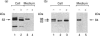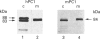Abstract
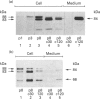
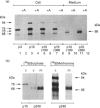
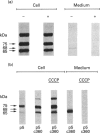
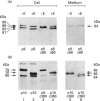
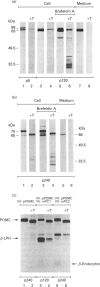
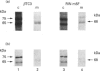
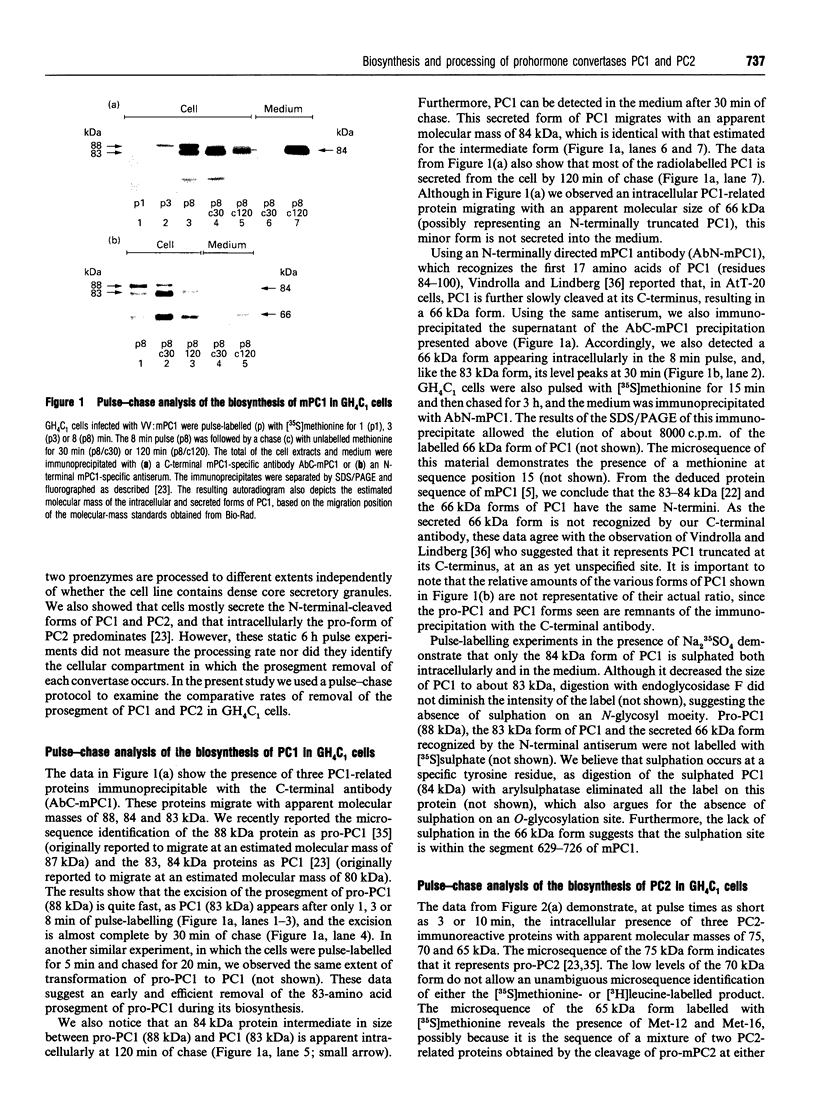
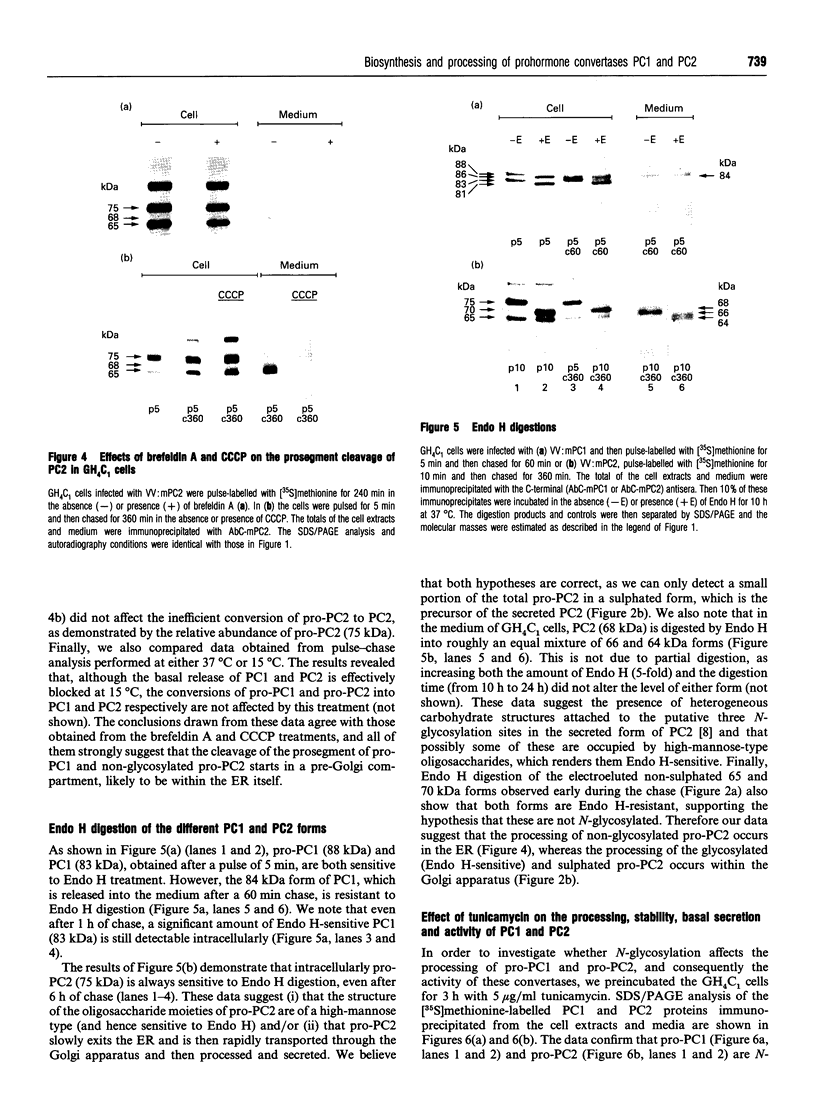
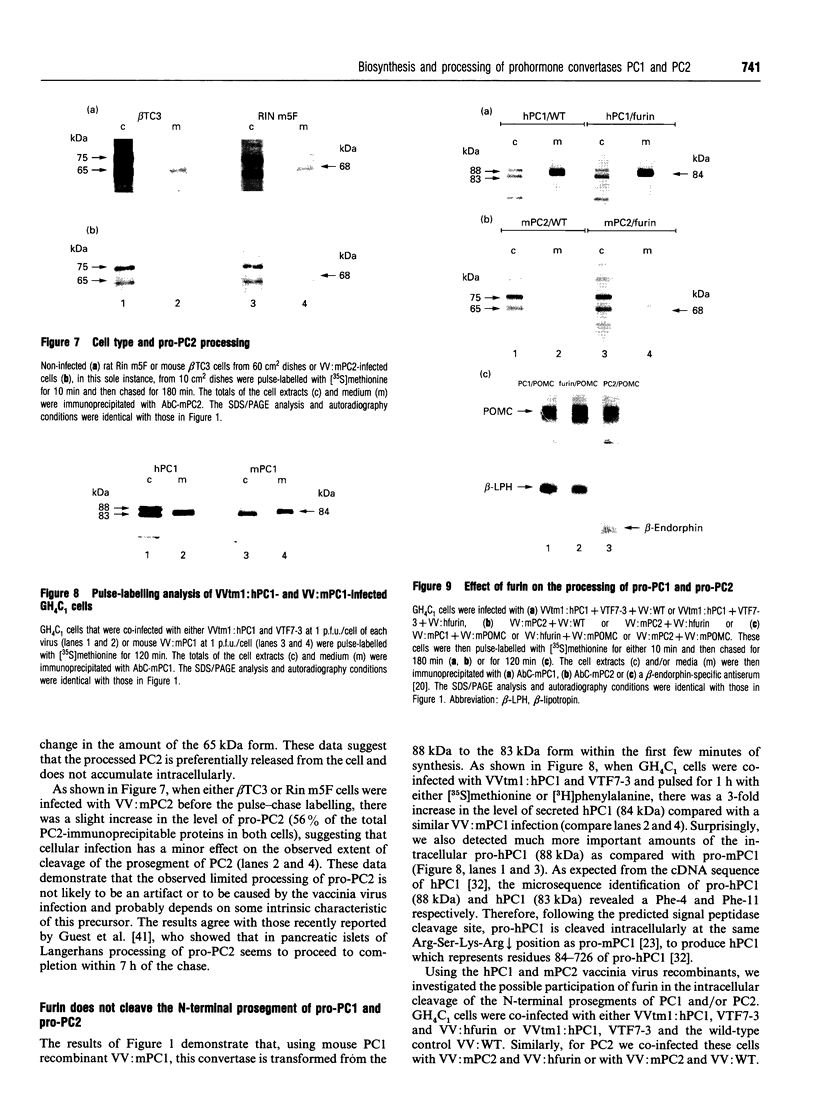
We present herein the pulse-chase analysis of the biosynthesis of the prohormone convertases PC1 and PC2 in the endocrine GH4C1 cells infected with vaccinia virus recombinants expressing these convertases. Characterization of the pulse-labelled enzymes demonstrated that pro-PC1 (88 kDa) is cleaved into PC1 (83 kDa) and pro-PC2 (75 kDa) into PC2 (68 kDa). Secretion of glycosylated and sulphated PC1 (84 kDa) occurs about 30 min after the onset of biosynthesis, whereas glycosylated and sulphated PC2 (68 kDa) is detected in the medium after between 1 and 2 h. Furthermore, in the case of pro-PC2 only, we observed that a fraction of this precursor escapes glycosylation. A small proportion (about 5%) of the intracellular glycosylated pro-PC2 (75 kDa) is sulphated, and it is this glycosylated and sulphated precursor that is cleaved into the secretable 68 kDa form of PC2. Major differences in the carbohydrate structures of PC1 and PC2 are demonstrated by the resistance of the secreted PC1 to endoglycosidase H digestion and sensitivity of the secreted PC2 to this enzyme. Inhibition of N-glycosylation with tunicamycin caused a dramatic intracellular degradation of these convertases within the endoplasmic reticulum, with the net effect of a reduction in the available activity of PC1 and PC2. These results emphasize the importance of N-glycosylation in the folding and stability of PC1 and PC2. Pulse-labelling experiments in uninfected mouse beta TC3 and rat Rin m5F insulinoma cells, which endogenously synthesize PC2, showed that, as in infected GH4C1 cells, pro-PC2 predominates intracellularly. In order to define the site of prosegment cleavage, pulse-chase analysis was performed at low temperature (15 degrees C) or after treatment of GH4C1 cells with either brefeldin A or carbonyl cyanide m-chlorophenylhydrazone. These results demonstrated that the onset of the conversions of pro-PC1 into PC1 and non-glycosylated pro-PC2 into PC2 (65 