Abstract
Metastasis to the clitoris is extremely rare. Here, we report a highly unusual case of high-grade squamous cell carcinoma of the cervix metastasizing to the clitoris a year following surgery and chemoradiotherapy. The patient presented with a painless clitoral mass identified through physical examination. Magnetic resonance imaging (MRI) showed a diffusely enhancing clitoral mass with hyperintense signals on diffusion-weighted imaging (DWI) and fluid-sensitive T2-weighted (T2W) sequences. This malignant tumor was detected by 18fluorine-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) due to its high FDG uptake. Pathological examination confirmed clitoral metastasis. Clitoral metastasis, although exceedingly rare, should be considered in cervical cancer patients presenting with clitoral masses on physical examination and imaging, particularly in those with advanced stages. Our case report is unique because it represents a recurrence in a patient initially diagnosed with early-stage cancer.
Keywords: Cervical cancer, Metastasis, Clitoris, 18F-FDG PET/CT, MRI
Introduction
Cancer of the clitoris can manifest as both primary and secondary malignancies [1]. Primary malignancy of the clitoris is classified as vulvar carcinoma. However, secondary malignancy of the clitoris is an extremely rare condition. Previous reports of secondary carcinoma of the clitoris most frequently originated from urinary system cancers (bladder and kidney), followed by endometrial and gastrointestinal cancers [2]. Only 6 reported cases of cervical cancer metastasizing to the clitoris have been identified in the literature [3]. 18F-FDG PET/CT is often used for cervical cancer staging, while MRI can help demonstrate the extent of the tumor for surgical and radiation therapy planning.
Case presentation
A 77-year-old female patient presented with post-menopausal bleeding and a negative preoperative work up for cancer. She underwent a robotic-assisted total laparoscopic hysterectomy and bilateral salpingo-oophorectomy, and on final pathology was found to have an incidental FIGO (International Federation of Gynecology and Obstetrics) Stage IB2 invasive squamous carcinoma of the cervix. Following this, she completed concurrent weekly Cisplatin 40 mg/m2 and external pelvic radiotherapy followed by vaginal brachytherapy. A post-treatment 18F-FDG PET/CT demonstrated no residual disease.
One-year post-surgery and chemoradiotherapy, the patient presented with a new clitoral mass. She denied experiencing clitoral pain or fever. Upon pelvic examination, a firm 2 x 1 cm mass was palpated at the base of the patient's clitoris. The clitoral mass appeared discrete, mobile, and nontender. Vaginal examination revealed smooth vaginal walls with no vaginal masses and an absent cervix. There was no clinically suspicious inguinofemoral adenopathy.
A pelvic MRI was conducted using a 1.5 Tesla magnet MRI with and without contrast, revealing a solid mass in the region of the clitoris measuring 1.3 x 2.5 x 3.0 cm. It appeared hypointense on T1-weighted imaging (T1WI) and was difficult to detect without intravenous contrast (Fig. 1). Following the intravenous contrast administration of 0.1 mmol/kg of gadolinium at a rate of 2 mL/second, there was diffuse enhancement of the clitoral mass on fat-saturated T1WI (Fig. 2).
