Abstract
Background
Romosozumab is a novel monoclonal antibody that binds to sclerostin, and has dual effects of increasing bone formation and decreasing bone resorption, giving it a unique mechanism of action. The objective of this study was to perform a systematic review and meta-analysis based on existing worldwide data on treatment effects and safety of romosozumab in randomized controlled trials.
Methods
A systematic search was carried out on four databases including PubMed, Embase, Web of Science and Cochrane Central Register of Controlled Trials (CENTRAL). The keywords used for search was “(romosozumab) AND (osteoporosis OR safety)”. Randomized controlled trial or post-hoc studies of the included randomized controlled trial which studied the effects and safety of romosozumab were included. The quality of selected studies was assessed with the Cochrane collaboration tool and the PEDro scale.
Results
20 studies were included for qualitative analysis. 14 studies were included for meta-analysis. In total, there were 13,507 (n = 13,507) participants with 637 men and 12,870 women from original cohorts. The overall mean difference was in favor of romosozumab treatment for lumbar spine (10.04 (95 % confidence interval (CI) = 7.51–12.57; p < 0.00001)), total hip (4.04 (95 % CI = 3.10–4.99; p < 0.00001)) and femoral neck bone mineral density (3.77 (95 % CI = 2.90–4.64; p < 0.00001)) at 12 months. There was significantly less likelihood of new vertebral fractures with romosozumab compared to control (odds ratio (OR) 0.42 (95 % CI = 0.20–0.89); p = 0.02) at 12 months of treatment. There was significantly less likelihood of new vertebral fracture at 24 months with 12 months of romosozumab followed by sequential treatment with anti-resorptive compared to control with only anti-resorptive agent use (OR 0.36 (95 % CI = 0.18–0.71); p = 0.003). There was no significant difference in serious adverse events and fatal adverse events with use of romosozumab compared with control in our meta-analyses. There were no significant differences in serious cardiovascular events in Asian population of romosozumab with control group with 12 months of romosozumab treatment followed by 24 months of anti-resorptive agent with OR 1.09 (95 % CI = 0.40–2.96; P = 0.86). There was no significant difference between romosozumab group and control group for the median time to radiographic healing. Our qualitative analysis on Quantitative Computed Tomography (QCT), Finite element analysis (FEA) and bone biopsy analyses demonstrated that romosozumab improved parameters and measures in these domains as well.
Conclusion
In conclusion, our study showed that romosozumab was an effective agent to treat osteoporosis with high quality evidence. There were no significant differences in the adverse events, serious adverse events, fatal adverse events identified. Further subgroup analysis of cardiovascular events and cardiovascular death in the total population showed no differences either.
The translational potential of this article
Given the results, romosozumab is an effective agent to treat patients with very-high risk of osteoporotic fractures.
Keywords: Cardiovascular, Osteoporosis, Osteoporotic fracture, Romosozumab, Safety
Graphical abstract
The translational potential of this article: Given the results, romosozumab is an effective agent to treat patients with very-high risk of osteoporotic fractures.
1. Introduction
Osteoporosis causes more than 8.9 million fractures annually and has become a worldwide health problem [1]. In fact, an osteoporotic fracture is estimated to occur every 3 s, and with the aging population, the number of osteoporotic fractures are estimated to increase rapidly worldwide [2]. After the occurrence of an osteoporotic fracture, the imminent risk of a fracture is high and existing global data shows it to be approximately 11.58 %, and more importantly, half of all refractures occur in the first 2 years after the initial event [1]. The risk of mortality is also higher in patients with subsequent fractures, and the treatment of osteoporosis can decrease mortality [3].
Previous meta-regression analyses have shown that improving bone mineral density is strongly associated with fracture risk reduction [4]. Therefore, current clinical guidelines have suggested the use of anabolic agents initially followed by an anti-resorptive agent for very-high risk patients including those with a recent osteoporotic fracture [[5], [6], [7]]. The rationale of this is to have a greater effect in a short period of time to best reduce an imminent risk of fracture, which is then maintained with the anti-resorptive agent. In fact, a previous study showed that 1 year of romosozumab followed by 1 year of denosumab approximated the effects of 7 years of continuous denosumab [8], illustrating as an example, the attractiveness of sequential therapy.
Romosozumab is a novel monoclonal antibody that binds to sclerostin, and has dual effects of increasing bone formation and decreasing bone resorption [9], giving it a unique mechanism of action. Numerous randomized controlled trials have been conducted on romosozumab in treating osteoporosis, where the largest ones include the Fracture Study in Postmenopausal Women with Osteoporosis (FRAME) and the Active-Controlled Fracture Study in Postmenopausal Women with Osteoporosis at High Risk (ARCH) trials [9,10], demonstrating the efficacy of the anti-osteoporotic agent. It is known that there is a warning for romosozumab in which there is potential risk of serious cardiovascular events such as myocardial infarction and stroke. In the FRAME trial, it was found that there was no significant difference in cardiovascular events with romosozumab compared to placebo [9]. However, in the ARCH trial, there was a 2.5 % cardiovascular adverse event in the romosozumab group compared to 1.9 % in the alendronate group after 12 months of treatment [10]. It remains unclear whether this observation is due to the use of sclerostin inhibition in an older population causing higher cardiovascular risk, or by the protective effect of alendronate or by chance alone [11]. Therefore, there is a need for more real-world evidence still [12].
Importantly, recently there have been more randomized controlled trials that have been published providing more clinical evidence and data on romosozumab. Studies have even been performed in using romosozumab for the treatment of fracture healing related outcomes [13,14]. Therefore, the objective of this study was to perform a systematic review and meta-analysis based on existing worldwide data on treatment effects and safety of romosozumab in randomized controlled trials.
2. Methodology
2.1. Search strategy
A systematic search was carried out on four databases including PubMed, Embase, Web of Science and Cochrane Central Register of Controlled Trials (CENTRAL), with last access date on 10 July 2024. The keywords used for search was “(romosozumab) AND (osteoporosis OR safety)”. The search was limited to articles in English language. No restriction was applied on publication dates. Additionally, reference lists on relevant articles were checked manually for potential eligible articles. This systematic review and meta-analysis was reported based on the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statements [15].
2.2. Search criteria
The inclusion criteria were: 1) randomized controlled trial or post-hoc studies of the included randomized controlled trial which studied the effects and safety of romosozumab, 2) use of romosozumab as an intervention/treatment for participants, 3) dosage of 210 mg romosozumab 4) follow-up period for at least 6 months. The exclusion criteria were: 1) no full text, 2) article not in English, 3) conference abstract, 4) outcomes not directly related to the efficacy or the safety of romosozumab, 5) missing data, 6) review papers, 7) included a second course of romosozumab.
2.3. Selection of studies
Article selection was conducted by two independent reviewers. All references searched by keywords from the four databases were imported into a reference management tool and all duplicate references were removed. References were screened out by title and abstracts according to the established inclusion and exclusion criteria. The full-text article of the remaining references were retrieved for further eligibility assessment. Disagreements for article selection were settled by discussion until consensus was reached. If discrepancies still existed, it would be resolved by a third reviewer.
2.4. Data extraction
Data extraction of the eligible studies was conducted by two independent reviewers and was presented in tables in a standardized form. The following data were extracted: The study characteristics include the surname of the first author, year of publication, country/region of the study and study design. Participant characteristics included population type, sample size (N), age in mean with standard deviation (mean ± SD) and gender. The intervention characteristics including dosage of romosozumab and control group, administration method and period of treatment, and follow-up data were collected. Outcome data, adverse event and other key findings after treatment were also collected.
2.5. Quality assessment of studies
The quality of the selected studies was assessed with the Cochrane collaboration tool and the PEDro scale by two independent reviewers. For the Cochrane collaboration tool, the risk of bias in 7 domains were assessed including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, incomplete outcome data, selective reporting and other sources of bias [16]. The risk of bias of each domain was classified as “low risk”, “high risk” or “unclear”. The risk of bias within studies and across studies were depicted by a risk of bias graph and a risk of bias summary respectively. For the PEDRo scale, a total of 11 items were assessed for methodology quality. Studies with total scores of ≥6 are considered as “good” quality, 4–5 are “fair” quality, and <4 are of “poor” quality [17]. Trials of poor quality would be excluded from analysis.
