Abstract
47,XXX (Triple X syndrome) is a sex chromosome aneuploidy characterized by the presence of a supernumerary X chromosome in affected females and is associated with a variable cognitive, behavioral, and psychiatric phenotype. The effect of a supernumerary X chromosome in affected females on intracortical microstructure is currently unknown. Therefore, we conducted 7 Tesla structural MRI and compared T1 (ms), as a proxy for intracortical myelin (ICM), across laminae of 21 adult women with 47,XXX and 22 age-matched typically developing females using laminar analyses. Relationships between phenotypic traits and T1 values in 47,XXX were also investigated. Adults with 47,XXX showed higher bilateral T1 across supragranular laminae in the banks of the superior temporal sulcus, and in the right inferior temporal gyrus, suggesting decreases of ICM primarily within the temporal cortex in 47,XXX. Higher social functioning in 47,XXX was related to larger inferior temporal gyrus ICM content. Our findings indicate an effect of a supernumerary X chromosome in adult-aged women on ICM across supragranular laminae within the temporal cortex. These findings provide insight into the role of X chromosome dosage on ICM across laminae. Future research is warranted to further explore the functional significance of altered ICM across laminae in 47,XXX.
Keywords: 47,XXX; 7 T; intracortical myelin; laminae; T1
Introduction
Triple X syndrome is a relatively common sex chromosome aneuploidy (SCA) characterized by the presence of a supernumerary X chromosome, resulting in a karyotype of 47,XXX in affected females, and has an estimated incidence of about one in 1,000 female newborns (Otter et al. 2010). 47,XXX is not typically associated with facial dysmorphology or distinct physical features and the phenotype is generally mild (Tartaglia et al. 2010). Therefore, it is estimated that only 16% of cases are clinically diagnosed (Viuff et al. 2015). It is hypothesized that overexpression of genes on the X chromosome that escape X-inactivation, as well as incomplete X chromosome inactivation, may result in the phenotypic traits associated with 47,XXX (Nielsen et al. 2020; Raznahan and Disteche 2021). However, minor physical findings can be present in some individuals with 47,XXX including clinodactyly, epicanthal folds, and tall stature, with body segment proportions typically showing a short sitting height and long legs (Tartaglia et al. 2010). Sex hormone levels are usually normal in individuals with 47,XXX (Green et al. 2018; Skuse et al. 2018). Deficits in children and adolescents with 47,XXX have been found in several domains, including motor skills, speech, receptive and expressive language, educational achievement, and interpersonal relationships (Leggett et al. 2010; Lenroot et al. 2014; Urbanus et al. 2021; Capelli et al. 2023). Additionally, women with 47,XXX are at increased risk for developing autism spectrum disorder (ASD) and attention-deficit hyperactivity disorder, as well as other psychiatric disorders including psychotic, anxiety, and depressive disorders (Otter et al. 2010; Green et al. 2018; van Rijn 2019; Berglund et al. 2022; Sánchez et al. 2023). Previous studies in adult women with 47,XXX showed psychiatric disorders in about 50% of the participating cases (Freilinger et al. 2018; Otter et al. 2022). Children and adolescents with 47,XXX often present with a variable cognitive phenotype, including mild learning disabilities and manifestations of executive dysfunction (Tartaglia et al. 2010; van Rijn and Swaab 2015; van Rijn et al. 2016). Lower mean full-scale IQ (FSIQ) has been reported in children, adolescents, and adults with 47,XXX, with the normal curve shifted to the left compared to healthy controls. Verbal IQ (VIQ) is generally more impaired compared to performance IQ (PIQ) (Otter et al. 2010; Tartaglia et al. 2010; Otter et al. 2022). Lastly, social functioning and social cognition deficits have been reported in children and adults with 47,XXX (Lee et al. 2012; van Rijn et al. 2014; Wilson et al. 2019; Otter et al. 2021).
In the last three decades, interest in the neurobiological effects in SCAs, including 47,XXX, has increased, including effects on brain structure and brain function as they can serve as promising models for examining the effects of sex chromosomes on brain development and clinical disease in the general population. Previous neuroimaging studies have revealed alterations in brain gray matter structure and function in children, adolescents, and adults with 47,XXX (Warwick et al. 1999; Patwardhan et al. 2001; Lenroot et al. 2014; Fish et al. 2016; Reardon et al. 2016; Mankiw et al. 2017; Nadig et al. 2018; Serrarens et al. 2022; Serrarens et al. 2023). More specifically, individuals with 47,XXX showed alterations of total brain volume (Warwick et al. 1999; Patwardhan et al. 2001; Lenroot et al. 2014; Fish et al. 2016; Reardon et al. 2016; Mankiw et al. 2017; Nadig et al. 2018), cortical gray matter volume (Lenroot et al. 2014), cortical white matter volume (Lenroot et al. 2014), subcortical nuclei volume (Reardon et al. 2016; Nadig et al. 2018; Serrarens et al. 2022), cortical thickness and surface area (Lenroot et al. 2014; Serrarens et al. 2022), and cortical folding (Fish et al. 2016). Moreover, adult individuals with 47,XXX showed altered frontoparietal functional connectivity at rest (Serrarens et al. 2023). However, intracortical (gray matter) microstructure has not been investigated in children, adolescents, or adults with 47,XXX. Moreover, studies investigating intracortical microstructure in other SCAs, including 45,X0 (Turner syndrome), 47,XXY (Klinefelter syndrome), and 47,XYY (XYY syndrome), are still lacking.
The human cerebral cortex is a complex structure comprising 6 morphologically and functionally distinct layers, also known as laminae, that differ in the density and arrangement of neuronal cells, and their pattern of myelination (Trampel et al. 2019). Myelin is a lipid- and protein-rich sheath formed by oligodendrocytes and wrapped around axons in the nervous system that greatly enhances the speed of action potential propagation, provides nutritional support for axons, and is necessary for maintaining proper brain function (Nave 2010; Orthmann et al. 2020; Guo et al. 2023). White matter myelin has been found to play a key role in cognitive functioning (O'Muircheartaigh et al. 2014; Chevalier et al. 2015; Dai et al. 2019; Gong et al. 2023), and abnormalities in white matter myelination have been linked with multiple sclerosis (Lemus et al. 2018), Alzheimer’s disease (Nasrabady et al. 2018), and several psychiatric disorders (Lewandowski et al. 2014; Ho et al. 2021). Although primarily concentrated in white matter, myelinated axons are also present in the cortex. Recently, there has been a growing interest in assessing the content of intracortical myelin (ICM), and advances in neuroimaging techniques have enabled the noninvasive visualization of ICM content in vivo. Several MRI contrasts are sensitive to ICM content (Glasser and Van Essen 2011; Cohen-Adad et al. 2012; De Martino et al. 2015), and studies have demonstrated alterations in ICM in patients with multiple sclerosis (Barletta et al. 2021), Alzheimer’s disease (Pelkmans et al. 2019), and several psychiatric disorders (Baranger et al. 2021; Suh et al. 2023; Zhang et al. 2023; Chen et al. 2024). Quantitative T1 maps are also sensitive to ICM content (Stüber et al. 2014; Waehnert et al. 2016). T1, which is the time constant (in ms) governing the recovery of the longitudinal component of the magnetization following radio-frequency excitation, is an MRI parameter that is closely related to tissue myelination (Koenig et al. 1990). It has been suggested that myelin and compounds colocalized to myelin influence the longitudinal T1, with cortical regions with higher myelination showing reduced T1 (ms) (Haast et al. 2016). T1 maps have, for example, been used to visualize myelination patterns in the auditory and visual cortices, regions that are characterized by high myelination and low T1 (Waehnert et al. 2016). Moreover, T1 map values have also been shown to vary across cortical laminae (Tardif et al. 2015; Waehnert et al. 2016; Sprooten et al. 2019).
To the best of our knowledge, there have been no ICM studies conducted in 47,XXX. Therefore, it remains unclear whether intracortical microstructure across laminae is affected in individuals with 47,XXX. Hence the effect of X chromosome dosage on ICM across laminae is unknown. The present study aimed to compare quantitative T1 maps of gray matter across laminae between adult individuals with 47,XXX and typically developing females using ultra-high field (7 Tesla) structural MRI. Furthermore, given previous evidence of lower IQ, impaired social functioning and social cognition, we explored whether the variability of these (cognitive) outcomes was related to variability in T1 across laminae in 47,XXX.
Materials and methods
All procedures in this study were performed in accordance with the ethical standards established by the respective national and institutional committees regarding human experimentation and in accordance with the Declaration of Helsinki. All procedures involving human subjects were approved by the Medical Ethics Committee of the Maastricht University Medical Centre, Maastricht, the Netherlands (METC143051/NL46871.068.14). Written informed consent was obtained from all participants.