kDa) occur in a pre-Golgi compartment, presumably within the endoplasmic reticulum. In contrast, pulse labelling in the presence of Na(2)35SO4 demonstrated that the processing of glycosylated and sulphated pro-PC2 occurs within the Golgi apparatus. In order to test the possibility that zymogen processing is performed by furin, we co-expressed this convertase with either pro-PC1 or pro-PC2. The data demonstrated the inability of furin to cleave either proenzyme.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Huttner W. B. Tyrosine sulfation is a trans-Golgi-specific protein modification. J Cell Biol. 1987 Dec;105(6 Pt 1):2655–2664. doi: 10.1083/jcb.105.6.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Elliott M. M., Keller D. S. ATP-coupled transport of vesicular stomatitis virus G protein between the endoplasmic reticulum and the Golgi. J Biol Chem. 1986 Nov 5;261(31):14681–14689. [PubMed] [Google Scholar]
- Barr P. J. Mammalian subtilisins: the long-sought dibasic processing endoproteases. Cell. 1991 Jul 12;66(1):1–3. doi: 10.1016/0092-8674(91)90129-m. [DOI] [PubMed] [Google Scholar]
- Benjannet S., Reudelhuber T., Mercure C., Rondeau N., Chrétien M., Seidah N. G. Proprotein conversion is determined by a multiplicity of factors including convertase processing, substrate specificity, and intracellular environment. Cell type-specific processing of human prorenin by the convertase PC1. J Biol Chem. 1992 Jun 5;267(16):11417–11423. [PubMed] [Google Scholar]
- Benjannet S., Rondeau N., Day R., Chrétien M., Seidah N. G. PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3564–3568. doi: 10.1073/pnas.88.9.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch N. P., Tracer H. L., Hakes D. J., Loh Y. P. Coordinate regulation of mRNA levels of pro-opiomelanocortin and the candidate processing enzymes PC2 and PC3, but not furin, in rat pituitary intermediate lobe. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1311–1319. doi: 10.1016/0006-291x(91)91716-p. [DOI] [PubMed] [Google Scholar]
- Chaudhuri B., Latham S. E., Helliwell S. B., Seeboth P. A novel Kex2 enzyme can process the proregion of the yeast alpha-factor leader in the endoplasmic reticulum instead of in the Golgi. Biochem Biophys Res Commun. 1992 Feb 28;183(1):212–219. doi: 10.1016/0006-291x(92)91630-9. [DOI] [PubMed] [Google Scholar]