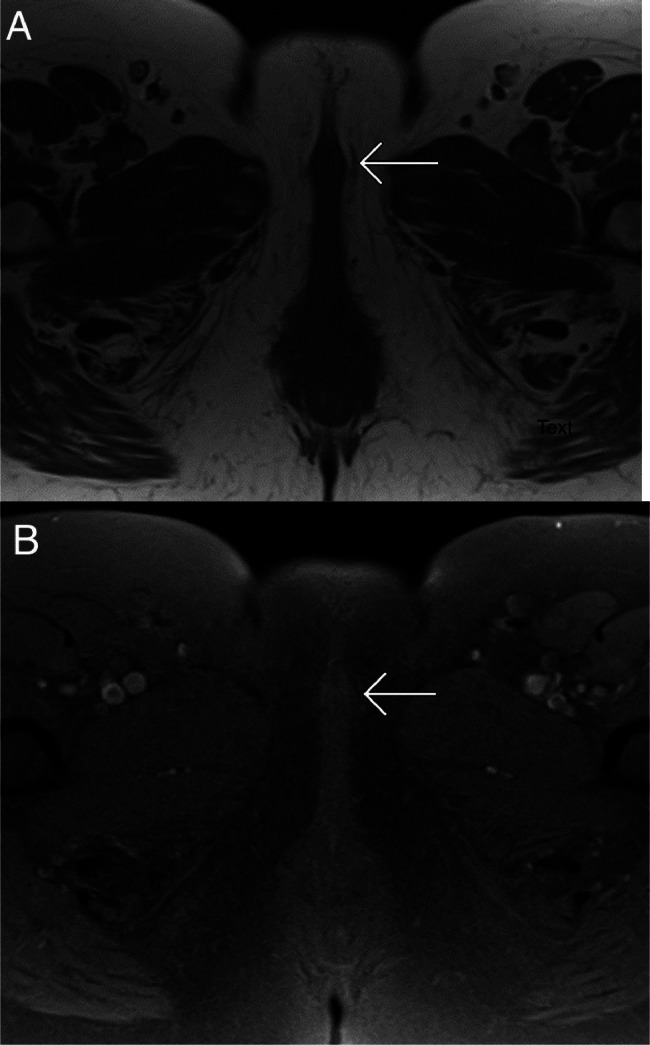
Fig. 1.
MRI of the pelvis at the level of the clitoris. Noncontrast axial T1WI (A) and noncontrast axial T1WI with fat saturation (B) show a subtle mass in the clitoris (white arrows). The clitoral metastasis was difficult to detect without intravenous contrast.
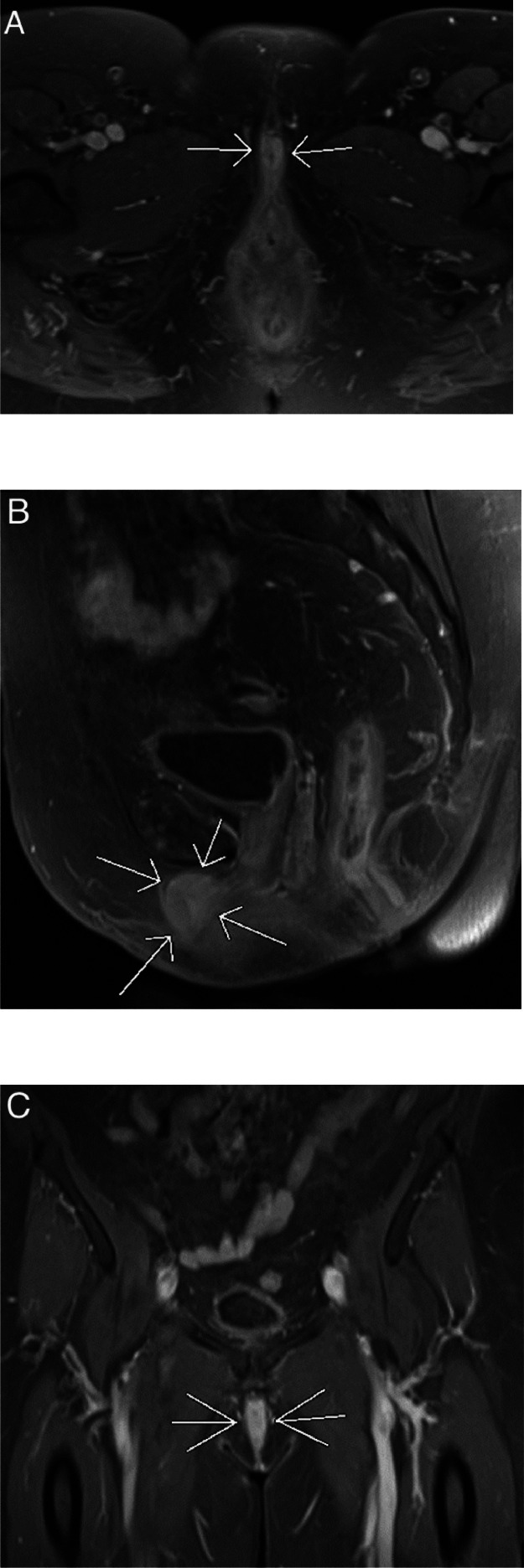
Fig. 2.