2.6. Data analysis
Assessment outcomes of efficacy and safety of romosozumab were analysed in our meta-analysis. The efficacy included areal bone mineral density (BMD) (percentage) in lumbar spine, total hip and femoral neck at month 6 and month 12 of treatment which was expressed in mean ± SD. As BMD data in some of the included studies were expressed in mean change (%) with 95 % confidence interval (CI) only. The SD in these studies was estimated with the equation: SQRT (total sample size)*(Upper CI-Lower CI)/(T.INV.2 T(0.05, Total sample size-1)*2) [18] in excel file or via Review Manager (RevMan) Calculator. The mean difference with 95 % CI between the romosozumab group, 210 mg once per month (QM) subcutaneously (SC) and control group was computed. Subgroup analysis for the active and placebo control was performed for each of the three sites in 2 time points. To reduce unit-of-analysis error, for McClung et al., 2014 [19] with multiple comparison of romosozumab against placebo, alendronate and teriparatide, only comparison of romosozumab against placebo was included in the pooled meta-analysis. The efficacy also included the incidence of new vertebral fractures at month 12 of treatment and also month 24 (additional 12 month of the transition effect of romosozumab to other osteoporotic drugs). Given the current existing data we were also able to perform subgroup analysis for Asian population for several outcomes. For month 24, subgroup analysis of total population and Asian population was performed. The odds ratio and 95 % CI between the romosozumab group (210 mg QM SC) and control group was computed.
In terms of assessment of safety, number of adverse events were analysed. For studies with romosozumab group (210 mg QM SC), total adverse events at month 12 and month 24 were performed. Subgroup analysis on adverse events, serious adverse events and fatal adverse events by different control groups were performed. Furthermore, cardiovascular adverse events analysis was further focused on. The cardiovascular adverse event within 12 months from all available studies was analysed. Subgroup analysis on serious cardiovascular event and cardiovascular death were also conducted. Analysis of cardiovascular adverse events in Asian population with 12 month romosozumab treatment followed by 24 months anti-resorptive agent was conducted. For fracture healing-related studies, analysis was performed as per the 4 doses on Day 1, Week 2, Week 6 and Week 12. Total adverse event at month 12 was performed. Adverse event was analysed with odds ratio and 95 % CI.
Data was used in studies with larger sample size if the same cohort was studied in different studies. Only studies with available data were included in meta-analysis. The effects on outcomes were analysed by Review Manager (RevMan 5.4, The Cochrane Centre, The Cochrane Collaboration). Heterogeneity was evaluated by I2 index or Cochran's Q test, where the I2 index >50 % or p-value <0.1 represented significant heterogeneity between studies. The random effect models were used in the forest plots where there was significant heterogeneity. Otherwise, fixed effect models were used in the forest plots. Sensitivity analyses was also conducted to investigate the effects of including certain type of participants (such as with disease) on the overall results. Qualitative analysis was performed for studies that were not included for meta-analysis.
3. Results
3.1. Search results
2592 records were identified through database searching (n = 2592) and 3 additional records were identified through other manual searching. Duplicate records were removed and 1524 records remained. The 1745 records were then screened by title and abstract, and 1706 records were excluded based on exclusion criteria. The remaining 39 full-text articles were assessed for eligibility and 19 articles were excluded based on exclusion criteria. 20 studies were included for qualitative analysis. 14 studies were included for meta-analysis (Fig. 1).
Figure 1.
PRISMA flowchart.
3.2. Study characteristics
The 20 studies were published from 2014 to 2023. 13 studies were randomized controlled trials [9,13,14,[19], [20], [21], [22], [23], [24], [25], [26], [27], [28]], in which 11 studies were the original cohorts [9,13,14,19,[22], [23], [24], [25], [26], [27], [28]] and 2 studies were the extension studies for studying the transitional effects of romosozumab to another osteoporotic drugs [20,21]. The remaining 7 were post-hoc analyses from the larger trial [[29], [30], [31], [32], [33], [34], [35]]. For the 13 randomized controlled trials, 5 studies [13,14,19,20,22] were phase 2 clinical trials, 6 studies [9,21,[23], [24], [25], [26]] were phase 3 clinical trials, 2 studies were pilot studies [27,28]. In which, active-control group was used with alendronate in 3 studies [19,20,24], teriparatide in 3 studies [19,20,23] or denosumab in 2 studies [27,28], and 9 studies [9,13,14,[19], [20], [21], [22],25,26] used placebo-control groups. In total, there were 13,507 (n = 13,507) participants with 637 men and 12,870 women in the original cohorts, 8 studies included postmenopausal women only [9,19,[22], [23], [24],[26], [27], [28]], 1 included men only [25] and 2 included both men and women [13,14]. Together with post-hoc studies, 7 studies focused on participants from Asian regions only [22,[26], [27], [28],32,33,35], while the remaining studies included a global population. Sample sizes ranged from 35 to 7180. 18 studies with outcomes related to osteoporosis, and 2 studies were related to fracture healing [13,14]. The follow-up period ranged from 2 months to 36 months. The cohort information of the randomized controlled trial and its post-hoc studies were summarized in Table 1. Detailed characteristics of the included studies were summarized in Supplementary Table 1.
Table 1.
Cohort information of included studies.