- Chege N. W., Pfeffer S. R. Compartmentation of the Golgi complex: brefeldin-A distinguishes trans-Golgi cisternae from the trans-Golgi network. J Cell Biol. 1990 Sep;111(3):893–899. doi: 10.1083/jcb.111.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crine P., Gossard F., Seidah N. G., Blanchette L., Lis M., Chrétien M. Concomitant synthesis of beta-endorphin and alpha-melanotropin from two forms of pro-opiomelanocortin in the rat pars intermedia. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5085–5089. doi: 10.1073/pnas.76.10.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan W. E., Day N. C., Schäfer M. K., Day R., Seidah N. G., Chrétien M., Akil H., Watson S. J. Neuroanatomical and functional studies of peptide precursor-processing enzymes. Enzyme. 1991;45(5-6):285–300. doi: 10.1159/000468902. [DOI] [PubMed] [Google Scholar]
- Day R., Schafer M. K., Watson S. J., Chrétien M., Seidah N. G. Distribution and regulation of the prohormone convertases PC1 and PC2 in the rat pituitary. Mol Endocrinol. 1992 Mar;6(3):485–497. doi: 10.1210/mend.6.3.1316544. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G. Progress in unraveling pathways of Golgi traffic. Annu Rev Cell Biol. 1985;1:447–488. doi: 10.1146/annurev.cb.01.110185.002311. [DOI] [PubMed] [Google Scholar]
- Germain D., Dumas F., Vernet T., Bourbonnais Y., Thomas D. Y., Boileau G. The pro-region of the Kex2 endoprotease of Saccharomyces cerevisiae is removed by self-processing. FEBS Lett. 1992 Mar 16;299(3):283–286. doi: 10.1016/0014-5793(92)80132-z. [DOI] [PubMed] [Google Scholar]
- Guest P. C., Arden S. D., Bennett D. L., Clark A., Rutherford N. G., Hutton J. C. The post-translational processing and intracellular sorting of PC2 in the islets of Langerhans. J Biol Chem. 1992 Nov 5;267(31):22401–22406. [PubMed] [Google Scholar]
- Hatsuzawa K., Hosaka M., Nakagawa T., Nagase M., Shoda A., Murakami K., Nakayama K. Structure and expression of mouse furin, a yeast Kex2-related protease. Lack of processing of coexpressed prorenin in GH4C1 cells. J Biol Chem. 1990 Dec 25;265(36):22075–22078. [PubMed] [Google Scholar]
- Hosaka M., Nagahama M., Kim W. S., Watanabe T., Hatsuzawa K., Ikemizu J., Murakami K., Nakayama K. Arg-X-Lys/Arg-Arg motif as a signal for precursor cleavage catalyzed by furin within the constitutive secretory pathway. J Biol Chem. 1991 Jul 5;266(19):12127–12130. [PubMed] [Google Scholar]