An MRI of the pelvis was performed with intravenous contrast administration, utilizing T1WI with fat saturation. Axial (A), sagittal (B), and coronal (C) images clearly delineate a diffusely enhancing clitoral mass measuring 1.3 × 2.5 × 3.0 cm (white arrows).
It was mildly hyperintense on T2-weighted imaging (T2WI), but was much more conspicuous on short tau inversion recovery (STIR) imaging, which is a fluid-sensitive MRI sequence (Fig. 3). Additionally, it exhibited restricted diffusion on diffusion-weighted imaging (DWI) (Fig. 4).
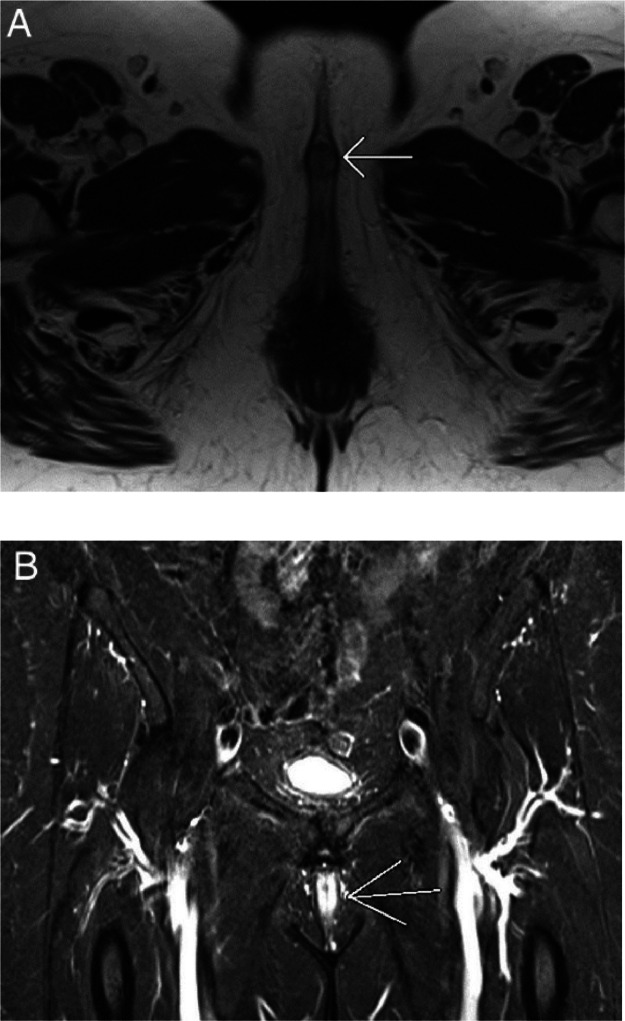
Fig. 3.
T2WI of the pelvis. Axial T2WI (A) demonstrates a well-circumscribed mass in the clitoris (white arrow). Coronal STIR (B), which is a highly fluid-sensitive sequence with excellent fat suppression, clearly demonstrates a mass in the clitoris due to its high water content (white arrow).