| Romosozumab, 210 mg once per month | ||||
|---|---|---|---|---|
| Cohort (NCT no.) | Population type | Author, year | Study design | Intervention/Analysis |
| NCT00896532 | Ambulatory postmenopausal women aged 55–85 years with low BMD from 28 study centers in Argentina, Austria, Belgium, Canada, Denmark, Spain & the United States [19] | McClung et al., 2014 [19] | Phase 2 RCT | Romosozumab, Placebo, Alendronate or Teriparatide (12 months) |
| McClung et al., 2018 [20] | Phase 2 RCT | 1. Romosozumab, Placebo, Alendronate or Teriparatide (24 months) & 2. Denosumab or Placebo (12 months) |
||
| Genant et al., 2017 [29] | Post-hoc analysis | Analysis on a subset of participants with QCT | ||
| Keaveny et al., 2017 [30] | Post-hoc analysis | Analysis on the subset of participants with QCT and FEA | ||
| The Fracture Study in Postmenopausal Women with Osteoporosis (FRAME) (NCT01575834) |
Postmenopausal women with osteoporosis (T-score between −2.5 and −3.5 at total hip/femoral neck) [9] | Cosman et al., 2016 [9] | Phase 3 RCT | 1. Romosozumab or Placebo (12 months) & 2. Denososumab (12 months) |
| Lewiecki et al., 2019 [21] | Phase 3 RCT | 1. Romosozumab or Placebo (12 months) & 2. Denososumab (12 months) & 3. Denososumab (12 months) |
||
| Chavassieux et al., 2019 [31] | Post-hoc analysis | Analysis on histomorphometry and microcomputed tomography‐based microarchitectural endpoints with transiliac bone biopsies | ||
| Miyauchi et al., 2019 [32] | Post-hoc analysis | Analysis on Japanese subgroup | ||
| Miyauchi et al., 2021 [33] | Post-hoc analysis | Analysis Japanese women with high fracture risk only | ||
| Eriksen et al., 2022 [34] | Post-hoc analysis | Analysis on bone biopsies of 2 months of romosozumab on the surface extent of modeling-based bone formation and remodeling-based bone formation | ||
| NCT01992159 | Ambulatory postmenopausal Japanese women with osteoporosis from 24 centers in Japan [22] |
Ishibshi et al., 2017 [22] | Phase 2 RCT | Romosozumab or Placebo (12 months) |
| STRUCTURE (NCT01796301) | Ambulatory postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy from 46 sites in North America, Latin America & Europe [23] | Langdahl et al., 2017 [23] | Phase 3 RCT | Romosozumab or Teriparatide (12 months) |
| Active-Controlled Fracture Study in Postmenopausal Women with Osteoporosis at High Risk (ARCH) (NCT01631214) |
Ambulatory postmenopausal women who had BMD T-score ≤ −2.5 at total hip/femoral neck & either ≥1 moderate/severe vertebral fractures or ≥ 2 mild vertebral fractures OR BMD T-score ≤ −2.0 at total hip/femoral neck & either ≥2 moderate/severe vertebral fractures or 1 proximal femur fracture sustained 3–24 months before randomization [24] |
Sagg et al., 2017 [24] | Phase 3 RCT | 1. Romosozumab or Alendronate (12 months) 2. Alendronate in both groups (24 months) |
| Lau et al., 2020 [35] | Post-hoc analysis | Analysis on East Asian population only | ||
| BRIDGE (NCT02186171) | Men aged 55–90 years with BMD T-score ≤ −2.5 OR ≤ −1.5 with a history of a fragility nonvertebral/vertebral fracture from 31 centers in Europe, Latin America, Japan & North America [25] |
Lewiecki et al., 2018 [25] | Phase 3 RCT | Romosozumab or Placebo (12 months) |
| NCT02791516 | Postmenopausal women with osteoporosis from 10 centers in Korea [26] | Baek et al., 2021 [26] | Phase 3 RCT | Romosozumab or Placebo (6 months) |
| TGE1220-064 | Patients with rheumatoid arthritis and severe osteoporosis (BMD T-score ≤ −2.5 and a previous fragility fracture OR BMD T-score ≤ −3.3 at LS and vertebral fracture ≥2 from clinical trial center of the author's institution [27] | Mochizuki et al., 2021 [27] | Pilot RCT | Romosozumab or Denosumab (6 months) |
| Mochizuki et al., 2023 [28] | Pilot RCT | 1. Romosozumab or Denosumab (6 months) 2. Denosumab (6 months) |
||
|
Romosozumab, 210 mg (4 doses) | ||||
|---|---|---|---|---|
| Cohort | Population type | Author, year | Study design | Intervention |
| NCT00907296 | Skeletally mature adults with fresh unilateral tibial diaphyseal fracture and had definitive fracture fixation with an IM nail (aged 18–82 years) [14] | Bhandari et al., 2020 [14] | Phase 2 RCT | Romosozumab (70 mg, 140 mg, 210 mg for 2, 3, 4 doses in each dosage respectively), 4 doses including Day 1, Week 2, Week 6 and Week 12, SC or Placebo |
| NCT01081678 | Patients with an acute, unilateral, low-energy hip fracture, and treated with open reduction and internal fixation (aged 55–95 years) [13] | Schemitsch et al., 2020 [13] | Phase 2 RCT | Romosozumab (70 mg, 140 mg, 210 mg for 2, 3, 4 doses in each dosage respectively), 4 doses including Day 1, Week 2, Week 6 and Week 12, SC or Placebo |
BMD, bone mineral density; RCT, randomized controlled trial; QCT, Quantitative Computed Tomography; FEA, finite element analysis; QM, once per month; SC, subcutaneous injection, IM nail, intramedullary nail.
3.3. Quality of selected studies
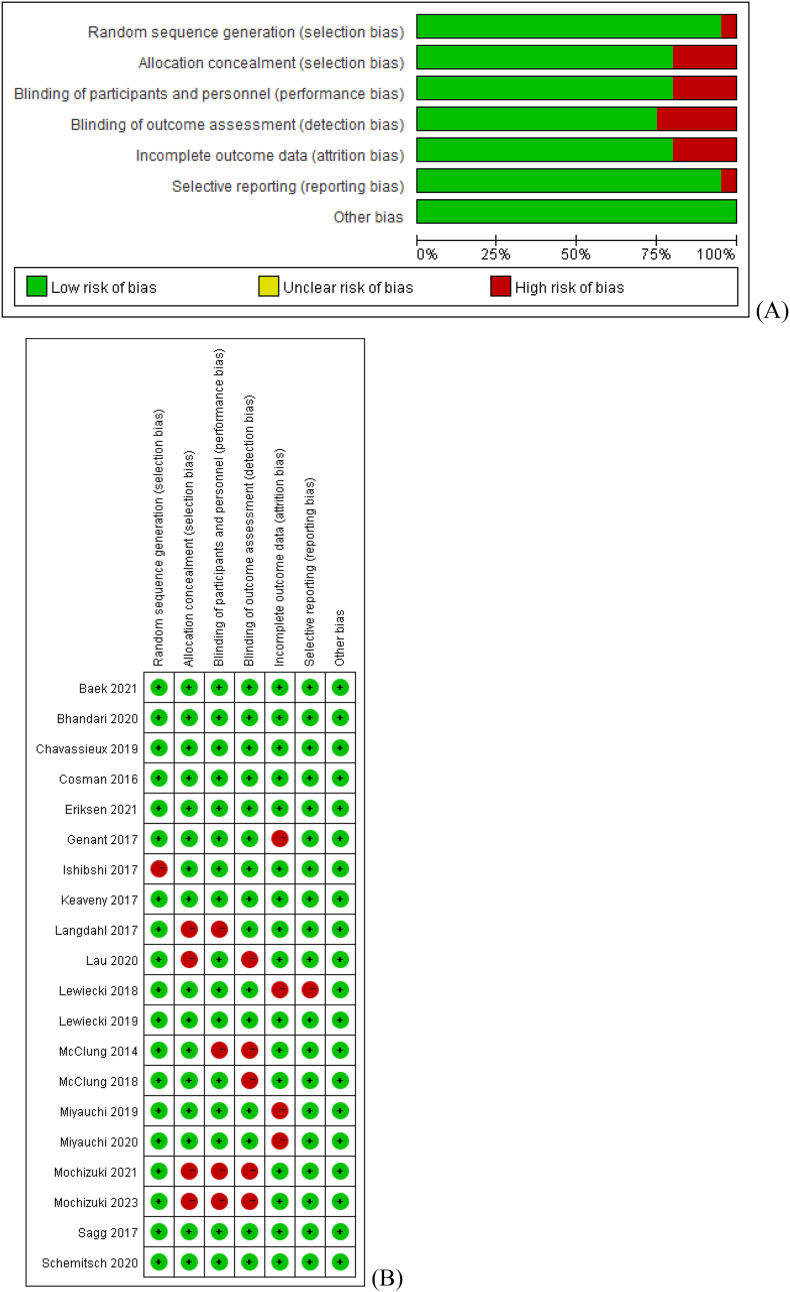
The Cochrane risk of bias tool was used for assessing the risk of bias of the 20 included studies. For random sequence generation and selective reporting, 1 study was classified as ‘high risk’, and the other 19 studies were classified as ‘low risk’ in each domain. For allocation concealment, blinding (participants and personnel) and incomplete outcome data, 4 studies were classified as 'high risk’, and the remaining 16 studies were classified as ‘low risk’ in each domain. For blinding (outcome assessment), 5 studies were classified as ‘high risk’, and the remaining 15 studies were classified as ‘low risk’. For other bias, all 20 studies were classified as ‘low risk’. The risk of bias graph and risk of bias summary are shown in Fig. 2. For the PEDRo scale, all studies had a score ≥7 and were considered as ‘good’ quality (Table 2).
Figure 2.
(A) Risk of bias graph of included randomized controlled trials (B) Risk of bias summary included randomized controlled trials.
Table 2.