- Jean F., Basak A., Rondeau N., Benjannet S., Hendy G. N., Seidah N. G., Chrétien M., Lazure C. Enzymic characterization of murine and human prohormone convertase-1 (mPC1 and hPC1) expressed in mammalian GH4C1 cells. Biochem J. 1993 Jun 15;292(Pt 3):891–900. doi: 10.1042/bj2920891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer M. C., Tucker J. E., Joh R., Landsberg K. E., Saltman D., Barr P. J. Identification of a second human subtilisin-like protease gene in the fes/fps region of chromosome 15. DNA Cell Biol. 1991 Dec;10(10):757–769. doi: 10.1089/dna.1991.10.757. [DOI] [PubMed] [Google Scholar]
- Kirchmair R., Egger C., Gee P., Hogue-Angeletti R., Fischer-Colbrie R., Laslop A., Winkler H. Differential subcellular distribution of PC1, PC2 and furin in bovine adrenal medulla and secretion of PC1 and PC2 from this tissue. Neurosci Lett. 1992 Aug 31;143(1-2):143–145. doi: 10.1016/0304-3940(92)90252-3. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990 Aug 24;62(4):611–614. doi: 10.1016/0092-8674(90)90104-m. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Lodish H. F. Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell. 1986 Sep 12;46(6):929–937. doi: 10.1016/0092-8674(86)90075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazure C., Seidah N. G., Pélaprat D., Chrétien M. Proteases and posttranslational processing of prohormones: a review. Can J Biochem Cell Biol. 1983 Jul;61(7):501–515. doi: 10.1139/o83-066. [DOI] [PubMed] [Google Scholar]
- Lazzarino D. A., Gabel C. A. Biosynthesis of the mannose 6-phosphate recognition marker in transport-impaired mouse lymphoma cells. Demonstration of a two-step phosphorylation. J Biol Chem. 1988 Jul 25;263(21):10118–10126. [PubMed] [Google Scholar]
- Lindberg I. The new eukaryotic precursor processing proteinases. Mol Endocrinol. 1991 Oct;5(10):1361–1365. doi: 10.1210/mend-5-10-1361. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L., Tipper C., Amherdt M., Orci L., Klausner R. D. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991 Nov 1;67(3):601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Kong N., Snider M., Strous G. J. Hepatoma secretory proteins migrate from rough endoplasmic reticulum to Golgi at characteristic rates. Nature. 1983 Jul 7;304(5921):80–83. doi: 10.1038/304080a0. [DOI] [PubMed] [Google Scholar]