Participants
Twenty-one adults with 47,XXX and 22 age-matched typically developing females, aged 18 to 59, were included in this study. The Dutch (NL) and Flemish (B) individuals with 47,XXX were recruited through the 47,XXX support group, clinicians, clinical geneticists, pediatricians, and gynecologists. Typically developing females were recruited independently through local advertisement. General inclusion criteria were (i) 18 years or older of age, (ii) mental capacity to give informed consent, and (iii) a sufficient command of the Dutch language. Individuals with 47,XXX were included if a 47,XXX karyotype or a mosaic 46,XX/47,XXX karyotype with at least 85% cells with an extra X chromosome was genetically confirmed. Exclusion criteria for all study participants were (i) being under legal guardianship, (ii) contraindications for MRI, and (iii) pregnancy.
Instruments
A shortened version of the Dutch Wechsler Adult Intelligence Scale, Third Edition (WAIS-III; Velthorst et al. 2013) was administered to all participants to estimate the level of intellectual functioning. The Emotion Recognition Task (ERT) of the Cambridge Neuropsychological Test Automated Battery (CANTAB; Cambridge Cognition, Cambridge, UK; see www.cambridgecognition.com) was used to assess social cognition in all participants. The total number of correctly identified emotions, with a maximum score of 180, was included as the outcome measure of the ERT (Cambridge Cognition 2014). The Dutch translation of the informant/observer version of the Social Responsiveness Scale for adults (SRS-A) was used to assess social responsiveness (which is considered a screening instrument for ASD) in all participants (Constantino et al. 2012). The SRS-A questionnaire is subdivided into 4 subscales including (i) social awareness, (ii) social communication, (iii) social motivation, and (iv) rigidity and repetitive behavior. SRS-A scales are reported as T-scores with scores <40 indicating high social functioning, scores between 40 and 59 indicating normal social functioning, scores between 60 and 75 indicating mild to moderate social deficits, and scores ≥76 indicating severe deficits.
MRI data acquisition
MRI data acquisition was carried out at Scannexus B. V. (https://scannexus.nl) on a Siemens Magnetom 7 T scanner (Siemens Healthineers, Erlangen, Germany) using a 1Tx/32Rx commercial head coil (Nova Medical Inc., Wilmington, MA, USA). Anatomical data were acquired using a 3D-MP2RAGE sequence (Marques et al. 2010); repetition time (TR) = 5,000 ms; echo time (TE) = 2.51 ms; inversion times TI1/TI2 = 900/2,750 ms; α1/α2 = 5°/3°; phase partial Fourier = 6/8; GRAPPA = 2 with 24 reference lines; bandwidth = 248 Hz/Px; nominal voxel size = 0.7 × 0.7 × 0.7 mm3; acquisition time = 10:57 min. When using a 3D-MP2RAGE sequence, images at the two inversion times (TI1/TI2) are used to calculate the UNI (or T1w), and the quantitative T1 maps. These maps can be obtained directly from the scanner.
Preprocessing of imaging data
Preprocessing of anatomical data was first carried out using presurfer scripts (https://github.com/srikash/presurfer, Kashyap 2021) to first remove the background noise from the MP2RAGE UNI (T1w) image (presurf_MPRAGEise.m) and then, an accurate brainmask (“stripmask”) was obtained using SPM12’s unified segmentation approach (presurf_UNI.m) (Ashburner and Friston 2005). The stripmasks were visually inspected in each participant and manually corrected using ITK-SNAP (Yushkevich et al. 2016) in cases in which the automatic masking was suboptimal (Kashyap et al. 2021). The T1w image and corrected mask were supplied as input to the recon-all pipeline of FreeSurfer (v7.3.2) to perform segmentation and cortical surface reconstruction (https://surfer.nmr.mgh.harvard.edu/, Fischl 2012) in native submillimeter resolution (https://surfer.nmr.mgh.harvard.edu/fswiki/SubmillimeterRecon).
Laminar analysis
The pial and white surfaces from FreeSurfer were further processed using FreeSurfer and LayNii (v2.4.0.; Huber et al. 2021) (https://github.com/srikash/surf_laynii/, Kashyap 2023). In brief, the white and pial surfaces were shifted toward the white matter and cerebrospinal fluid, respectively, by 30% of the cortical thickness using FreeSurfer’s mris_expand tool to account for any small discrepancies in the placement of the boundaries when using 3D-MP2RAGE for automatic segmentation (Fujimoto et al. 2014; Kashyap et al. 2021). The surfaces were then transformed into volumetric space, upscaled, and relabeled as per LayNii requirements. Finally, a total of nine intracortical equivolume laminae were delineated using LayNii’s LN2_LAYERS (Waehnert et al. 2014; Huntenburg et al. 2017), where the first lamina is the deepest and closest to the white matter boundary, and the last lamina is the most superficial and closest to the pial surface. The number of nine laminae was chosen based on a compromise between computational feasibility and smoothness (please see https://layerfmri.com/2019/02/22/how-many-layers-should-i-reconstruct/).
Correction of quantitative T1
B1+ maps acquired during scanning were used to correct the quantitative T1 map images (Marques and Gruetter 2013; Haast et al. 2018) using publicly available scripts (https://github.com/JosePMarques/MP2RAGE-related-scripts). The corrected T1 maps and FreeSurfer’s parcellation schemes based on the Desikan–Killiany atlas (Desikan et al. 2006) were also upsampled to 0.3 mm isotropic resolution for further laminar analysis. Quantitative T1 laminar profiles were obtained from 68 (34 left and 34 right hemisphere) cortical regions of interest. Mean T1 values (ms) were sampled from each region of interest of the Desikan–Killiany atlas for the 9 intracortical laminae.
Statistical analysis
Statistical analyses were performed in R, version 3 (R Core Team 2020). First, differences in group demographics including age, FSIQ, VIQ, and PIQ were examined using Mann–Whitney U tests and independent-samples t-tests according to the normality of data distribution. Second, ERT scores were transformed into standardized Z-scores to identify outliers (Z-scores smaller than −3 or larger than 3) and no outliers were detected. Normally distributed raw scores on the ERT of the CANTAB were compared between groups using the independent-samples t-test. Normally and non-normally distributed total SRS-A T-scores and T-scores for SRS-A subscales were compared using independent-samples t-tests and Mann–Whitney U tests, respectively. Group differences in T1 values were examined using multiple linear regression models via the lm function in R, with per region of interest and per lamina each mean T1 value as the dependent variable and group (i.e. diagnosis) as the independent variable, adjusted for FSIQ. Cohen’s d effect size estimates were derived from the t-statistic of the group variable from the multiple linear regression model. Bonferroni correction was applied to correct for multiple comparisons [0.05/(2(hemispheres)]. Consequently, a P-value < 0.025 was considered significant. In case cognitive outcome measure scores or social functioning scores were significantly different between individuals with 47,XXX and typically developing females, relationships between these cognitive and social functioning parameters and mean T1 values extracted from significant region of interest laminae were calculated using Pearson’s or Spearman’s rank correlation coefficients, separately for individuals with 47,XXX and typically developing females.
Results
Demographics
Sample demographics are presented in Table 1. There was no significant difference in age between groups. Individuals with 47,XXX had a significantly lower FSIQ, VIQ, and PIQ compared to typically developing females.
Table 1.
Sample demographics.
| 47,XXX Mean (SD) |
TD females Mean (SD) |
Statistic | P | |||
|---|---|---|---|---|---|---|
| N | N | |||||
| Age | 21 | 30.10 (11.88) | 22 | 33.82 (12.38) | U = 277 | 0.2682 |
| FSIQ | 21 | 85.81 (10.44) | 22 | 99.73 (12.32) | t = 3.99 | <0.001 |
| VIQa | 21 | 80.81 (12.34) | 22 | 95.18 (12.96) | t = 3.72 | <0.001 |
| PIQa | 21 | 87.67 (14.53) | 22 | 102.82 (18.00) | t = 3.03 | 0.004 |
Numbers in bold reflect significant between group differences. TD, typically developing; FSIQ, full-scale intelligence quotient; VIQ, verbal intelligence quotient; PIQ, performance intelligence quotient.
aNo significant difference between VIQ and PIQ in either 47,XXX or 46,XX (typically developing females).
Social cognition and social responsiveness
Social cognition and SRS-A T-scores are summarized in Table 2. Individuals with 47,XXX had significantly lower ERT scores compared to age-matched typically developing females. Women with 47,XXX scored significantly higher on 3 SRS-A subscales: social awareness, social communication, and social motivation, as well as on total SRS-A score. There was no significant difference between groups in score of SRS-A subscale rigidity and repetitive behavior.
Table 2.