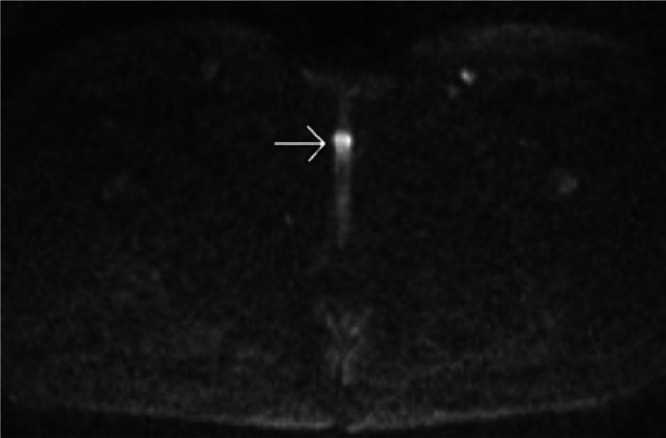
Fig. 4.
An axial MRI of the pelvis with DWI shows diffusion restriction (bright signal intensity) in the clitoral mass (white arrow).
An 18F-FDG PET/CT showed an isolated, hypermetabolic clitoral mass with a maximum SUV (standardized uptake value) of 13.1 (Fig. 5). No local recurrence was observed at the vaginal apex, and there was no evidence of pelvic or retroperitoneal lymphadenopathy or distant metastasis on 18F-FDG PET/CT.
Fig. 5.
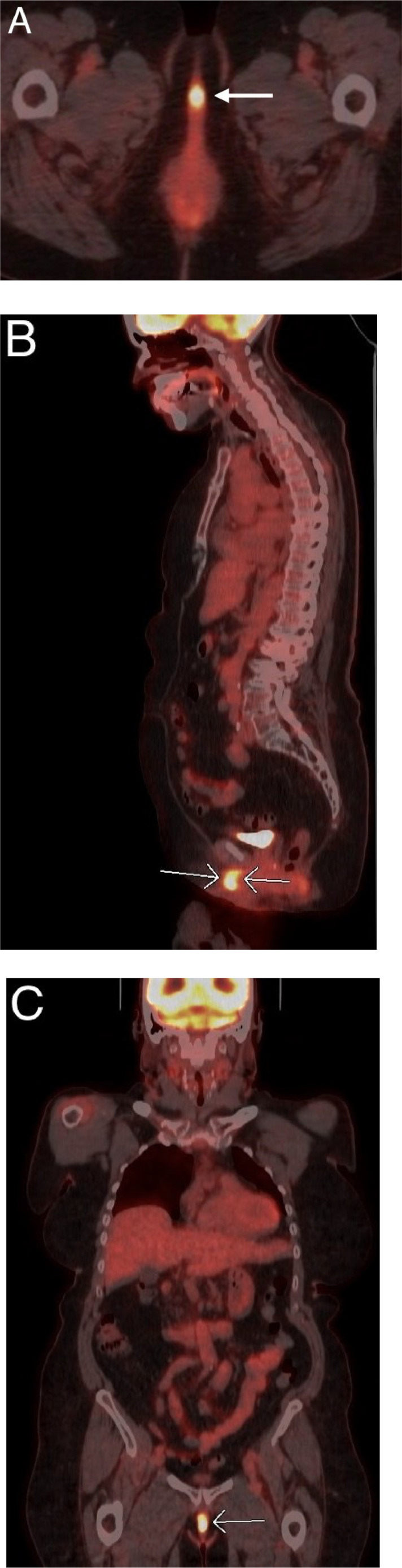
An 18F-FDG PET/CT of the body from the skull base to the thigh shows a localized hypermetabolic clitoral mass with a maximum SUV of 13.1 in axial (A), sagittal (B), and coronal (C) planes. There was no imaging evidence of distant metastasis detected elsewhere in the body on this 18F-FDG PET/CT.