- Lusson J., Vieau D., Hamelin J., Day R., Chrétien M., Seidah N. G. cDNA structure of the mouse and rat subtilisin/kexin-like PC5: a candidate proprotein convertase expressed in endocrine and nonendocrine cells. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6691–6695. doi: 10.1073/pnas.90.14.6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Elroy-Stein O., Mizukami T., Alexander W. A., Fuerst T. R. Product review. New mammalian expression vectors. Nature. 1990 Nov 1;348(6296):91–92. doi: 10.1038/348091a0. [DOI] [PubMed] [Google Scholar]
- Nagahama M., Ikemizu J., Misumi Y., Ikehara Y., Murakami K., Nakayama K. Evidence that differentiates between precursor cleavages at dibasic and Arg-X-Lys/Arg-Arg sites. J Biochem. 1991 Nov;110(5):806–811. doi: 10.1093/oxfordjournals.jbchem.a123664. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Kim W. S., Torii S., Hosaka M., Nakagawa T., Ikemizu J., Baba T., Murakami K. Identification of the fourth member of the mammalian endoprotease family homologous to the yeast Kex2 protease. Its testis-specific expression. J Biol Chem. 1992 Mar 25;267(9):5897–5900. [PubMed] [Google Scholar]
- Rehemtulla A., Dorner A. J., Kaufman R. J. Regulation of PACE propeptide-processing activity: requirement for a post-endoplasmic reticulum compartment and autoproteolytic activation. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8235–8239. doi: 10.1073/pnas.89.17.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebroek A. J., Schalken J. A., Leunissen J. A., Onnekink C., Bloemers H. P., Van de Ven W. J. Evolutionary conserved close linkage of the c-fes/fps proto-oncogene and genetic sequences encoding a receptor-like protein. EMBO J. 1986 Sep;5(9):2197–2202. doi: 10.1002/j.1460-2075.1986.tb04484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist G. L., Nicholas H. B., Jr Analysis of sequence requirements for protein tyrosine sulfation. Protein Sci. 1993 Feb;2(2):215–222. doi: 10.1002/pro.5560020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Schalken J. A., Roebroek A. J., Oomen P. P., Wagenaar S. S., Debruyne F. M., Bloemers H. P., Van de Ven W. J. fur gene expression as a discriminating marker for small cell and nonsmall cell lung carcinomas. J Clin Invest. 1987 Dec;80(6):1545–1549. doi: 10.1172/JCI113240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A., Fransen J. A., Matter K., Kreis T. E., Ginsel L., Hauri H. P. Identification of an intermediate compartment involved in protein transport from endoplasmic reticulum to Golgi apparatus. Eur J Cell Biol. 1990 Dec;53(2):185–196. [PubMed] [Google Scholar]