Between-group differences in social cognition and social responsiveness.
| 47,XXX Mean (SD) |
TD females Mean (SD) |
Statistic | P | |||
|---|---|---|---|---|---|---|
| N | N | |||||
| Social cognition | ||||||
| ERT score | 21 | 101.33 (17.56) | 22 | 119.59 (14.05) | t = 3.77 | <0.001 |
| Social responsiveness | ||||||
| Social awareness score | 20 | 56.85 (10.73) | 22 | 49.36 (11.24) | t = −2.20 | 0.033 |
| Social communication score | 20 | 55.85 (7.89) | 22 | 46.86 (7.82) | t = −3.71 | <0.001 |
| Social motivation score | 20 | 54.75 (8.08) | 22 | 45.64 (7.34) | U = 87.5 | <0.001 |
| Rigidity and repetitive behavior score | 20 | 53.50 (9.70) | 22 | 48.86 (10.45) | U = 152.5 | 0.090 |
| Social functioning total score | 20 | 56.05 (8.02) | 22 | 47.32 (9.47) | t = −3.21 | 0.003 |
Numbers in bold reflect significant between group differences. TD, typically developing; ERT, emotion recognition task.
T1 profiles across intracortical laminae
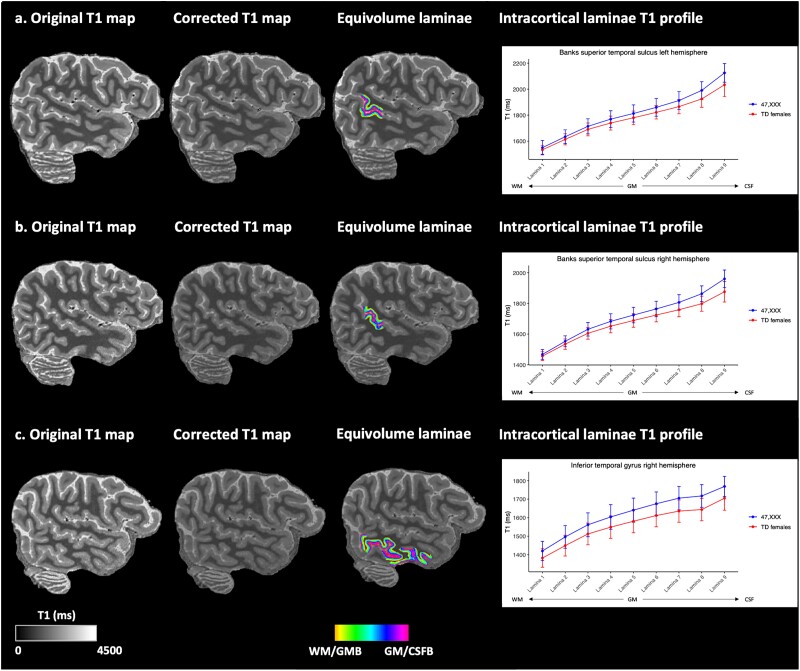
Laminar analyses applied to T1 maps showed significantly higher mean T1 in lamina 9 of the banks of the superior temporal sulcus (Cohen’s d = 0.745) of the left hemisphere, as well as higher mean T1 in laminae 8 and 9 of the banks of the superior temporal sulcus (Cohen’s d = 0.924; 1.038 respectively) of the right hemisphere in 47,XXX compared to typically developing females (Table 3 and Fig. 1A and B). In addition, 47,XXX subjects showed higher mean T1 in laminae 7 and 8 of the inferior temporal gyrus (Cohen’s d = 0.709; 0.779, respectively) of the right hemisphere compared to typically developing females (Table 3 and Fig. 1C).
Table 3.
Results for mean T1 map values of significant regions of interest in 47,XXX compared to typically developing females.
| Region of interest | Hemisphere | Lamina | Cohen’s d (47,XXX – TD females) |
Standard
error |
95% CI | p-value | |
|---|---|---|---|---|---|---|---|
| Banks superior temporal sulcus | Left | 9 | 0.745 | 0.316 | 0.126 | 1.365 | 0.019 |
| Banks superior temporal sulcus | Right | 8 9 |
0.924 1.038 |
0.322 0.326 |
0.294 0.399 |
1.555 1.676 |
0.004 0.002 |
| Inferior temporal gyrus | Right | 7 8 |
0.709 0.779 |
0.315 0.317 |
0.091 0.157 |
1.326 1.400 |
0.025 0.015 |
TD, typically developing.
Fig. 1.
Original T1 maps, corrected T1 maps, equivolume laminae projected on corrected T1 maps, and intracortical laminae T1 profiles of 47,XXX and typically developing (TD) females of a) banks of the superior temporal sulcus of the left hemisphere, b) banks of the superior temporal sulcus of the right hemisphere and c) inferior temporal gyrus of the right hemisphere. Intracortical laminae T1 profiles show mean T1 (ms) values and standard deviations for intracortical (gray matter) laminae 1 to 9, with lamina 1 closest to the white matter/gray matter boundary and lamina 9 closest to the gray matter/cerebrospinal fluid boundary. WM: white matter; GM: gray matter; CSF: cerebrospinal fluid; WM/GMB: white matter/gray matter boundary; GM/CSFB: gray matter/cerebrospinal fluid boundary.
Relationship with IQ, social cognition, and social behavior
We found significant positive correlations between mean T1 values in lamina 7 of the right hemisphere inferior temporal gyrus and social awareness scores (r = 0.496, P = 0.026), social communication scores (r = 0.512, P = 0.021), social motivation scores (r = 0.489, P = 0.029), and social functioning total scores (r = 0.581, P = 0.007) within individuals with 47,XXX, but not in typically developing females. In addition, we found significant positive correlations between mean T1 values in lamina 8 of the right hemisphere inferior temporal gyrus and social awareness scores (r = 0.466, P = 0.038), social communication scores (r = 0.513, P = 0.021), social motivation scores (r = 0.470, P = 0.037), and social functioning total scores (r = 0.560, P = 0.010) within individuals with 47,XXX, but not in typically developing females. However, only the positive correlation between lamina 7 mean T1 of the inferior temporal gyrus of the right hemisphere and social functioning total scores did survive correction for multiple comparisons [P = 0.002; = 0.05/5 (laminae) × 5 (social cognition and social functioning tasks)]. Correlations between T1 values and IQ and ERT scores were not present in 47,XXX.
Discussion
To the best of our knowledge, this is the first MRI study investigating ICM in 47,XXX using ultra-high field 7 T structural MRI. Using laminar analyses applied to quantitative T1 maps, we demonstrated significantly lower ICM across supragranular laminae in 47,XXX bilaterally in the banks of the superior temporal sulcus and in the right inferior temporal gyrus. Moreover, better social functioning was associated with larger ICM in supragranular laminae of the right inferior temporal gyrus in adult individuals with 47,XXX.
We reported significantly higher T1 across supragranular cortical laminae in 47,XXX bilaterally in the banks of the superior temporal sulcus and in the inferior temporal gyrus of the right hemisphere with high effect sizes, possibly indicating less ICM content in these cortical gray matter structures. Given that intracortical T1 values of these cortical brain regions are within the expected range of gray matter T1 at 7 T (Kashyap et al. 2018; Sanchez Panchuelo et al. 2021; Gulban et al. 2022), there is no possibility of potentially having sampled nongray matter tissue (e.g. cerebrospinal fluid) in these supragranular cortical laminae. Based on findings of previous neuroimaging studies, the superior temporal sulcus is associated with speech, language processing, and social cognition (Redcay 2008; Saitovitch et al. 2012; Specht and Wigglesworth 2018; Wilson et al. 2018; Nourski et al. 2021), and the inferior temporal gyrus is associated with visual information processing, language, emotion regulation, and social cognition (Lin et al. 2020; Balgova et al. 2022). Myelination of axons, which increases the speed of signal transmission between neurons and facilitates information integration, has been found to play a key role in the development of many aspects of cognition, including language ability and social cognition (O'Muircheartaigh et al. 2014), and alterations in cortical myelin have been demonstrated in patients with depressive disorders (Baranger et al. 2021; Zhang et al. 2023), bipolar disorder (Suh et al. 2023), schizophrenia (Wei et al. 2020), Alzheimer’s disease (Pelkmans et al. 2019), and multiple sclerosis (Mangeat et al. 2018; Barletta et al. 2021). Therefore, abnormalities in myelin can lead to dysregulation of neuronal circuits and may (partially) underly the behavioral phenotype associated with 47,XXX. Speech, language, and visual information processing abilities of 47,XXX individuals were not assessed in the current study. However, speech and language deficits have previously been described in children and adolescents with 47,XXX (Leggett et al. 2010; Urbanus et al. 2021; Capelli et al. 2023). Using macromolecular proton fraction as a marker of myelin, significant positive correlations between early language skills and myelin density were shown in typically developing toddlers in gray matter of frontal, parietal, and temporal lobes using neuroimaging data (Corrigan et al. 2022). Therefore, more research is necessary to investigate the contribution of altered superior temporal sulcus and inferior temporal gyrus ICM content across laminae to speech and language problems in 47,XXX.