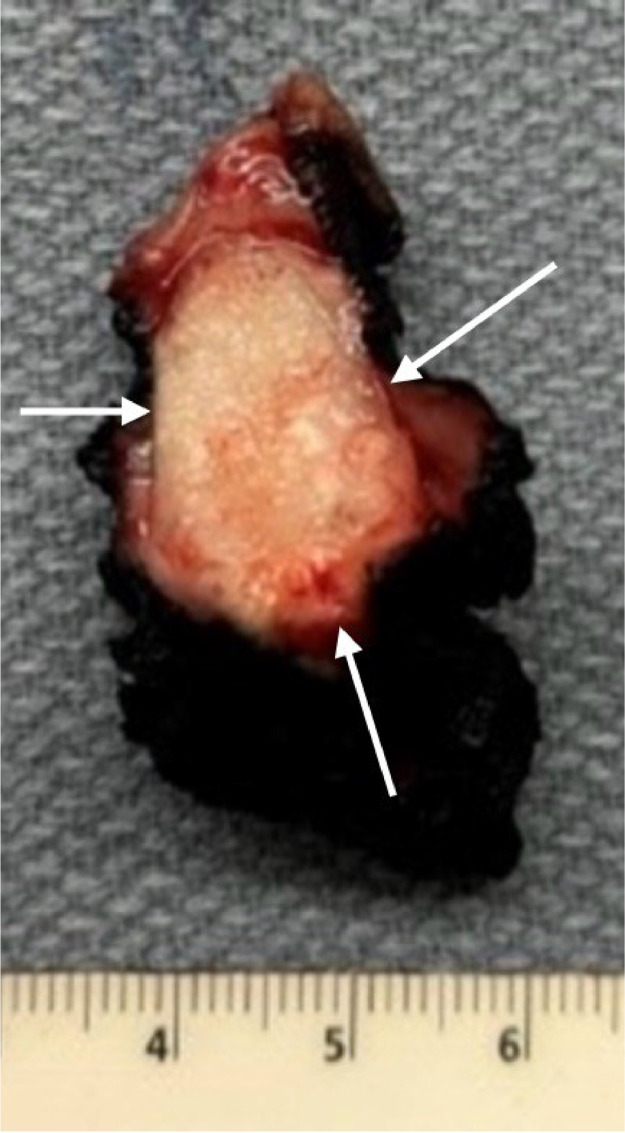
The patient underwent partial radical vulvectomy with resection of the clitoral mass (Fig. 6). The pathological diagnosis revealed a moderately differentiated, invasive recurrent cervical squamous cell carcinoma involving the submucosal and deep soft tissue of the clitoris with lymphovascular invasion (Fig. 7). She received adjuvant radiotherapy to the inguinofemoral lymph nodes and resection bed and remains disease-free 1 year after completion of treatment.
Fig. 6.
The gross surgical specimen demonstrates a clitoral mass, which appears pale-tan, firm, ill-defined, and glistening (white arrows). .
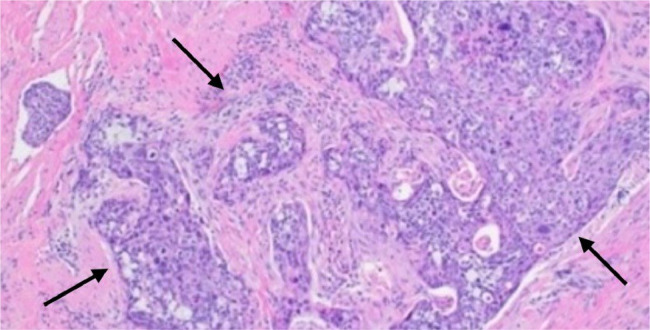
Fig. 7.
Hematoxylin and eosin (H&E) stained section of the clitoral tumor with photomicrograph (100X) shows invasive, moderately differentiated squamous cell carcinoma (black arrows) with lymphovascular invasion.
Discussion
Cervical cancer ranks as the fourth most common cancer among females globally, leading to over 300,000 deaths worldwide [4]. The causative agent of cervical cancer is persistent infection with high-risk subtypes of the Human Papillomavirus (HPV). The viral oncoproteins E5, E6, and E7 collaborate with host factors to initiate and perpetuate the malignant transformation [5]. Cervical cancer typically spreads by local extension, which can directly invade the vagina, parametrium, uterus, bladder, and rectum. It may also spread to the regional lymph nodes in the pelvis. In advanced disease, cervical cancer metastasizes through the bloodstream to distant sites. When diagnosed at an early stage, the 5-year relative survival rate is 91%. However, when diagnosed after spreading to nearby tissues, organs, or regional lymph nodes, the 5-year relative survival rate decreases to 60%. Furthermore, when cervical cancer is diagnosed after it has spread to a distant part of the body, the 5-year relative survival rate decreases drastically to 19% [6]. In a large population-based study, the typical single-site hematogenous metastasis locations were found to include lung (37.9%), bone (16.7%), liver (12.5%), and brain (1.6%), with 31.3% of hematogenous metastasis occurring in multiple organs [7].
