and malonyl-CoA. J Biol Chem. 1984 Nov 10;259(21):13084–13088. [PubMed] [Google Scholar]
- Fiol C. J., Bieber L. L. Sigmoid kinetics of purified beef heart mitochondrial carnitine palmitoyltransferase. Effect of pH and malonyl-CoA. J Biol Chem. 1984 Nov 10;259(21):13084–13088. [PubMed] [Google Scholar]
- Gandour R. D., Colucci W. J., Stelly T. C., Brady P. S., Brady L. J. Active-site probes of carnitine acyltransferases. Inhibition of carnitine acetyltransferase by hemiacetylcarnitinium, a reaction intermediate analogue. Biochem Biophys Res Commun. 1986 Jul 31;138(2):735–741. doi: 10.1016/s0006-291x(86)80558-7. [DOI] [PubMed] [Google Scholar]
- Gandour R. D., Colucci W. J., Stelly T. C., Brady P. S., Brady L. J. Hemipalmitoylcarnitinium, a strong competitive inhibitor of purified hepatic carnitine palmitoyltransferase. Arch Biochem Biophys. 1988 Dec;267(2):515–520. doi: 10.1016/0003-9861(88)90058-6. [DOI] [PubMed] [Google Scholar]
- Gandour R. D., Leung O. T., Greway A. T., Ramsay R. R., Nic a'Bháird N., Fronczek F. R., Bellard B. M., Kumaravel G. (+)-Hemipalmitoylcarnitinium strongly inhibits carnitine palmitoyltransferase-I in intact mitochondria. J Med Chem. 1993 Jan 22;36(2):237–242. doi: 10.1021/jm00054a007. [DOI] [PubMed] [Google Scholar]
- Ishii S., Ishii H., Watanabe T., Suga T. Purification and properties of peroxisomal carnitine palmitoyltransferase in chick embryo liver. Biochim Biophys Acta. 1987 Jun 22;924(3):530–542. doi: 10.1016/0304-4165(87)90169-3. [DOI] [PubMed] [Google Scholar]
- Kopec B., Fritz I. B. Properties of a purified carnitine palmitoyltransferase, and evidence for the existence of other carnitine acyltransferases. Can J Biochem. 1971 Aug;49(8):941–948. doi: 10.1139/o71-136. [DOI] [PubMed] [Google Scholar]
- Lilly K., Bugaisky G. E., Umeda P. K., Bieber L. L. The medium-chain carnitine acyltransferase activity associated with rat liver microsomes is malonyl-CoA sensitive. Arch Biochem Biophys. 1990 Jul;280(1):167–174. doi: 10.1016/0003-9861(90)90532-4. [DOI] [PubMed] [Google Scholar]
- Miyazawa S., Ozasa H., Osumi T., Hashimoto T. Purification and properties of carnitine octanoyltransferase and carnitine palmitoyltransferase from rat liver. J Biochem. 1983 Aug;94(2):529–542. doi: 10.1093/oxfordjournals.jbchem.a134384. [DOI] [PubMed] [Google Scholar]
- Pauly D. F., McMillin J. B. Importance of acyl-CoA availability in interpretation of carnitine palmitoyltransferase I kinetics. J Biol Chem. 1988 Dec 5;263(34):18160–18167. [PubMed] [Google Scholar]
- Ramsay R. R., Derrick J. P., Friend A. S., Tubbs P. K. Purification and properties of the soluble carnitine palmitoyltransferase from bovine liver mitochondria. Biochem J. 1987 Jun 1;244(2):271–278. doi: 10.1042/bj2440271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay R. R. The soluble carnitine palmitoyltransferase from bovine liver. A comparison with the enzymes from peroxisomes and from the mitochondrial inner membrane. Biochem J. 1988 Jan 1;249(1):239–245. doi: 10.1042/bj2490239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Effects of fasting and malonyl CoA on the kinetics of carnitine palmitoyltransferase and carnitine octanoyltransferase in intact rat liver mitochondria. FEBS Lett. 1981 Sep 28;132(2):166–168. doi: 10.1016/0014-5793(81)81152-0. [DOI] [PubMed] [Google Scholar]
- Smith R. H., Powell G. L. The critical micelle concentration of some physiologically important fatty acyl-coenzyme A's as a function of chain length. Arch Biochem Biophys. 1986 Jan;244(1):357–360. doi: 10.1016/0003-9861(86)90124-4. [DOI] [PubMed] [Google Scholar]
- Stephens T. W., Cook G. A., Harris R. A. Effect of pH on malonyl-CoA inhibition of carnitine palmitoyltransferase I. Biochem J. 1983 May 15;212(2):521–524. doi: 10.1042/bj2120521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldegiorgis G., Bremer J., Shrago E. Substrate inhibition of carnitine palmitoyltransferase by palmitoyl-CoA and activation by phospholipids and proteins. Biochim Biophys Acta. 1985 Nov 14;837(2):135–140. doi: 10.1016/0005-2760(85)90236-x. [DOI] [PubMed] [Google Scholar]