Although our results showed significantly worse scores on social cognition (ERT) in adults with 47,XXX compared to typically developing females, we did not show any significant associations between ERT scores and T1 across laminae in the superior temporal sulcus or inferior temporal gyrus in 47,XXX. However, other aspects of social cognition, including theory of mind and joint attention, were not assessed in the present study. Therefore, future studies are necessary to investigate potential relationships between other aspects of social cognition and altered ICM in the superior temporal sulcus and inferior temporal gyrus in adult individuals with 47,XXX. Nevertheless, we reported a significant positive correlation between inferior temporal gyrus intracortical T1 and total social functioning scores in 47,XXX, indicating that higher social functioning in 47,XXX is related to larger ICM content. A previous study investigating ICM trajectories of social–emotional brain regions, including the superior temporal sulcus, in typically developing toddlers using the myelin water fraction reported a steep increase in ICM content in delineated brain regions throughout the first 3 years of life (Schneider et al. 2021). Moreover, they showed a significant correlation between this pattern of myelination and their social–emotional development as observed and rated by parents (Schneider et al. 2021). In addition, social cognitive training in typically developing individuals was associated with decreases in T1 in superficial depths in the parietal and temporal cortices and sensory-motor areas (Valk et al. 2023). Previous studies in individuals with psychiatric disorders have not directly related ICM to social functioning or social cognition. Therefore, more research is necessary to investigate the contribution of altered ICM in the superior temporal sulcus and inferior temporal gyrus to social functioning and social cognition in 47,XXX.
Alterations of ICM in the temporal cortex as reported here in 47,XXX have also been shown in individuals with schizophrenia. More specifically, increases in ICM were shown in supragranular laminae of the parietal–temporal cortex including the supramarginal and superior temporal gyri in individuals with schizophrenia compared to typically developing individuals (Wei et al. 2020). Psychotic symptoms and psychotic disorders have been reported in individuals with 47,XXX (Otter et al. 2010; Green et al. 2018; Otter et al. 2022). Thus, these results may indicate potentially shared neurodevelopmental pathways contributing to the 47,XXX phenotype and idiopathic psychotic disorders. Alterations in brain morphology and brain function in the superior temporal sulcus (Boddaert et al. 2004; Redcay 2008; Saitovitch et al. 2012; Nomi and Uddin 2015) and inferior temporal gyrus (Cai et al. 2018; Kim et al. 2021) have previously also been reported in individuals with ASD. Yet, most of these studies have focused disproportionately on males with ASD rather than females with ASD. However, these parallels in altered brain (micro)structure and brain function between individuals with 47,XXX and those with ASD may suggest potentially shared neurodevelopmental pathways underlying both conditions. Nevertheless, an investigation of ICM in young children with ASD using the T1w/T2w ratio as an estimate of ICM content found no significant differences between individuals with ASD and typically developing children, also not while controlling for sex (Chen et al. 2022). Yet, children with ASD showed altered developmental timing of myelination across several posterior cortical regions (Chen et al. 2022). This alteration may suggest long-term effects that may manifest as differences in ICM in adulthood in ASD. It might be interesting for future studies to compare ICM across laminae using T1 between individuals with 47,XXX with and without ASD across different developmental periods. Unfortunately, we could not examine these potential differences as a result of an insufficient sample size resulting in decreased power to detect statistically significant differences between individuals with 47,XXX with and without ASD.
In a previous study using a largely overlapping sample, we showed smaller subcortical nuclei volumes and lower surface area in the superior temporal gyrus and superior frontal gyrus of the right hemisphere in adult women with 47,XXX (Serrarens et al. 2022). Here, we demonstrate altered ICM across supragranular laminae bilaterally in the banks of the superior temporal sulcus, which separates the superior temporal gyrus from the middle temporal gyrus. It has been hypothesized that surface area and ICM are neurodevelopmentally related (Cafiero et al. 2019). Although we did not observe significant differences in surface area of the banks of the superior temporal sulcus in 47,XXX in our previous study, further investigation is warranted to investigate the potential relationship between ICM and surface area in 47,XXX.
The deeper layers of the cortex closer to the white matter boundary are generally the most heavily myelinated and have an MRI signal that is distinct from the signal in the superficial gray matter, which has fewer myelinated fibers (Rowley et al. 2015). Yet, here we showed ICM alterations in superficial cortical laminae of the superior temporal sulcus and inferior temporal gyrus in 47,XXX. Given that superficial cortical laminae tend to contain the supragranular layers that are rich in feed-forward sensory connections on pyramidal neurons and contain neurons that project their axons to other cortical areas of the same hemisphere (associative), decreased myelination in 47,XXX may lead to disrupted neuronal connectivity with other cortical regions (Wei et al. 2020). ICM content in deeper cortical laminae (infragranular) of the superior temporal sulcus and inferior temporal gyrus was not statistically altered in individuals with 47,XXX. However, medium effect sizes were observed for deeper cortical laminae in the banks of the superior temporal sulcus and the inferior temporal gyrus in the right hemisphere, also showing higher T1 values in 47,XXX compared to typically developing females. Decreasing T1 value trajectories were observed from supragranular to infragranular laminae, trajectories that are comparable to the results of previous studies using T1 map values (Tardif et al. 2015; Waehnert et al. 2016; Sprooten et al. 2019; Gulban et al. 2022). Future studies including larger sample sizes are required to further investigate ICM in deeper cortical laminae in the superior temporal sulcus and inferior temporal gyrus in 47,XXX.
Using diffusion tensor imaging data, X chromosome dosage effects on white matter microstructure were previously shown in individuals with 45,X0 (Molko et al. 2004; Holzapfel et al. 2006; Yamagata et al. 2011; Xie et al. 2015) and 47,XXY (Goddard et al. 2015). Altered radial diffusivity, which is an indirect measure of white matter myelination, was reported in children with 45,X0 in widespread white matter regions and tracts (Yamagata et al. 2011). Additionally, children and adolescents with 47,XXY showed altered radial diffusivity in the anterior corona radiata and sagittal striatum (Goddard et al. 2015). Combined, these results suggest X chromosome dosage effects on white matter microstructure across the lifespan. The results in 47,XXX presented here increase our understanding of X chromosome dosage effects on gray matter microstructure, more specifically on ICM across laminae. However, comparison data from structural MRI studies investigating ICM in other SCAs, including 45,X0 and 47,XXY, are currently not available. To elucidate the direct impact of X chromosome dosage on ICM across laminae in more depth, future studies including a more diverse group of SCAs are required. Moreover, longitudinal studies investigating ICM across laminae in SCAs, including 47,XXX, across developmental trajectories are warranted.
Strengths and limitations
Our study is the first to investigate ICM across laminae in individuals with 47,XXX. Our work in 47,XXX presented here offers a better understanding of how X chromosome dosage impacts ICM. Another important strength of this study is the use of ultra-high field 7 T MRI data. MRI at 7 T offers increased signal-to-noise ratio and increased contrast-to-noise ratio compared to 3 T MRI, allowing imaging with submillimeter spatial resolutions and mapping of laminar profiles, improving the delineation of anatomical structures, providing clearer tissue boundaries which results in improved segmentation accuracy and minimizing partial volume effects (Marques et al. 2010; Choi et al. 2011; Bahrami et al. 2017; Vachha and Huang 2021). In addition, our study is the first to examine relationships between ICM across laminae and IQ, and social cognition and social functioning in 47,XXX. Despite the strong merits and novelty of our study, some limitations should be mentioned as well. First, our sample size was relatively small, resulting in decreased power to detect statistically significant differences. When having a small sample size, P values are particularly vulnerable to small deviations in the number of outcomes (Mitani and Haneuse 2020). Since this is the first explorative study investigating intracortical myelin across laminae in 47,XXX, we chose to be comprehensive and report statistical significance at P < 0.025 (corrected for the number of hemispheres). The relatively small sample size could be a potential explanation for our not observing alterations in ICM across deeper laminae of the banks of the superior temporal sulcus and the inferior temporal gyrus. However, 47,XXX is considered an underrepresented and understudied population (Tuke et al. 2019). Given the variability in clinical phenotype and the assumption that many individuals with 47,XXX are not clinically diagnosed, recruitment of large sample sizes is difficult and requires international collaborative consortia. Ascertainment bias is also a well-known limitation in research in genetic disorders in general. This also applies to 47,XXX studies as patients presenting more severe phenotypes are more likely to be clinically diagnosed, and recognized and enrolled in research. Therefore, our sample may not be representative of all individuals with 47,XXX. Moreover, the cross-sectional nature of our data makes it difficult to assess possible age-varying patterns of ICM across laminae in 47,XXX, stressing the need for longitudinal studies investigating ICM across laminae and its potential relationships with clinical symptoms in 47,XXX. Individuals with 47,XXX had a confirmed 47,XXX diagnosis through DNA testing, which was verified by their general practitioner. However, genetic/genomic information was not collected for this study. Therefore, future studies are necessary to investigate the effect of these factors on intracortical myelin in 47,XXX. While X chromosome dosage effects on ICM across laminae were elucidated in 47,XXX at adult age, alterations in brain structure may also be the result of indirect sex hormonal effects. Sex hormone levels in 47,XXX are usually normal, but were not investigated in this study, and therefore, future research is warranted. We acknowledge the inherent limitation that T1 values do not directly represent myelin concentrations, but rather serve as a close approximation. T1 values are also influenced by other factors, such as iron or susceptibility. However, work by Stüber (Stüber et al. 2014) suggests that the value of T1 within the cortex is mostly dominated by myelin content. In laminar analysis using MRI, cortical laminae that are used to calculate laminar profiles do not directly correspond to cytoarchitectonic layers (in a histological sense). The geometrical depth might not consistently align with a cortical lamina based on cytoarchitecture across regions or individuals, as demonstrated by variations in laminar volumes along functional gradients by Wagstyl and colleagues (Wagstyl et al. 2020). Lastly, 7 T MRI is fraught with challenges posed by transmit field (B1+)-related inhomogeneities that need to be corrected using additionally acquired data and/or image processing. In the present study, we were able to correct quantitative T1 map images for B1+ inhomogeneities in postprocessing using a dedicated B1+ acquisition. Future studies can consider employing methods outlined here or recent advances in MR acquisition methods like pTx and Universal Pulses to remediate some of these issues associated with imaging inhomogeneities (Gras et al. 2016; Choi et al. 2024).