It is highly unusual for cervical cancer not to spread locally to adjacent tissues, organs, or regional lymph nodes, but rather to spread to the clitoris, as in our case report. Only 6 reported cases in the literature (Table 1) have demonstrated the spread of cervical cancer to the clitoris, and all 6 of these cases were initially diagnosed with locally advanced disease and distant metastasis (FIGO stages IIIB, IVB, IIIB, IVB, IVB, and IIIA) [3]. Our case report is unique in that it involved an early-stage cancer, FIGO stage IB2, with tumor recurrence a year later in the clitoris. Restaging pelvic MRI and 18F-FDG PET/CT demonstrated clitoral metastasis as the only site of tumor recurrence. There was no evidence of the local tumor involving the vaginal vault, parametrium, or regional lymph nodes. Additionally, there were no distant metastases to the lung, bone, liver, and brain as demonstrated by 18F-FDG PET/CT.
Table 1.
Summary of reported cases of clitoral metastasis from cervical cancer.
| Author | FIGO Stage | Histology | Clinical Findings |
|---|---|---|---|
| Hino Y [3] | IIIA | Adenocarcinoma | Clitoral pain and enlargement |
| Marek CB [11] | IIIB | Squamous Cell Carcinoma | Clitoral enlargement |
| Bonneau C [12] | IVB | Squamous Cell Carcinoma | Clitoral pain and enlargement |
| Jiang S [2] | IIIB | Squamous Cell Carcinoma | Clitoral pain and enlargement |
| Papoutsis D [13] | IVB | Adenocarcinoma | Clitoral pain |
| Lu YY [14] | IVB | Squamous Cell Carcinoma | Information not available |
| The present case | IB2 | Squamous Cell Carcinoma | Clitoral enlargement |
Imaging may offer an accurate method to detect tumor relapse early, enabling timely salvage treatment when it may be most effective [8]. 18F-FDG PET/CT is the study of choice for the evaluation of cervical cancer to determine the extent of disease, accurately detecting both local and distant involvement. It provides functional and anatomical information to be obtained in a single examination. 18F-FDG PET/CT offers superior sensitivity and specificity compared to CT and other anatomical imaging methods, as it reports on regional glucose metabolism. It outperforms other imaging modalities in assessing lymph nodes status and distant metastasis [9]. In surgically staged patients, 18F-FDG PET/CT has demonstrated a sensitivity of over 80% and a specificity of over 90% for detecting regional lymph nodes metastasis. Higher FDG uptake in both the primary tumor and regional lymph node metastasis strongly predicts a worse outcome [6].
MRI, due to its exquisite soft tissue resolution, can provide a more detailed, accurate depiction of the size and extent of a clitoral tumor, facilitating treatment planning. An MRI of a clitoral cancer typically reveals high signal intensity on T2WI, robust enhancement on postcontrast T1WI, and restricted diffusion on DWI of the lesion. A tumor that responds to therapy often exhibits a decrease in size of the mass over time, with low signal intensity on T2WI due to post-treatment fibrosis, reduced enhancement, and absence of diffusion restriction on DWI [10]. In our experience with this case and the corresponding literature, we found DWI and postcontrast fat-saturated T1WI to be the most useful MRI sequences in detecting the clitoral tumor. In the era of image-guided adaptive radiotherapy, accurately defining disease areas using MRI is crucial to avoid irradiating normal tissue.