- Seidah N. G., Day R., Benjannet S., Rondeau N., Boudreault A., Reudelhuber T., Schafer M. K., Watson S. J., Chrétien M. The prohormone and proprotein processing enzymes PC1 and PC2: structure, selective cleavage of mouse POMC and human renin at pairs of basic residues, cellular expression, tissue distribution, and mRNA regulation. NIDA Res Monogr. 1992;126:132–150. [PubMed] [Google Scholar]
- Seidah N. G., Day R., Hamelin J., Gaspar A., Collard M. W., Chrétien M. Testicular expression of PC4 in the rat: molecular diversity of a novel germ cell-specific Kex2/subtilisin-like proprotein convertase. Mol Endocrinol. 1992 Oct;6(10):1559–1570. doi: 10.1210/mend.6.10.1448111. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Day R., Marcinkiewicz M., Benjannet S., Chrétien M. Mammalian neural and endocrine pro-protein and pro-hormone convertases belonging to the subtilisin family of serine proteinases. Enzyme. 1991;45(5-6):271–284. doi: 10.1159/000468901. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Fournier H., Boileau G., Benjannet S., Rondeau N., Chrétien M. The cDNA structure of the porcine pro-hormone convertase PC2 and the comparative processing by PC1 and PC2 of the N-terminal glycopeptide segment of porcine POMC. FEBS Lett. 1992 Oct 5;310(3):235–239. doi: 10.1016/0014-5793(92)81339-n. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Gaspar L., Mion P., Marcinkiewicz M., Mbikay M., Chrétien M. cDNA sequence of two distinct pituitary proteins homologous to Kex2 and furin gene products: tissue-specific mRNAs encoding candidates for pro-hormone processing proteinases. DNA Cell Biol. 1990 Jul-Aug;9(6):415–424. doi: 10.1089/dna.1990.9.415. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Hamelin J., Gaspar A. M., Day R., Chrétien M. The cDNA sequence of the human pro-hormone and pro-protein convertase PC1. DNA Cell Biol. 1992 May;11(4):283–289. doi: 10.1089/dna.1992.11.283. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Marcinkiewicz M., Benjannet S., Gaspar L., Beaubien G., Mattei M. G., Lazure C., Mbikay M., Chrétien M. Cloning and primary sequence of a mouse candidate prohormone convertase PC1 homologous to PC2, Furin, and Kex2: distinct chromosomal localization and messenger RNA distribution in brain and pituitary compared to PC2. Mol Endocrinol. 1991 Jan;5(1):111–122. doi: 10.1210/mend-5-1-111. [DOI] [PubMed] [Google Scholar]