Conclusion
In conclusion, our results indicate an effect of a supernumerary X chromosome in adult-aged women on ICM across supragranular laminae of the banks of the superior temporal sulcus, and the inferior temporal gyrus. These findings provide insight into the role of X chromosome dosage on ICM across laminae. Future research is warranted to further explore the functional significance of altered ICM across laminae in 47,XXX.
Acknowledgments
We would like to thank all the women for participating in this study. We would also like to thank Truda Driesen for coordinating the study.
Contributor Information
Chaira Serrarens, Department of Psychiatry and Neuropsychology, Mental Health and Neuroscience Institute (MHeNS), Maastricht University, Maastricht, 6200 MD, The Netherlands.
Julia Ruiz-Fernandez, Department of Psychiatry and Neuropsychology, Mental Health and Neuroscience Institute (MHeNS), Maastricht University, Maastricht, 6200 MD, The Netherlands; INSERM U1299, Centre Borelli UMR 9010, ENS-Paris-Saclay, Université Paris Saclay, Paris, France.
Maarten Otter, Department of Psychiatry and Neuropsychology, Mental Health and Neuroscience Institute (MHeNS), Maastricht University, Maastricht, 6200 MD, The Netherlands; Medical Department, SIZA, Arnhem, 6800 AM, The Netherlands.
Bea C M Campforts, Department of Psychiatry and Neuropsychology, Mental Health and Neuroscience Institute (MHeNS), Maastricht University, Maastricht, 6200 MD, The Netherlands.
Constance T R M Stumpel, Department of Clinical Genetics and School for Oncology and Developmental Biology, Maastricht University Medical Centre, Maastricht, 6229 ER, The Netherlands.
David E J Linden, Department of Psychiatry and Neuropsychology, Mental Health and Neuroscience Institute (MHeNS), Maastricht University, Maastricht, 6200 MD, The Netherlands.
Therese A M J van Amelsvoort, Department of Psychiatry and Neuropsychology, Mental Health and Neuroscience Institute (MHeNS), Maastricht University, Maastricht, 6200 MD, The Netherlands.
Sriranga Kashyap, Department of Cognitive Neuroscience, Faculty of Psychology and Neuroscience, Maastricht University, Maastricht, 6229 EV, The Netherlands; Krembil Brain Institute, University Health Network, Toronto, ON M5T 2S8, Canada.
Claudia Vingerhoets, Department of Psychiatry and Neuropsychology, Mental Health and Neuroscience Institute (MHeNS), Maastricht University, Maastricht, 6200 MD, The Netherlands; ‘s Heeren Loo Zorggroep, Amersfoort, 3818 LA, The Netherlands.
Author contributions
Chaira Serrarens (Data curation, Formal analysis, Methodology, Software, Visualization, Writing—original draft, Writing—review & editing), Julia Ruiz-Fernandez (Data curation, Formal analysis, Methodology, Software, Writing—review & editing), Maarten Otter (Conceptualization, Investigation, Project administration, Resources, Writing—review & editing), Bea C.M. Campforts (Investigation, Project administration, Resources, Writing—review & editing), Constance T.R.M. Stumpel (Conceptualization, Project administration, Supervision, Writing—review & editing), David Linden (Supervision, Writing—review & editing), T.A.M.J. van Amelsvoort (Conceptualization, Project administration, Supervision, Writing—review & editing), Sriranga Kashyap (Formal analysis, Methodology, Software, Supervision, Visualization, Writing—original draft, Writing—review & editing), and Claudia Vingerhoets (Methodology, Supervision, Visualization, Writing—original draft, Writing—review & editing).
Funding
The work of C.S. was supported by the National Institute of Mental Health (NIMH 5U01MH119740-05). STEVIG, Oostrum, the Netherlands, contributed financially to the work of co-author M.O.
Conflict of interest statement: None declared.
References
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005:26(3):839–851. 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bahrami K, Shi F, Rekik I, Gao Y, Shen D. 7T-guided super-resolution of 3T MRI. Med Phys. 2017:44(5):1661–1677. 10.1002/mp.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balgova E, Diveica V, Walbrin J, Binney RJ. The role of the ventrolateral anterior temporal lobes in social cognition. Hum Brain Mapp. 2022:43(15):4589–4608. 10.1002/hbm.25976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranger DAA, Halchenko YO, Satz S, Ragozzino R, Iyengar S, Swartz HA, Manelis A. Aberrant levels of cortical myelin distinguish individuals with depressive disorders from healthy controls. NeuroImage Clin. 2021:32:102790. 10.1016/j.nicl.2021.102790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barletta V, Herranz E, Treaba CA, Mehndiratta A, Ouellette R, Mangeat G, Granberg T, Sloane JA, Klawiter EC, Cohen-Adad J, et al. Quantitative 7-tesla imaging of cortical myelin changes in early multiple sclerosis. Front Neurol. 2021:12:714820. 10.3389/fneur.2021.714820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund A, Stochholm K, Gravholt CH. The comorbidity landscape of 47,XXX syndrome: a nationwide epidemiologic study. Genet Med. 2022:24(2):475–487. 10.1016/j.gim.2021.10.012. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, Barthélémy C, Mouren MC, Artiges E, Samson Y, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. NeuroImage. 2004:23(1):364–369. 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Cafiero R, Brauer J, Anwander A, Friederici AD. The concurrence of cortical surface area expansion and white matter myelination in human brain development. Cereb Cortex. 2019:29(2):827–837. 10.1093/cercor/bhy277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Hu X, Guo K, Yang P, Situ M, Huang Y. Increased left inferior temporal gyrus was found in both low function autism and high function autism. Front Psychiatry. 2018:9:542. 10.3389/fpsyt.2018.00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambridge Cognition ERTE . 2014. In: CANTAB Research Suite 6 Test Administration Guide: Ch 10. In.