PEDRo scale of included studies.
| Author, year | Eligibility Criteria | Randomised Allocation | Concealed Allocation | Baseline Comparability | Blinded Participants | Blinded Therapists | Blinded Assessors | Adequate follow-up | Intention-to-treat Analysis | Between-group Comparisons | Point estimates and variability | Total Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| McClung et al., 2014 [19] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 7 |
| Cosman et al., 2016 [9] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 |
| Genant et al., 2017 [29] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 8 |
| Ishibshi et al., 2017 [22] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9 |
| Keaveny et al., 2017 [30] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9 |
| Langdahl et al., 2017 [23] | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Sagg et al., 2017 [24] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 |
| Lewiecki et al., 2018 [25] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 10 |
| McClung et al., 2018 [20] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 9 |
| Chavassieux et al., 2019 [31] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 10 |
| Lewiecki et al., 2019 [21] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 |
| Miyauchi et al., 2019 [32] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 10 |
| Bhandari et al., 2020 [14] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 9 |
| Lau et al., 2020 [35] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 7 |
| Miyauchi et al., 2021 [33] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 9 |
| Schemitsch et al., 2020 [13] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 10 |
| Baek et al., 2021 [26] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 10 |
| Eriksen et al., 2022 [34] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 8 |
| Mochizuki et al., 2021 [27] | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Mochizuki et al., 2023 [28] | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
3.4. Systematic review and meta-analysis results
3.4.1. Lumbar spine, total hip and femoral neck BMD at 12 months of treatment
7 studies assessed romosozumab on lumbar spine BMD (%) after 12 months of treatment. Romosozumab to the 4 placebo-control groups showed significant improvements after 12 months of treatment (mean difference (MD) = 12.86 (95 % confidence interval (CI) = 10.72–15.01; p < 0.00001). Romosozumab to the 3 active-control groups also showed significant improvement after 12 months of treatment (MD = 6.19 (95 % CI = 2.74–9.64; p = 0.0004). The overall MD was 10.04 (95 % CI = 7.51–12.57; p < 0.00001) in favor of romosozumab (Fig. 3A). Subgroup analysis for romosozumab to the active-control group with alendronate showed significant improvement (MD = 8.12 (95 % CI = 6.69–9.55; p < 0.00001). Romosozumab to the active-control group with teriparatide also showed significant improvement (MD = 4.33 (95 % CI = 3.50–5.17; p < 0.00001) (Fig. S1).
Figure 3.
Forest plot of BMD change (%) from baseline and at month 12 of treatment in romosozumab group, 210 mg once per month subcutaneously and control group at three sites, (A) Lumbar spine, (B) Total hip and (C) Femoral neck. Subgroup analysis for placebo control and active control for the three sites.
6 studies assessed romosozumab on total hip BMD (%) after 12 months of treatment. Romosozumab to 3 placebo-control groups showed significant improvements after 12 months of treatment (mean difference (MD) = 5.20 (95 % CI = 3.75–6.66; p < 0.00001). Romosozumab to the 3 active-control groups also showed significant improvement after 12 months of treatment (MD = 3.18 (95 % CI = 2.46–3.90; p < 0.00001). The overall MD was 4.04 (95 % CI = 3.10–4.99; p < 0.00001) in favor of romosozumab (Fig. 3B). Subgroup analysis for romosozumab to the active-control group with alendronate showed significant improvement (MD = 2.88 (95 % CI = 1.71–4.04; p < 0.00001). Romosozumab to the active-control group with teriparatide showed significant improvement (MD = 3.19 (95 % CI = 2.62–3.75); p < 0.00001 (Fig. S2).
6 studies assessed romosozumab on femoral neck BMD (%) after 12 months of treatment. Romosozumab to the 3 placebo-control groups showed significant improvements after 12 months of treatment (mean difference (MD) = 4.69 (95 % CI = 3.41–5.98; p < 0.00001). Romosozumab to the 3 active-control groups also showed significant improvement after 12 months of treatment (MD = 3.14 (95 % CI = 2.47–3.81; p < 0.00001). The overall MD was 3.77 (95 % CI = 2.90–4.64; p < 0.00001) in favor of romosozumab (Fig. 3C). Subgroup analysis for romosozumab to the active-control group with alendronate showed significant improvement (MD = 3.89 (95 % CI = 2.33–5.44); p < 0.00001. Romosozumab to the active group with teriparatide showed significant improvement (MD = 3.13 (95 % CI = 2.40–3.87); p < 0.00001 (Fig. S3).
3.4.2. Lumbar spine, total hip and femoral neck BMD at 6 months of treatment
5 studies assessed romosozumab on lumbar spine BMD (%) after 6 months of treatment. Romosozumab to the 3 placebo-control groups showed significant improvements after 6 months of treatment (MD = 9.79 (95 % CI = 7.19–12.39; p < 0.00001). Romosozumab to the 2 active-control groups also showed significant improvement after 6 months of treatment (MD = 3.62 (95 % CI = 2.83–4.41; p < 0.00001). The overall MD was 7.14 (95 % CI = 3.54–10.74; p = 0.0001) in favor of romosozumab (Fig. 4A). Subgroup analysis for romosozumab to the active-control group with teriparatide showed significant improvement (MD = 3.62 (95 % CI 2.93–4.31); p < 0.00001 (Fig. S4).
Figure 4.
Forest plot of BMD change (%) from baseline and at month 6 of treatment in romosozumab group, 210 mg once per month subcutaneously and control group at three sites, (A) Lumbar spine, (B) Total hip and (C) Femoral neck. Subgroup analysis for placebo control and active control for the three sites.
5 studies assessed romosozumab on total hip BMD (%) after 6 months of treatment. Romosozumab to the 3 placebo-control groups showed significant improvements after 6 months of treatment (MD = 2.99 (95 % CI = 2.36–3.63; p < 0.00001). Romosozumab to the 2 active-control groups did not show significant improvement after 6 months of treatment (MD = 1.72 (95 % CI = −1.21-4.65; p = 0.25). The overall MD was 2.65 (95 % CI = 1.89–3.41; p < 0.00001) in favor of romosozumab (Fig. 4B). Subgroup analysis for romosozumab to the active-control group with teriparatide showed significant improvement (MD = 2.80 (95 % CI 2.12–3.48); p < 0.00001 (Fig. S5).
5 studies assessed romosozumab on femoral neck BMD (%) after 6 months of treatment. Romosozumab to the 3 placebo-control groups showed significant improvements after 6 months of treatment (MD = 2.41 (95 % CI = 1.66–3.16; p < 0.00001). The 2 active-control groups did not show significant improvement after 6 months of treatment (MD = 1.49 (95 % CI = −2.13-5.10; p = 0.42). The overall MD was 2.26 (95 % CI = 1.29–3.22; p < 0.00001) in favor of romosozumab (Fig. 4C). Subgroup analysis for romosozumab to the active-control group with teriparatide showed significant improvement (MD = 2.33 (95 % CI 0.57–4.09); p = 0.010 (Fig. S6).
3.4.3. Incidence of new vertebral fractures at 12 months of treatment
3 studies assessed romosozumab on the incidence of new vertebral fractures at 12 months of treatment. Results showed that there was significantly less likelihood of new vertebral fractures with the use of romosozumab compared to control (odds ratio (OR) 0.42 (95 % CI = 0.20–0.89); p = 0.02) (Fig. 5A).
Figure 5.
Forest plot of incidence of new vertebral fracture in romosozumab group, once per month subcutaneously and control group (A) at month 12 of treatment and (B) at month 24 in which all studies included romosozumab or control treatment in the first 12 month, and then switched to another active drugs for another 12 months with aim of studying the transition effects of romosozumab, subgroup analysis for Asian population at month 24.
3.4.4. Sequential treatment with romosozumab to anti-resorptive
4 studies assessed romosozumab on the incidence of new vertebral fractures at 24 months with 12 months of romosozumab followed by sequential treatment with anti-resorptive. Results from total population (2 studies) showed that there was significantly less likelihood of new vertebral fracture with the use of romosozumab followed by anti-resorptive compared to control of only anti-resorptive agent use (OR 0.36 (95 % CI = 0.18–0.71); p = 0.003). Subgroup analysis for Asian populations (2 studies) showed that there was significantly less likelihood with the use of romosozumab followed by anti-resorptive compared to control of only anti-resorptive agent use with OR 0.36 (95 % CI = 0.15–0.86); p = 0.02 (Fig. 5B).