- Smeekens S. P., Avruch A. S., LaMendola J., Chan S. J., Steiner D. F. Identification of a cDNA encoding a second putative prohormone convertase related to PC2 in AtT20 cells and islets of Langerhans. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):340–344. doi: 10.1073/pnas.88.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. P., Steiner D. F. Identification of a human insulinoma cDNA encoding a novel mammalian protein structurally related to the yeast dibasic processing protease Kex2. J Biol Chem. 1990 Feb 25;265(6):2997–3000. [PubMed] [Google Scholar]
- Tartakoff A. M. Temperature and energy dependence of secretory protein transport in the exocrine pancreas. EMBO J. 1986 Jul;5(7):1477–1482. doi: 10.1002/j.1460-2075.1986.tb04385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L., Leduc R., Thorne B. A., Smeekens S. P., Steiner D. F., Thomas G. Kex2-like endoproteases PC2 and PC3 accurately cleave a model prohormone in mammalian cells: evidence for a common core of neuroendocrine processing enzymes. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5297–5301. doi: 10.1073/pnas.88.12.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet T., Tessier D. C., Richardson C., Laliberté F., Khouri H. E., Bell A. W., Storer A. C., Thomas D. Y. Secretion of functional papain precursor from insect cells. Requirement for N-glycosylation of the pro-region. J Biol Chem. 1990 Sep 25;265(27):16661–16666. [PubMed] [Google Scholar]
- Vindrola O., Lindberg I. Biosynthesis of the prohormone convertase mPC1 in AtT-20 cells. Mol Endocrinol. 1992 Jul;6(7):1088–1094. doi: 10.1210/mend.6.7.1508222. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Nakagawa T., Ikemizu J., Nagahama M., Murakami K., Nakayama K. Sequence requirements for precursor cleavage within the constitutive secretory pathway. J Biol Chem. 1992 Apr 25;267(12):8270–8274. [PubMed] [Google Scholar]
- Wells J. A., Ferrari E., Henner D. J., Estell D. A., Chen E. Y. Cloning, sequencing, and secretion of Bacillus amyloliquefaciens subtilisin in Bacillus subtilis. Nucleic Acids Res. 1983 Nov 25;11(22):7911–7925. doi: 10.1093/nar/11.22.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox C. A., Fuller R. S. Posttranslational processing of the prohormone-cleaving Kex2 protease in the Saccharomyces cerevisiae secretory pathway. J Cell Biol. 1991 Oct;115(2):297–307. doi: 10.1083/jcb.115.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]