- Capelli E, Silibello G, Provera A, Dallara F, Ajmone PF, Monti F, Scionti N, Zanchi P, Costantino MA, Vizziello PG, et al. Speech sound development in 18-month-old children with sex chromosome trisomies. Am J Speech-Lang Pathol. 2023:32(1):287–297. 10.1044/2022_AJSLP-22-00118. [DOI] [PubMed] [Google Scholar]
- Chen B, Linke A, Olson L, Kohli J, Kinnear M, Sereno M, Müller RA, Carper R, Fishman I. Cortical myelination in toddlers and preschoolers with autism spectrum disorder. Dev Neurobiology. 2022:82(3):261–274. 10.1002/dneu.22874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Patel Z, Liu S, Bock NA, Frey BN, Suh JS. A systematic review of abnormalities in intracortical myelin across psychiatric illnesses. J Affect Disord Rep. 2024:15:100689. 10.1016/j.jadr.2023.100689. [DOI] [Google Scholar]
- Chevalier N, Kurth S, Doucette MR, Wiseheart M, Deoni SCL, Dean DC, O’Muircheartaigh J, Blackwell KA, Munakata Y, LeBourgeois MK. Myelination is associated with processing speed in early childhood: preliminary insights. PLoS One. 2015:10(10):e0139897. 10.1371/journal.pone.0139897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Cunningham DT, Aguila F, Corrigan JD, Bogner J, Mysiw WJ, Knopp MV, Schmalbrock P. DTI at 7 and 3 T: systematic comparison of SNR and its influence on quantitative metrics. Magn Reson Imaging. 2011:29(6):739–751. 10.1016/j.mri.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Choi C-H, Webb A, Orzada S, Kelenjeridze M, Shah NJ, Felder J. A review of parallel transmit arrays for ultra-high field MR imaging. IEEE Rev Biomed Eng. 2024:17:351–368. 10.1109/RBME.2023.3244132. [DOI] [PubMed] [Google Scholar]
- Cohen-Adad J, Polimeni JR, Helmer KG, Benner T, McNab JA, Wald LL, Rosen BR, Mainero C. T2* mapping and B0 orientation-dependence at 7T reveal cyto- and myeloarchitecture organization of the human cortex. NeuroImage. 2012:60(2):1006–1014. 10.1016/j.neuroimage.2012.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino J, Gruber C, Noens I, De la Marche M, Scholte E. SRS-A the social responsiveness scale for adults (Dutch manual). In: Hogrefe; 2012. [Google Scholar]
- Corrigan NM, Yarnykh VL, Huber E, Zhao TC, Kuhl PK. Brain myelination at 7 months of age predicts later language development. NeuroImage. 2022:263:119641. 10.1016/j.neuroimage.2022.119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Hadjipantelis P, Wang JL, Deoni SCL, Müller HG. Longitudinal associations between white matter maturation and cognitive development across early childhood. Hum Brain Mapp. 2019:40(14):4130–4145. 10.1002/hbm.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino F, Moerel M, Xu J, van de Moortele P-F, Ugurbil K, Goebel R, Yacoub E, Formisano E. High-resolution mapping of Myeloarchitecture In vivo: localization of auditory areas in the human brain. Cereb Cortex. 2015:25(10):3394–3405. 10.1093/cercor/bhu150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006:31(3):968–980. 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. NeuroImage. 2012:62(2):774–781. 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish AM, Cachia A, Fischer C, Mankiw C, Reardon PK, Clasen LS, Blumenthal JD, Greenstein D, Giedd JN, Mangin J-F, et al. Influences of brain size, sex, and sex chromosome complement on the architecture of human cortical folding. Cereb Cortex. 2016:27(12):5557–5567. 10.1093/cercor/bhw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freilinger P, Kliegel D, Hänig S, Oehl-Jaschkowitz B, Henn W, Meyer J. Behavioral and psychological features in girls and women with triple-X syndrome. Am J Med Genet A. 2018:176(11):2284–2291. 10.1002/ajmg.a.40477. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Polimeni JR, van der Kouwe AJW, Reuter M, Kober T, Benner T, Fischl B, Wald LL. Quantitative comparison of cortical surface reconstructions from MP2RAGE and multi-echo MPRAGE data at 3 and 7T. NeuroImage. 2014:90:60–73. 10.1016/j.neuroimage.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Van Essen DC. Mapping human cortical AreasIn VivoBased on myelin content as revealed by T1- and T2-weighted MRI. J Neurosci. 2011:31(32):11597–11616. 10.1523/JNEUROSCI.2180-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard MN, van Rijn S, Rombouts SARB, Swaab H. White matter microstructure in a genetically defined group at increased risk of autism symptoms, and a comparison with idiopathic autism: an exploratory study. Brain Imaging Behav. 2015:10(4):1280–1288. 10.1007/s11682-015-9496-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z, Bilgel M, Kiely M, Triebswetter C, Ferrucci L, Resnick SM, Spencer RG, Bouhrara M. Lower myelin content is associated with more rapid cognitive decline among cognitively unimpaired individuals. Alzheimers Dement. 2023:19(7):3098–3107. 10.1002/alz.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras V, Vignaud A, Amadon A, Le Bihan D, Boulant N. Universal pulses: a new concept for calibration-free parallel transmission. Magn Reson Med. 2016:77(2):635–643. 10.1002/mrm.26148. [DOI] [PubMed] [Google Scholar]
- Green T, Flash S, Reiss AL. Sex differences in psychiatric disorders: what we can learn from sex chromosome aneuploidies. Neuropsychopharmacology. 2018:44(1):9–21. 10.1038/s41386-018-0153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulban OF, Bollmann S, Huber L, Wagstyl K, Goebel R, Poser BA, Kay K, Ivanov D. Mesoscopic in vivo human T2* dataset acquired using quantitative MRI at 7 Tesla. NeuroImage. 2022:264:119733. 10.1016/j.neuroimage.2022.119733. [DOI] [PubMed] [Google Scholar]
- Guo Y, Wu H, Dong D, Zhou F, Li Z, Zhao L, Long Z. Stress and the brain: emotional support mediates the association between myelination in the right supramarginal gyrus and perceived chronic stress. Neurobiol Stress. 2023:22:100511. 10.1016/j.ynstr.2022.100511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haast RAM, Ivanov D, Formisano E, Uludaǧ K. Reproducibility and reliability of quantitative and weighted T1 and T2* mapping for myelin-based cortical parcellation at 7 Tesla. Front Neuroanat. 2016:10:112. 10.3389/fnana.2016.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haast RAM, Ivanov D, Uludağ K. The impact of B1+ correction on MP2RAGE cortical T1 and apparent cortical thickness at 7T. Hum Brain Mapp. 2018:39(6):2412–2425. 10.1002/hbm.24011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TC, Sisk LM, Kulla A, Teresi GI, Hansen MM, Wu H, Gotlib IH. Sex differences in myelin content of white matter tracts in adolescents with depression. Neuropsychopharmacology. 2021:46(13):2295–2303. 10.1038/s41386-021-01078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzapfel M, Barnea-Goraly N, Eckert MA, Kesler SR, Reiss AL. Selective alterations of white matter associated with visuospatial and sensorimotor dysfunction in Turner syndrome. J Neurosci. 2006:26(26):7007–7013. 10.1523/JNEUROSCI.1764-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber L, Poser BA, Bandettini PA, Arora K, Wagstyl K, Cho S, Goense J, Nothnagel N, Morgan AT, van den Hurk J, et al. LayNii: a software suite for layer-fMRI. NeuroImage. 2021:237:118091. 10.1016/j.neuroimage.2021.118091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntenburg JM, Bazin P-L, Goulas A, Tardif CL, Villringer A, Margulies DS. A systematic relationship between functional connectivity and intracortical myelin in the human cerebral cortex. Cereb Cortex. 2017:27(2):981–997. 10.1093/cercor/bhx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap S. 2021. srikash/presurfer: ondu.
- Kashyap S. 2023. srikash/surf_laynii: ondu (v0.1).