In the study by Lewiecki et al. [21], subjects received romosozumab or placebo for 12 months (same cohort as FRAME study), then followed by 24 months of denosumab. Compared with placebo-to-denosumab group, the mean difference in relative increase in BMD percentage changes from baseline were significantly greater in romosozumab-to-denosumab group at 36 months by 10.5 % at lumbar spine, 5.2 % at total hip, and 4.8 % at femoral neck. It had also been shown that 1 year of romosozumab followed by 1 year of denosumab approximated the effects of 7 years of continuous denosumab [8]. Another study by Miyauchi et al. [33] conducted a post-hoc analysis from FRAME study which focused on Japanese subjects with high risk of fracture, 79.6 % of romosozumab-to-denosumab group attained a lumbar spine BMD T-score larger than 2.5 at 36 months, compared with 21.6 % in placebo-to-denosumab group, the difference was significant (p < 0.001). McClung et al. [20] studied the treatment effects of 24 months of romosozumab followed by 12 months of denosumab and found that in the romosozumab-to-denosumab group, BMD continued to increase with mean change in lumbar spine (+2.6 %), total hip (+1.9 %) and femoral neck (+1.4 %) at 36 months. On the other hand, in the romosozumab-to-placebo group, mean change in BMD was decreased by 5.4 % at total hip and returned to the pretreatment level at 36 months.
3.4.5. Adverse events, serious adverse events, and fatal adverse events within 12 months of treatment
6 studies evaluated adverse events of romosozumab compared with control within 12 months of treatment. Results showed that there was less likelihood of adverse events with romosozumab compared to control group (OR 0.90 (95 % CI = 0.82–0.98; p = 0.01) (Fig. 6). In ARCH [24], FRAME [9], BRIDGE [25] and McClung et al. [19] study, romosozumab group had a lower proportion of any adverse event compared to the control group, only ARCH study with an OR not crossing 1 Subgroup analysis for romosozumab to the active-control group with alendronate showed there was less likelihood of any adverse event in the romosozumab group than the alendronate group with OR 0.84 (95 % CI = 0.73–0.98; p = 0.02 (Fig. S7).
Figure 6.
Forest plot of adverse event in romosozumab group, once per month subcutaneously and control group at month 12 of treatment. Subgroup analysis of any adverse event, serious adverse event and fatal adverse event.
5 studies evaluated serious adverse events and fatal adverse events in which there were no significant difference between romosozumab and control (Fig. 6). The pooled OR 1.02 (95 % CI = 0.90–1.14; p = 0.80). All studies had a 95 % confidence interval crossing 1. Subgroup analysis for romosozumab to the active-control group with alendronate showed there was less likelihood of serious adverse event in the romosozumab group than the alendronate group with OR 0.93 (95 % CI = 0.77–1.11; p = 0.40, but the result was not significant (Fig. S8). For fatal adverse event, there was higher likelihood of fatal adverse event with romosozumab compared with control group with OR 1.29 (95 % CI = 0.88–1.90; p = 0.19), but the result was not statistically significant. Subgroup analysis for active group with alendronate showed there was higher likelihood of fatal adverse event in the romosozumab group than the alendronate group with OR 1.42 (95 % CI = 0.81–2.48; p = 0.22), but the result was not significant (Fig. S9).
3.4.6. Adverse events, serious adverse events, and fatal adverse events within 24 months of treatment
2 studies evaluated adverse events of romosozumab compared with control group at 24 months. For any adverse event, there was less likelihood of any adverse event with romosozumab compared with control group, with OR 0.95 (95 % CI = 0.84–1.09; p = 0.48), but this was not significant. For serious adverse event, there was higher likelihood of serious adverse event with romosozumab compared with control group, with OR 1.05 (95 % CI = 0.92–1.19; p = 0.49), but this was not significant. For fatal adverse event, there was higher likelihood of fatal adverse event with romosozumab compared with control group, with OR 1.08 (95 % CI = 0.73–1.60; p = 0.70), but this was not significant (Fig. 7).
Figure 7.
Forest plot of adverse event in romosozumab group, 210 mg once per month subcutaneously and control group at month 24 of treatment. Subgroup analysis of any adverse event, serious adverse event and fatal adverse event.
3.4.7. Serious cardiovascular adverse events, and cardiovascular death within 12 months of treatment
3 studies evaluated serious cardiovascular events and cardiovascular death of romosozumab with control group within 12 months of treatment. For serious cardiovascular event, there was higher likelihood of serious cardiovascular event with romosozumab compared with control group, with OR 1.21 (95 % CI = 0.90–1.63; p = 0.20), but this was not significant. For cardiovascular death, there was higher likelihood of fatal adverse event with romosozumab compared with control group, with OR 1.24 (95 % CI = 0.76–2.04; p = 0.40), but this was not significant (Fig. 8).
Figure 8.
Forest plot of cardiovascular adverse event within 12 months. Subgroup analysis of adjudicated serious cardiovascular event (included events adjudicated as positive by the independent adjudication committee only) and cardiovascular death.
3.4.8. Serious cardiovascular event with 12 months of romosozumab treatment and 24 months of anti-resorptive in Asian population
2 studies evaluated serious cardiovascular events in Asian population of romosozumab with control group within 36 months, with OR 1.09 (95 % CI = 0.40–2.96; p = 0.86). In these two studies, romosozumab administration time were both 12 months, followed by 24 months of alendronate [35] or 24 months of denosumab [32] treatment. There were no significant differences found (Fig. 9).
Figure 9.
Forest plot of serious cardiovascular event with 12 months of romosozumab treatment and 24 months of anti-resorptive agent in Asian population. Romosozumab administration time were both 12 months, followed by 24 months of alendronate (Lau et al., 2020) or 24 months of denosumab (Miyauchi et al., 2019).
3.4.9. Radiographic healing outcomes with treatment and associated adverse events
2 studies evaluated radiographic healing on fracture. There was no significant difference between romosozumab group and control group for the median time to radiographic healing. Furthermore, the adverse events, serious adverse events and fatal adverse events were comparable between both groups (Figure 10, Figure 11).
Figure 10.
Forest plot of median time to radiographic healing. The estimate of the treatment effect in romosozumab group (210 mg, 4 doses: Day 1, Week 2, Week 6 and Week 12, subcutaneously) and placebo control group was expressed as hazard ratio and 95 % confidence interval with respect to time to revision-surgery-free healing.
Figure 11.
Forest plot of adverse event in romosozumab group (210 mg, 4 doses: Day 1, Week 2, Week 6 and Week 12, subcutaneously) and placebo control group at month 12. Subgroup analysis of any adverse event, serious adverse event and fatal adverse event.
3.4.10. Sensitivity analysis
Sensitivity analysis conducted by removing studies that included participants with specific disease in the results of meta-analysis including BMD percentage change (lumbar spine, total hip and femoral neck), incidence of new vertebral fractures and adverse events. Heterogeneity decreased to 0 % in the meta-analysis results of total hip and femoral neck BMD after 12 months of treatment, and also the adverse events within 12 months of treatment, whilst other results did not show change in the heterogeneity. However, there was no overall change in the direction of effect or significance.
3.4.11. Quantitative Computed Tomography (QCT) & finite element analysis (FEA)
Apart from DXA which assessed areal BMD, QCT assessed the skeleton by separate measures of cortical and trabecular BMD, and measurement of volumetric BMD. Compared with placebo and teriparatide groups at month 12, treatment with romosozumab showed a significant increase in integral vBMD (volumetric bone mineral density) and BMC (bone mineral content) at the lumbar spine and total hip from baseline [29]. The mean percentage change from baseline at month 12 in integral lumbar spine vBMD in romosozumab, placebo and teriparatide groups were +17.7 %, −0.8 % and +12.9 %, respectively. The mean percentage change from baseline at month 12 in total hip vBMD in romosozumab, placebo and teriparatide groups were +4.1 %, +0.3 % and +1.2 %, respectively. Furthermore, compared with placebo, cortical thickness increased significantly with romosozumab at the lumbar spine.
In another study that assessed FEA, the strength of bone for a compression overload at the spine and the proximal femur was estimated [30]. The change of vertebral strength from baseline at month 12 was significantly higher in the romosozumab group by 31.2 % compared with placebo and the change of femoral strength was higher in romosozumab group by 3.7 % compared with placebo. The study further showed that the strengthening effect in romosozumab group at month 12 was contributed by both cortical and trabecular bone compartments. At the lumbar spine, strength was associated with cortical compartment increase from baseline with romosozumab, teriparatide and placebo at +28.8 %, +16.2 % and −2.0 %, respectively. This showed a significantly greater increase in cortical strength in romosozumab group compared with teriparatide and placebo group.