Conclusions
Cervical cancer metastasizing to the clitoris is very rare. Knowledge of this possibility can aid in diagnosing clitoral metastasis when a patient presents with a clitoral mass through the use of pelvic examination, 18F-FDG PET/CT, and MRI.
Patient consent
A written, informed consent for publication of the case was obtained from the patient.
Footnotes
Competing Interests: The authors have no declaration of interest.
References
- 1.Czernobilsky B, Gat A, Evron R, Dgani R, Ben-Hur H, Lifschitz-Mercer B. Carcinoma of the clitoris: a histologic study with cytokeratin profile. Int J Gynecol Pathol. 1995;14:274–278. doi: 10.1097/00004347-199507000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Jiang S, Sheng XG, Song QQ, Lu CH, Pan CX, Li QS. Metastasis of a cervical carcinoma to the clitoris. Jiang J Obstet Gynaecol. 2015;35:213–214. doi: 10.3109/01443615.2014.937335. [DOI] [PubMed] [Google Scholar]
- 3.Hino Y, Yamada Y, Miura S, Okada F, Uchiyama T, Mabuchi S. Clitoral metastasis from uterine cervical cancer: a case report and review of the literature. Gynecol Oncol Rep. 2020;33:100591. doi: 10.1016/j.gore.2020.100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.“Cervical Cancer Prognosis and Survival Rates (NCI website).” National Cancer Institute, 2024, www.cancer.gov/types/cervical/survival.
- 5.Burmeister CA, Khan SF, Schäfer G, Mbatani N, Adams T, Moodley J, Prince S. Cervical cancer therapies: current challenges and future perspectives. Tumour Virus Res. 2022;13:200238. doi: 10.1016/j.tvr.2022.200238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrera FG, Prior JO. The role of PET/CT in cervical cancer. Front Oncol. 2013;3:34. doi: 10.3389/fonc.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou S, Peng F. Patterns of metastases in cervical cancer: a population-based study. J Clin Exp Pathol. 2020;13(7):1615–1623. [PMC free article] [PubMed] [Google Scholar]
- 8.Miccò M, Lupinelli M, Mangialardi M, Gui B, Manfredi R. Patterns of recurrent disease in cervical cancer. J Pers Med. 2022;12(5):755. doi: 10.3390/jpm12050755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grigsby PW. The prognostic value of PET and PET/CT in cervical cancer. Cancer Imaging. 2008;8(1):146–155. doi: 10.1102/1470-7330.2008.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miccò M, Russo L, Persiani S, Dolciami M, Manganaro L, Cunha TM, Janicas C, Rizzo S, Nicolic O, Garganese G, Tagliaferri L. MRI in the evaluation of locally advanced vulvar cancer treated with chemoradiotherapy and vulvar cancer recurrence: the 2021 revision of FIGO classification and the need for multidisciplinary management. Cancers (Basel) 2022;14(16):3852. doi: 10.3390/cancers14163852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marek CB, Hayden CR. Metastatic carcinoma of the clitoris. Am J Obstet Gynecol. 1950;60:443–444. doi: 10.1016/0002-9378(50)90491-1. [DOI] [PubMed] [Google Scholar]
- 12.Bonneau C, Rodrigues A, Poncelet C, Cornelis F, Chanelles O, Carbillon L, Bricou A. Clitoral metastasis from cervical carcinoma. J Obstet Gynaecol. 2011;31:359–360. doi: 10.3109/01443615.2011.556269. [DOI] [PubMed] [Google Scholar]
- 13.Papoutsis D, Haefner HK. Metastatic adenocarcinoma to the clitoris from the cervix. Am J Obstet Gynecol. 2015;213 doi: 10.1016/j.ajog.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 14.Lu YY, Lu CH, Wang HY. Clitoral metastasis from advanced cervical carcinoma on 18F-FDG-PET/CT. Clin Nucl Med. 2017;42:54–55. doi: 10.1097/RLU.0000000000001410. [DOI] [PubMed] [Google Scholar]