- Kashyap S, Ivanov D, Havlicek M, Poser BA, Uludağ K. Impact of acquisition and analysis strategies on cortical depth-dependent fMRI. NeuroImage. 2018:168:332–344. 10.1016/j.neuroimage.2017.05.022. [DOI] [PubMed] [Google Scholar]
- Kashyap S, Ivanov D, Havlicek M, Huber L, Poser BA, Uludağ K. Sub-millimetre resolution laminar fMRI using arterial spin labelling in humans at 7 T. PLoS One. 2021:16(4):e0250504. 10.1371/journal.pone.0250504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Lee JY, Jeong BC, Ahn JH, Kim JI, Lee ES, Kim H, Lee HJ, Han CE. Overconnectivity of the right Heschl's and inferior temporal gyrus correlates with symptom severity in preschoolers with autism spectrum disorder. Autism Res. 2021:14(11):2314–2329. 10.1002/aur.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig SH, Brown RD, Spiller M, Lundbom N. Relaxometry of brain: why white matter appears bright in MRI. Magn Reson Med. 1990:14(3):482–495. 10.1002/mrm.1910140306. [DOI] [PubMed] [Google Scholar]
- Lee NR, Wallace GL, Adeyemi EI, Lopez KC, Blumenthal JD, Clasen LS, Giedd JN. Dosage effects of X and Y chromosomes on language and social functioning in children with supernumerary sex chromosome aneuploidies: implications for idiopathic language impairment and autism spectrum disorders. J Child Psychol Psychiatry. 2012:53(10):1072–1081. 10.1111/j.1469-7610.2012.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett V, Jacobs P, Nation K, Scerif G, Bishop DVM. Neurocognitive outcomes of individuals with a sex chromosome trisomy: XXX, XYY, or XXY: a systematic review*. Dev Med Child Neurol. 2010:52(2):119–129. 10.1111/j.1469-8749.2009.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemus HN, Warrington AE, Rodriguez M. Multiple sclerosis: mechanisms of disease and strategies for myelin and axonal repair. Neurol Clin. 2018:36(1):1–11. 10.1016/j.ncl.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Blumenthal JD, Wallace GL, Clasen LS, Lee NR, Giedd JN. A case‐control study of brain structure and behavioral characteristics in 47,XXXsyndrome. Genes Brain Behav. 2014:13(8):841–849. 10.1111/gbb.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski KE, Ongür D, Sperry SH, Cohen BM, Sehovic S, Goldbach JR, Du F. Myelin vs axon abnormalities in white matter in bipolar disorder. Neuropsychopharmacology. 2014:40(5):1243–1249. 10.1038/npp.2014.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-H, Young IM, Conner AK, Glenn CA, Chakraborty AR, Nix CE, Bai MY, Dhanaraj V, Fonseka RD, Hormovas J, et al. Anatomy and white matter connections of the inferior temporal gyrus. World Neurosurg. 2020:143:e656–e666. 10.1016/j.wneu.2020.08.058. [DOI] [PubMed] [Google Scholar]
- Mangeat G, Badji A, Ouellette R, Treaba CA, Herranz E, Granberg T, Louapre C, Stikov N, Sloane JA, Bellec P, et al. Changes in structural network are associated with cortical demyelination in early multiple sclerosis. Hum Brain Mapp. 2018:39(5):2133–2146. 10.1002/hbm.23993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankiw C, Park MTM, Reardon PK, Fish AM, Clasen LS, Greenstein D, Giedd JN, Blumenthal JD, Lerch JP, Chakravarty MM, et al. Allometric analysis detects brain size-independent effects of sex and sex chromosome complement on human cerebellar organization. J Neurosci. 2017:37(21):5221–5231. 10.1523/JNEUROSCI.2158-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques JP, Gruetter R. New developments and applications of the MP2RAGE sequence - focusing the contrast and high spatial resolution R1 mapping. PLoS One. 2013:8(7):e69294. 10.1371/journal.pone.0069294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele P-F, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. NeuroImage. 2010:49(2):1271–1281. 10.1016/j.neuroimage.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Mitani AA, Haneuse S. Small data challenges of studying rare diseases. JAMA Netw Open. 2020:3(3):e201965. 10.1001/jamanetworkopen.2020.1965. [DOI] [PubMed] [Google Scholar]
- Molko N, Cachia A, Riviere D, Mangin JF, Bruandet M, LeBihan D, Cohen L, Dehaene S. Brain anatomy in Turner syndrome: evidence for impaired social and spatial-numerical networks. Cereb Cortex. 2004:14(8):840–850. 10.1093/cercor/bhh042. [DOI] [PubMed] [Google Scholar]
- Nadig A, Reardon PK, Seidlitz J, McDermott CL, Blumenthal JD, Clasen LS, Lalonde F, Lerch JP, Chakravarty MM, Raznahan A. Carriage of supernumerary sex chromosomes decreases the volume and alters the shape of limbic structures. eneuro. 2018:5(5):ENEURO.0265–ENEU18.2018. 10.1523/ENEURO.0265-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrabady SE, Rizvi B, Goldman JE, Brickman AM. White matter changes in Alzheimer’s disease: a focus on myelin and oligodendrocytes. Acta Neuropathol Commun. 2018:6(1):22. 10.1186/s40478-018-0515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave K-A. Myelination and support of axonal integrity by glia. Nature. 2010:468(7321):244–252. 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- Nielsen MM, Trolle C, Vang S, Hornshøj H, Skakkebæk A, Hedegaard J, Nordentoft I, Pedersen JS, Gravholt CH. Epigenetic and transcriptomic consequences of excess X‐chromosome material in 47,XXXsyndrome—A comparison with Turner syndrome and 46,XXfemales. Am J Med Genet C: Semin Med Genet. 2020:184(2):279–293. 10.1002/ajmg.c.31799. [DOI] [PubMed] [Google Scholar]
- Nomi JS, Uddin LQ. Face processing in autism spectrum disorders: from brain regions to brain networks. Neuropsychologia. 2015:71:201–216. 10.1016/j.neuropsychologia.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourski KV, Steinschneider M, Rhone AE, Kovach CK, Banks MI, Krause BM, Kawasaki H, Howard MA. Electrophysiology of the human superior temporal sulcus during speech processing. Cereb Cortex. 2021:31(2):1131–1148. 10.1093/cercor/bhaa281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Muircheartaigh J, Dean DC, Ginestet CE, Walker L, Waskiewicz N, Lehman K, Dirks H, Piryatinsky I, Deoni SCL. White matter development and early cognition in babies and toddlers. Hum Brain Mapp. 2014:35(9):4475–4487. 10.1002/hbm.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orthmann-Murphy J, Call CL, Molina-Castro GC, Hsieh YC, Rasband MN, Calabresi PA, Bergles DE. Remyelination alters the pattern of myelin in the cerebral cortex. elife. 2020:9:e56621. 10.7554/eLife.56621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter M, Schrander-Stumpel CTRM, Curfs LMG. Triple X syndrome: a review of the literature. Eur J Hum Genet. 2010:18(3):265–271. 10.1038/ejhg.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter M, Crins PML, Campforts BCM, Stumpel CTRM, van Amelsvoort TAMJ, Vingerhoets C. Social functioning and emotion recognition in adults with triple X syndrome. BJPsych Open. 2021:7(2):e51. 10.1192/bjo.2021.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter M, Campforts BCM, Stumpel CTRM, van Amelsvoort TAMJ, Drukker M. Triple X syndrome: psychiatric disorders and impaired social functioning as a risk factor. Eur Psychiatry. 2022:66(1):e7. 10.1192/j.eurpsy.2022.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan AJ, Brown WE, Bender BG, Linden MG, Eliez S, Reiss AL. Reduced size of the amygdala in individuals with 47,XXY and 47,XXX karyotypes. Am J Med Genet. 2001:114(1):93–98. 10.1002/ajmg.10154. [DOI] [PubMed] [Google Scholar]
- Pelkmans W, Dicks E, Barkhof F, Vrenken H, Scheltens P, van der Flier WM, Tijms BM. Gray matter T1-w/T2-w ratios are higher in Alzheimer's disease. Hum Brain Mapp. 2019:40(13):3900–3909. 10.1002/hbm.24638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2020. [Google Scholar]
- Raznahan A, Disteche CM. X-chromosome regulation and sex differences in brain anatomy. Neurosci Biobehav Rev. 2021:120:28–47. 10.1016/j.neubiorev.2020.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon PK, Clasen L, Giedd JN, Blumenthal J, Lerch JP, Chakravarty MM, Raznahan A. An Allometric analysis of sex and sex chromosome dosage effects on subcortical anatomy in humans. J Neurosci. 2016:36(8):2438–2448. 10.1523/JNEUROSCI.3195-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E. The superior temporal sulcus performs a common function for social and speech perception: implications for the emergence of autism. Neurosci Biobehav Rev. 2008:32(1):123–142. 10.1016/j.neubiorev.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Rowley CD, Bazin P-L, Tardif CL, Sehmbi M, Hashim E, Zaharieva N, Minuzzi L, Frey BN, Bock NA. Assessing intracortical myelin in the living human brain using myelinated cortical thickness. Front Neurosci. 2015:9:396. 10.3389/fnins.2015.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitovitch A, Bargiacchi A, Chabane N, Brunelle F, Samson Y, Boddaert N, Zilbovicius M. Social cognition and the superior temporal sulcus: implications in autism. Rev Neurol. 2012:168(10):762–770. 10.1016/j.neurol.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Sanchez Panchuelo RM, Mougin O, Turner R, Francis ST. Quantitative T1 mapping using multi-slice multi-shot inversion recovery EPI. NeuroImage. 2021:234:117976. 