3.5. Bone biopsy analysis
Lewiecki et al. [25] conducted histomorphometry of bone biopsies and showed a significant reduction in ratio of eroded surface to bone surface, and osteoclast surface to bone surface in cancellous bone compartment static resorption parameters in the romosozumab group at month 12 when compared with placebo. In another study, bone biopsies from FRAME study were analyzed [31]. In cortical bone, romosozumab group had a significant increase in osteoid volume at 2 months. Dynamic bone formation parameters in cortical bone were also significantly higher in the romosozumab group at 2 months. There was a significant decrease in bone resorption parameters in romosozumab group at 12 months when compared with placebo in cortical bone, and also endocortical bone. For the bone structure parameters, there was significant increase in bone volume to tissue volume in cortical bone, trabecular thickness and cortical thickness in romosozumab group at 12 months. Eriksen et al. [34] further investigated whether early increase in bone formation after romosozumab treatment was mainly modeling-based bone formation (MBBF) as shown in preclinical studies. Bone biopsies obtained from the FRAME study at 2 months were used to study the extent of MBBF and remodeling-based bone formation (RBBF) on bone surfaces. Results showed an increase in MBBF on endocortical and cancellous surfaces which mainly contributed to bone formation stimulation in postmenopausal women with osteoporosis in the first 2 months with romosozumab. This was indicated by a significant increase in median percentage of MBBF to total bone surface when compared to placebo on cancellous and endocortical surfaces, but not periosteal surfaces. Also, no significant difference was found regarding the surface extent of RBBF on cancellous, endocortical or periosteal surfaces.
4. Discussion
Osteoporosis affects nearly 50 million people worldwide, and it is expected that osteoporotic fractures will continue to increase. An osteoporotic fracture typically occurs after a low energy trauma event, such as a fall from standing height or less [7]. Common regions of an osteoporotic fracture include regions of the hip, spine, distal forearm and proximal humerus. However, it is not uncommon for patients with an osteoporotic fracture to have a bone mineral density (BMD) T-score > −2.5 [36,37]. More importantly, it is recommended to stratify patients by fracture risk stratification, which drives the choice of the initial agent. Recent guidelines have recommended the use of an anabolic agent followed by an anti-resorptive agent for patients with very-high risk of osteoporotic fracture [[5], [6], [7]]. These include patients that had a recent osteoporotic fracture due to the concept of imminent risk of fracture, where a subsequent fracture is not constant with time and occurs shortly after an initial one [1,5,38,39].
Romosozumab is an agent that has been used in patients with very-high risk of osteoporotic fracture. When given as an initial therapy for 1 year, there is marked increase in BMD over the total hip and lumbar spine with further improvements upon transitioning to an anti-resorptive agent [40]. Our meta-analysis had shown that within 6 months and 12 months of treatment, romosozumab compared to either placebo-control or active-control was significantly more effective in improving BMD over lumbar spine, total hip and femoral neck. Furthermore, more importantly, it was more effective in decreasing the incidence of vertebral fractures compared to control at 12 months of treatment. With sequential treatment, with romosozumab followed by an anti-resorptive agent, our results reinforced that it was significantly more effective than the use of anti-resorptive agent alone at 24 months. Subgroup analysis of the Asian population also concurred with this result.
The safety of romosozumab was investigated in our meta-analysis, and cardiovascular adverse events was also specifically analyzed. Compared with control group, adverse events appeared to have less likelihood in the romosozumab group within 12 months of treatment with a OR of 0.90. However, serious adverse events and fatal adverse events were comparable between groups. No difference was also detected for adverse events, serious adverse events and fatal adverse events at 24 months. Furthermore, specifically analyzing serious cardiovascular events and cardiovascular death showed that romosozumab was comparable to control group within 12 months. Analysis in the Asian population with 12 months romosozumab treatment followed by 24 months anti-resorptive also showed no differences either. Our meta-analysis results show that the use of romosozumab did not have higher rates of adverse events overall. However, in a pharmacovigilance study which evaluated the safety profile of romosozumab by extracting the cases from the Food and Drug Administration Adverse Event Reporting System (FAERS), elevated myocardial infarction (MI), stroke, or cardiovascular death (MACE) was found with use of romosozumab, particularly in Japan [41]. However, the result could be caused by the disproportionality analysis in the Japanese reports, with older patients, more males and higher proportion of patients with cardio-protective drugs amongst cases with MACE compared with reports in the United States [42]. Another meta-analysis on the results of FRAME, ARCH and BRIDGE studies together with the unpublished data, found that the use of romosozumab led to higher risk of cardiovascular disease [43], in which cardiac ischemic events with a pooled OR of 1.54 (95 % CI, 0.90–2.64), cerebrovascular events with OR 1.44 (95 % CI, 0.80–2.58), MACE (OR, 1.39; 95 %, 0.98–1.98) and serious cardiovascular events (OR, 1.21; 95 % CI, 0.90–1.63). However, some results were not statistically significant. Currently, the data regarding the cardiovascular risk and romosozumab is not consistent, and the results support the prescription recommendations of romosozumab on cardiovascular death, myocardial infarction and stroke. Further research data, especially in the Asian population and male populations, may be useful.
Our qualitative analysis on QCT, FEA and bone biopsy analyses demonstrated that romosozumab improved parameters and measures in these domains as well. These reinforce the effects of bone building and further explains the positive effects on BMD and fracture reduction. It is important to note that romosozumab is currently a Food and Drug Administration (FDA) approved agent to treat osteoporosis in postmenopausal women at high risk of fracture. However, we found that studies also assessed its efficacy in the clinical outcomes of fractures [13,14]. Our results show that the use of romosozumab was comparable to control group in the time to radiographic healing. The adverse event, serious adverse event and fatal adverse event were comparable as well in each of the analysis. Previous pre-clinical studies had shown success of romosozumab in enhancing fracture healing [44,45]. Further studies focusing on cases of delayed healing or non-union may be useful.
Although previous meta-analysis has been published in this topic [46,47], these were focused on postmenopausal osteoporosis. Previous studies also did not review the effects of romosozumab on QCT, FEA and bone biopsy analyses. Additional strengths of our study include analysis of only randomized controlled trials with more recently published trials. We have provided high quality evidence in which we have assessed the quality of studies. In our meta-analysis we have analyzed fracture healing outcome in clinical studies. We have also provided subgroup analysis specifically for cardiovascular events. It has been projected that for hip fractures alone, numbers will increase from 1,124,060 in 2018 to 2,563,488 in 2050 in Asia [48], which is also an estimated 50 % of all hip fractures in the world. Given the number of hip fractures that will increase in Asia, treating osteoporosis and the prevention of secondary fractures are warranted to decrease healthcare costs. Therefore, our study has also focused a subgroup analysis on the Asian population for cardiovascular events. The limitations of our study include the heterogeneity of studies, and for the subgroup analysis of adverse outcomes, there were limited studies and therefore subgroup analysis could not be performed in all the active-control groups. Further studies will be required for investigating the effect of romosozumab with comparison of different active-control groups. Besides, subgroup analysis of serious cardiovascular event and cardiovascular death in Asian population could not be performed as large-scale phase III clinical trials in the existing literature were limited in these regions. Furthermore, some studies had qualitative analysis only due to different nature of the article.
In conclusion, our study showed that romosozumab was an effective agent to treat osteoporosis with high quality evidence. There were no significant differences in the adverse events, serious adverse events, fatal adverse events identified. Further subgroup analysis of cardiovascular events and cardiovascular death in the total population showed no differences either.
Funding
This study was funded by the General Research Fund, HKSAR Research Grants Council (Ref: 14116223) and Early Career Scheme, HKSAR Research Grants Council (Ref: 24108519).
Disclosures
RMY Wong has received advisor/speaker fees from Amgen, and speaker honorarium from ZP Therapeutics – A Division of Zuellig Pharma Corporation. SW Law has received advisor/speaker fees from Amgen.
Authorship
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Each author certifies that this material or part thereof has not been published in another journal, that it is not currently submitted elsewhere, and that it will not be submitted elsewhere until a final decision regarding publication of the manuscript in Journal of Orthopaedic Translation has been made.
Conception and design of study: R.M.Y. Wong, N Zhang, W.H Cheung, S.W. Law; acquisition of data: P.Y. Wong, H.Y. Wong, M.K. Fong, analysis and/or interpretation of data: R.M.Y. Wong, P.Y. Wong, C. Liu. Drafting the manuscript: R.M.Y. Wong, P.Y Wong, revising the manuscript critically for important intellectual content: N. Zhang, W.H. Cheung<a name = "Line_supportingmanuscript_19"> Approval of the version of the manuscript to be published (the names of all authors must be listed).
Section I
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Section II
The authors whose names are listed immediately below report the following details of affiliation or involvement in an organization or entity with a financial or non-financial interest in the subject matter or materials discussed in this manuscript. Please specify the nature of the conflict on a separate sheet of paper if the space below is inadequate.
Declaration of competing interest
A conflict of interest occurs when an individual's objectivity is potentially compromised by a desire for financial gain, prominence, professional advancement or a successful outcome. The Editors of the Journal of Orthopaedic Translation strive to ensure that what is published in the Journal is as balanced, objective and evidence-based as possible. Since it can be difficult to distinguish between an actual conflict of interest and a perceived conflict of interest, the Journal requires authors to disclose all and any potential conflicts of interest.
Acknowledgements
All persons who have made substantial contributions to the work reported in the manuscript (e.g., technical help, writing and editing assistance, general support), but who do not meet the criteria for authorship, are named in the Acknowledgements and have given us their written permission to be named. If we have not included an Acknowledgements, then that indicates that we have not received substantial contributions from non-authors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2024.07.011.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Wong R.M.Y., Wong P.Y., Liu C., Wong H.W., Chung Y.L., Chow S.K.H., et al. The imminent risk of a fracture-existing worldwide data: a systematic review and meta-analysis. Osteoporos Int. 2022;33(12):2453–2466. doi: 10.1007/s00198-022-06473-0. [DOI] [PubMed] [Google Scholar]
- 2.Ganda K. Fracture liaison services: past, present and future : editorial relating to: the impact of Fracture Liaison Services on subsequent fractures and mortality: a systematic literature review and meta-analysis. Osteoporos Int. 2021;32(8):1461–1464. doi: 10.1007/s00198-021-05982-8. [DOI] [PubMed] [Google Scholar]
- 3.Bolland M.J., Grey A.B., Gamble G.D., Reid I.R. Effect of osteoporosis treatment on mortality: a meta-analysis. J Clin Endocrinol Metab. 2010;95(3):1174–1181. doi: 10.1210/jc.2009-0852. [DOI] [PubMed] [Google Scholar]
- 4.Black D.M., Bauer D.C., Vittinghoff E., Lui L.Y., Grauer A., Marin F., et al. Treatment-related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: meta-regression analyses of individual patient data from multiple randomised controlled trials. Lancet Diabetes Endocrinol. 2020;8(8):672–682. doi: 10.1016/S2213-8587(20)30159-5. [DOI] [PubMed] [Google Scholar]
- 5.Camacho P.M., Petak S.M., Binkley N., Diab D.L., Eldeiry L.S., Farooki A., et al. American association of clinical endocrinologists/American college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. 2020;26(Suppl 1):1–46. doi: 10.4158/GL-2020-0524SUPPL. [DOI] [PubMed] [Google Scholar]
- 6.Kanis J.A., Harvey N.C., McCloskey E., Bruyere O., Veronese N., Lorentzon M., et al. Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos Int. 2020;31(1):1–12. doi: 10.1007/s00198-019-05176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong R.M.Y., Cheung W.H., Chow S.K.H., Ng R.W.K., Li W., Hsu A.Y., et al. Recommendations on the post-acute management of the osteoporotic fracture - patients with "very-high" Re-fracture risk. J Orthop Translat. 2022;37:94–99. doi: 10.1016/j.jot.2022.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosman F., Crittenden D.B., Ferrari S., Khan A., Lane N.E., Lippuner K., et al. FRAME study: the foundation effect of building bone with 1 Year of romosozumab leads to continued lower fracture risk after transition to denosumab. J Bone Miner Res. 2018;33(7):1219–1226. doi: 10.1002/jbmr.3427. [DOI] [PubMed] [Google Scholar]
- 9.Cosman F., Crittenden D.B., Adachi J.D., Binkley N., Czerwinski E., Ferrari S., et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 10.Saag K.G., Petersen J., Brandi M.L., Karaplis A.C., Lorentzon M., Thomas T., et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377(15):1417–1427. doi: 10.1056/NEJMoa1708322. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari S.L. Osteoporosis: romosozumab to rebuild the foundations of bone strength. Nat Rev Rheumatol. 2018;14(3):128. doi: 10.1038/nrrheum.2018.5. [DOI] [PubMed] [Google Scholar]
- 12.Fixen C., Tunoa J. Romosozumab: a review of efficacy, safety, and cardiovascular risk. Curr Osteoporos Rep. 2021;19(1):15–22. doi: 10.1007/s11914-020-00652-w. [DOI] [PubMed] [Google Scholar]
- 13.Schemitsch E.H., Miclau T., Karachalios T., Nowak L.L., Sancheti P., Poolman R.W., et al. A randomized, placebo-controlled study of romosozumab for the treatment of hip fractures. J Bone Joint Surg Am. 2020;102(8):693–702. doi: 10.2106/JBJS.19.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhandari M., Schemitsch E.H., Karachalios T., Sancheti P., Poolman R.W., Caminis J., et al. Romosozumab in skeletally mature adults with a fresh unilateral tibial diaphyseal fracture: a randomized phase-2 study. J Bone Joint Surg Am. 2020;102(16):1416–1426. doi: 10.2106/JBJS.19.01008. [DOI] [PubMed] [Google Scholar]
- 15.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maher C.G., Sherrington C., Herbert R.D., Moseley A.M., Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–721. [PubMed] [Google Scholar]
- 18.Higgins J., Green Se. The Cochrane Collaboration; 2011. Cochrane handbook for systematic reviews of interventions. [updated March 2011] [Google Scholar]
- 19.McClung M.R., Grauer A., Boonen S., Bolognese M.A., Brown J.P., Diez-Perez A., et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370(5):412–420. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 20.McClung M.R., Brown J.P., Diez-Perez A., Resch H., Caminis J., Meisner P., et al. Effects of 24 Months of treatment with romosozumab followed by 12 Months of denosumab or placebo in postmenopausal women with low bone mineral density: a randomized, double-blind, phase 2, parallel group study. J Bone Miner Res. 2018;33(8):1397–1406. doi: 10.1002/jbmr.3452. [DOI] [PubMed] [Google Scholar]
- 21.Lewiecki E.M., Dinavahi R.V., Lazaretti-Castro M., Ebeling P.R., Adachi J.D., Miyauchi A., et al. One year of romosozumab followed by two years of denosumab maintains fracture risk reductions: results of the FRAME extension study. J Bone Miner Res. 2019;34(3):419–428. doi: 10.1002/jbmr.3622. [DOI] [PubMed] [Google Scholar]
- 22.Ishibashi H., Crittenden D.B., Miyauchi A., Libanati C., Maddox J., Fan M., et al. Romosozumab increases bone mineral density in postmenopausal Japanese women with osteoporosis: a phase 2 study. Bone. 2017;103:209–215. doi: 10.1016/j.bone.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Langdahl B.L., Libanati C., Crittenden D.B., Bolognese M.A., Brown J.P., Daizadeh N.S., et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet. 2017;390(10102):1585–1594. doi: 10.1016/S0140-6736(17)31613-6. [DOI] [PubMed] [Google Scholar]