10.1016/j.neuroimage.2021.117976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez XC, Montalbano S, Vaez M, Krebs MD, Byberg-Grauholm J, Mortensen PB, Børglum AD, Hougaard DM, Nordentoft M, Geschwind DH, et al. Associations of psychiatric disorders with sex chromosome aneuploidies in the Danish iPSYCH2015 dataset: a case-cohort study. Lancet Psychiatry. 2023:10(2):129–138. 10.1016/S2215-0366(23)00004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider N, Greenstreet E, Deoni SCL. Connecting inside out: development of the social brain in infants and toddlers with a focus on myelination as a marker of brain maturation. Child Dev. 2021:93(2):359–371. 10.1111/cdev.13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrarens C, Otter M, Campforts BCM, Stumpel CTRM, Jansma H, van Amelsvoort TAMJ, Vingerhoets C. Altered subcortical and cortical brain morphology in adult women with 47,XXX: a 7-tesla magnetic resonance imaging study. J Neurodev Disord. 2022:14(1):14. 10.1186/s11689-022-09425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrarens C, Kashyap S, Riveiro-Lago L, Otter M, Campforts BCM, Stumpel CTRM, Jansma H, Linden DEJ, van Amelsvoort TAMJ, Vingerhoets C. Resting-state functional connectivity in adults with 47,XXX: a 7Tesla MRI study. Cereb Cortex. 2023:33(9):5210–5217. 10.1093/cercor/bhac410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse D, Printzlau F, Wolstencroft J. Sex chromosome aneuploidies. Neurogenetics, Part I. 2018:147:355–376. 10.1016/B978-0-444-63233-3.00024-5. [DOI] [PubMed] [Google Scholar]
- Specht K, Wigglesworth P. The functional and structural asymmetries of the superior temporal sulcus. Scand J Psychol. 2018:59(1):74–82. 10.1111/sjop.12410. [DOI] [PubMed] [Google Scholar]
- Sprooten E, O'Halloran R, Dinse J, Lee WH, Moser DA, Doucet GE, Goodman M, Krinsky H, Paulino A, Rasgon A, et al. Depth-dependent intracortical myelin organization in the living human brain determined by in vivo ultra-high field magnetic resonance imaging. NeuroImage. 2019:185:27–34. 10.1016/j.neuroimage.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüber C, Morawski M, Schäfer A, Labadie C, Wähnert M, Leuze C, Streicher M, Barapatre N, Reimann K, Geyer S, et al. Myelin and iron concentration in the human brain: a quantitative study of MRI contrast. NeuroImage. 2014:93:95–106. 10.1016/j.neuroimage.2014.02.026. [DOI] [PubMed] [Google Scholar]
- Suh JS, Rowley CD, Sehmbi M, Tardif CL, Minuzzi L, Bock NA, Frey BN. Loss of age-related laminar differentiation of intracortical myelin in bipolar disorder. Cereb Cortex. 2023:33(12):7468–7476. 10.1093/cercor/bhad052. [DOI] [PubMed] [Google Scholar]
- Tardif CL, Schäfer A, Waehnert M, Dinse J, Turner R, Bazin P-L. Multi-contrast multi-scale surface registration for improved alignment of cortical areas. NeuroImage. 2015:111:107–122. 10.1016/j.neuroimage.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Tartaglia NR, Howell S, Sutherland A, Wilson R, Wilson L. A review of trisomy X (47,XXX). Orphanet J Rare Dis. 2010:5(1):8. 10.1186/1750-1172-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trampel R, Bazin P-L, Pine K, Weiskopf N. In-vivo magnetic resonance imaging (MRI) of laminae in the human cortex. NeuroImage. 2019:197:707–715. 10.1016/j.neuroimage.2017.09.037. [DOI] [PubMed] [Google Scholar]
- Tuke MA, Ruth KS, Wood AR, Beaumont RN, Tyrrell J, Jones SE, Yaghootkar H, Turner CLS, Donohoe ME, Brooke AM, et al. Mosaic Turner syndrome shows reduced penetrance in an adult population study. Genet Med. 2019:21(4):877–886. 10.1038/s41436-018-0271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanus E, Swaab H, Tartaglia N, Boada R, van Rijn S. A cross-sectional study of early language abilities in children with sex chromosome trisomy (XXY, XXX, XYY) aged 1–6 years. Child Neuropsychol. 2021:28(2):171–196. 10.1080/09297049.2021.1960959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn S. A review of neurocognitive functioning and risk for psychopathology in sex chromosome trisomy (47,XXY, 47,XXX, 47, XYY). Curr Opin Psychiatry. 2019:32(2):79–84. 10.1097/YCO.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn S, Swaab H. Executive dysfunction and the relation with behavioral problems in children with 47,XXY and 47,XXX. Genes Brain Behav. 2015:14(2):200–208. 10.1111/gbb.12203. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Stockmann L, van Buggenhout G, van Ravenswaaij-Arts C, Swaab H. Social cognition and underlying cognitive mechanisms in children with an extra X chromosome: a comparison with autism spectrum disorder. Genes Brain Behav. 2014:13(5):459–467. 10.1111/gbb.12134. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Barneveld P, Descheemaeker M-J, Giltay J, Swaab H. The effect of early life stress on the cognitive phenotype of children with an extra X chromosome (47,XXY/47,XXX). Child Neuropsychol. 2016:24(2):277–286. 10.1080/09297049.2016.1252320. [DOI] [PubMed] [Google Scholar]
- Vachha B, Huang SY. MRI with ultrahigh field strength and high-performance gradients: challenges and opportunities for clinical neuroimaging at 7 T and beyond. Eur Radiol Exp. 2021:5(1):35. 10.1186/s41747-021-00216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valk SL, Kanske P, B-y P, Hong S-J, Böckler A, Trautwein F-M, Bernhardt BC, Singer T. Functional and microstructural plasticity following social and interoceptive mental training. elife. 2023:12:e85188. 10.7554/eLife.85188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velthorst E, Levine SZ, Henquet C, de Haan L, van Os J, Myin-Germeys I, Reichenberg A. To cut a short test even shorter: reliability and validity of a brief assessment of intellectual ability in schizophrenia—a control-case family study. Cogn Neuropsychiatry. 2013:18(6):574–593. 10.1080/13546805.2012.731390. [DOI] [PubMed] [Google Scholar]
- Viuff MH, Stochholm K, Uldbjerg N, Nielsen BB, Gravholt CH. Only a minority of sex chromosome abnormalities are detected by a national prenatal screening program for down syndrome. Hum Reprod. 2015:30(10):2419–2426. 10.1093/humrep/dev192. [DOI] [PubMed] [Google Scholar]
- Waehnert MD, Dinse J, Weiss M, Streicher MN, Waehnert P, Geyer S, Turner R, Bazin PL. Anatomically motivated modeling of cortical laminae. NeuroImage. 2014:93:210–220. 10.1016/j.neuroimage.2013.03.078. [DOI] [PubMed] [Google Scholar]
- Waehnert MD, Dinse J, Schäfer A, Geyer S, Bazin P-L, Turner R, Tardif CL. A subject-specific framework for in vivo myeloarchitectonic analysis using high resolution quantitative MRI. NeuroImage. 2016:125:94–107. 10.1016/j.neuroimage.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Wagstyl K, Larocque S, Cucurull G, Lepage C, Cohen JP, Bludau S, Palomero-Gallagher N, Lewis LB, Funck T, Spitzer H, et al. BigBrain 3D atlas of cortical layers: cortical and laminar thickness gradients diverge in sensory and motor cortices. PLoS Biol. 2020:18(4):e3000678. 10.1371/journal.pbio.3000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwick MM, Doody GA, Lawrie SM, Kestelman JN, Best JJ, Johnstone EC. Volumetric magnetic resonance imaging study of the brain in subjects with sex chromosome aneuploidies. J Neurol Neurosurg Psychiatry. 1999:66(5):628–632. 10.1136/jnnp.66.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang Y, Li Y, Meng Y, Li M, Wang Q, Deng W, Ma X, Palaniyappan L, Zhang N, et al. Depth-dependent abnormal cortical myelination in first-episode treatment-naïve schizophrenia. Hum Brain Mapp. 2020:41(10):2782–2793. 10.1002/hbm.24977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Bautista A, McCarron A. Convergence of spoken and written language processing in the superior temporal sulcus. NeuroImage. 2018:171:62–74. 10.1016/j.neuroimage.2017.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AC, King J, Bishop DVM. Autism and social anxiety in children with sex chromosome trisomies: an observational study. Wellcome Open Res. 2019:4:32. 10.12688/wellcomeopenres.15095.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Zhang Z, Zhao Q, Zhang J, Zhong S, Bi Y, He Y, Pan H, Gong G. The effects of X chromosome loss on neuroanatomical and cognitive phenotypes during adolescence: a multi-modal structural MRI and diffusion tensor imaging study. Cereb Cortex. 2015:25(9):2842–2853. 10.1093/cercor/bhu079. [DOI] [PubMed] [Google Scholar]
- Yamagata B, Barnea-Goraly N, Marzelli MJ, Park Y, Hong DS, Mimura M, Reiss AL. White matter aberrations in Prepubertal Estrogen-naive girls with Monosomic Turner syndrome. Cereb Cortex. 2011:22(12):2761–2768. 10.1093/cercor/bhr355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Gao Y, Gerig G. ITK-SNAP: An interactive tool for semi-automatic segmentation of multi-modality biomedical images. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2016:3342–3345. [DOI] [PMC free article] [PubMed]
- Zhang S, She S, Qiu Y, Li Z, Mao D, Zheng W, Wu H, Huang R. Altered cortical myelin in the salience and default mode networks in major depressive disorder patients: a surface-based analysis. J Affect Disord. 2023:340:113–119. 10.1016/j.jad.2023.07.068. [DOI] [PubMed] [Google Scholar]