- 24.Saag K.G., Petersen J., Brandi M.L., Karaplis A.C., Lorentzon M., Thomas T., et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377(15):1417–1427. doi: 10.1056/NEJMoa1708322. [DOI] [PubMed] [Google Scholar]
- 25.Lewiecki E.M., Blicharski T., Goemaere S., Lippuner K., Meisner P.D., Miller P.D., et al. A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J Clin Endocrinol Metab. 2018;103(9):3183–3193. doi: 10.1210/jc.2017-02163. [DOI] [PubMed] [Google Scholar]
- 26.Baek K.H., Chung Y.S., Koh J.M., Kim I.J., Kim K.M., Min Y.K., et al. Romosozumab in postmenopausal Korean women with osteoporosis: a randomized, double-blind, placebo-controlled efficacy and safety study. Endocrinology and metabolism (Seoul, Korea) 2021;36(1):60–69. doi: 10.3803/EnM.2020.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mochizuki T., Yano K., Ikari K., Okazaki K. Effects of romosozumab or denosumab treatment on the bone mineral density and disease activity for 6 months in patients with rheumatoid arthritis with severe osteoporosis: an open-label, randomized, pilot study. Osteoporosis and Sarcopenia. 2021;7(3):110–114. doi: 10.1016/j.afos.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mochizuki T., Yano K., Ikari K., Hiroshima R., Okazaki K. Comparison of romosozumab versus denosumab treatment on bone mineral density after 1 year in rheumatoid arthritis patients with severe osteoporosis: a randomized clinical pilot study. Mod Rheumatol. 2023;33(3):490–495. doi: 10.1093/mr/roac059. [DOI] [PubMed] [Google Scholar]
- 29.Genant H.K., Engelke K., Bolognese M.A., Mautalen C., Brown J.P., Recknor C., et al. Effects of romosozumab compared with teriparatide on bone density and mass at the spine and hip in postmenopausal women with low bone mass. J Bone Miner Res. 2017;32(1):181–187. doi: 10.1002/jbmr.2932. [DOI] [PubMed] [Google Scholar]
- 30.Keaveny T.M., Crittenden D.B., Bolognese M.A., Genant H.K., Engelke K., Oliveri B., et al. Greater gains in spine and hip strength for romosozumab compared with teriparatide in postmenopausal women with low bone mass. J Bone Miner Res. 2017;32(9):1956–1962. doi: 10.1002/jbmr.3176. [DOI] [PubMed] [Google Scholar]
- 31.Chavassieux P., Chapurlat R., Portero-Muzy N., Roux J.P., Garcia P., Brown J.P., et al. Bone-forming and antiresorptive effects of romosozumab in postmenopausal women with osteoporosis: bone histomorphometry and microcomputed Tomography analysis after 2 and 12 Months of treatment. J Bone Miner Res. 2019;34(9):1597–1608. doi: 10.1002/jbmr.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyauchi A., Dinavahi R.V., Crittenden D.B., Yang W., Maddox J.C., Hamaya E., et al. Increased bone mineral density for 1 year of romosozumab, vs placebo, followed by 2 years of denosumab in the Japanese subgroup of the pivotal FRAME trial and extension. Arch Osteoporosis. 2019;14(1) doi: 10.1007/s11657-019-0608-z. (no pagination) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyauchi A., Hamaya E., Yang W., Nishi K., Libanati C., Tolman C., et al. Romosozumab followed by denosumab in Japanese women with high fracture risk in the FRAME trial. J Bone Miner Metabol. 2021;39(2):278–288. doi: 10.1007/s00774-020-01147-5. [DOI] [PubMed] [Google Scholar]
- 34.Eriksen E.F., Chapurlat R., Boyce R.W., Shi Y., Brown J.P., Horlait S., et al. Modeling-based bone formation after 2 Months of romosozumab treatment: results from the FRAME clinical trial. J Bone Miner Res. 2022;37(1):36–40. doi: 10.1002/jbmr.4457. [DOI] [PubMed] [Google Scholar]
- 35.Lau E.M.C., Dinavahi R., Woo Y.C., Wu C.H., Guan J., Maddox J., et al. Romosozumab or alendronate for fracture prevention in East Asian patients: a subanalysis of the phase III, randomized ARCH study. Osteoporos Int. 2020;31(4):677–685. doi: 10.1007/s00198-020-05324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khosla S., Cauley J.A., Compston J., Kiel D.P., Rosen C., Saag K.G., et al. Addressing the crisis in the treatment of osteoporosis: a path forward. J Bone Miner Res. 2017;32(3):424–430. doi: 10.1002/jbmr.3074. [DOI] [PubMed] [Google Scholar]
- 37.Chapurlat R., Bui M., Sornay-Rendu E., Zebaze R., Delmas P.D., Liew D., et al. Deterioration of cortical and trabecular microstructure identifies women with osteopenia or normal bone mineral density at imminent and long-term risk for fragility fracture: a prospective study. J Bone Miner Res. 2020;35(5):833–844. doi: 10.1002/jbmr.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong R.M.Y., Ho W.T., Wai L.S., Li W., Chau W.W., Chow K.S., et al. Fragility fractures and imminent fracture risk in Hong Kong: one of the cities with longest life expectancies. Arch Osteoporosis. 2019;14(1):104. doi: 10.1007/s11657-019-0648-4. [DOI] [PubMed] [Google Scholar]
- 39.Wong R.M.Y., Law S.W., Lee K.B., Chow S.K.H., Cheung W.H. Secondary prevention of fragility fractures: instrumental role of a fracture liaison service to tackle the risk of imminent fracture. Hong Kong Med J. 2019;25(3):235–242. doi: 10.12809/hkmj187593. [DOI] [PubMed] [Google Scholar]
- 40.Cosman F., Kendler D.L., Langdahl B.L., Leder B.Z., Lewiecki E.M., Miyauchi A., et al. Romosozumab and antiresorptive treatment: the importance of treatment sequence. Osteoporos Int. 2022;33(6):1243–1256. doi: 10.1007/s00198-021-06174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vestergaard Kvist A., Faruque J., Vallejo-Yagüe E., Weiler S., Winter E.M., Burden A.M. Cardiovascular safety profile of romosozumab: a pharmacovigilance analysis of the us Food and drug administration adverse event reporting System (FAERS) J Clin Med. 2021;10(8):1660. doi: 10.3390/jcm10081660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeuchi Y. Romosozumab and cardiovascular safety in Japan. Osteoporos Sarcopenia. 2021;7(3):89–91. doi: 10.1016/j.afos.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bovijn J., Krebs K., Chen C.Y., Boxall R., Censin J.C., Ferreira T., et al. Evaluating the cardiovascular safety of sclerostin inhibition using evidence from meta-analysis of clinical trials and human genetics. Sci Transl Med. 2020;12(549) doi: 10.1126/scitranslmed.aay6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ominsky M.S., Li C., Li X., Tan H.L., Lee E., Barrero M., et al. Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. J Bone Miner Res. 2011;26(5):1012–1021. doi: 10.1002/jbmr.307. [DOI] [PubMed] [Google Scholar]
- 45.Suen P.K., He Y.X., Chow D.H., Huang L., Li C., Ke H.Z., et al. Sclerostin monoclonal antibody enhanced bone fracture healing in an open osteotomy model in rats. J Orthop Res. 2014;32(8):997–1005. doi: 10.1002/jor.22636. [DOI] [PubMed] [Google Scholar]
- 46.Singh S., Dutta S., Khasbage S., Kumar T., Sachin J., Sharma J., et al. A systematic review and meta-analysis of efficacy and safety of Romosozumab in postmenopausal osteoporosis. Osteoporos Int. 2022;33(1):1–12. doi: 10.1007/s00198-022-06340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y., Cao Y., Zhang S., Zhang W., Zhang B., Tang Q., et al. Romosozumab treatment in postmenopausal women with osteoporosis: a meta-analysis of randomized controlled trials. Climacteric. 2018;21(2):189–195. doi: 10.1080/13697137.2018.1433655. [DOI] [PubMed] [Google Scholar]
- 48.Cheung C.L., Ang S.B., Chadha M., Chow E.S., Chung Y.S., Hew F.L., et al. An updated hip fracture projection in Asia: the Asian Federation of Osteoporosis Societies study. Osteoporos Sarcopenia. 2018;4(1):16–21. doi: 10.1016/j.afos.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.