Abstract
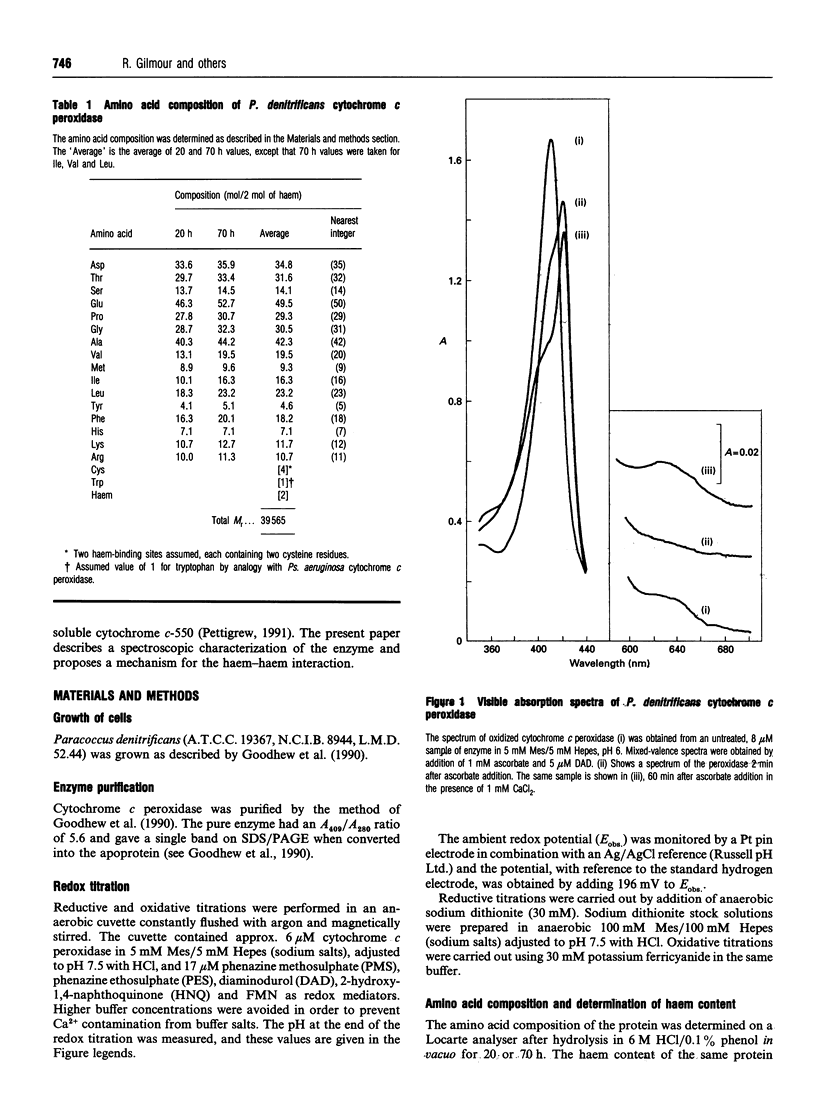
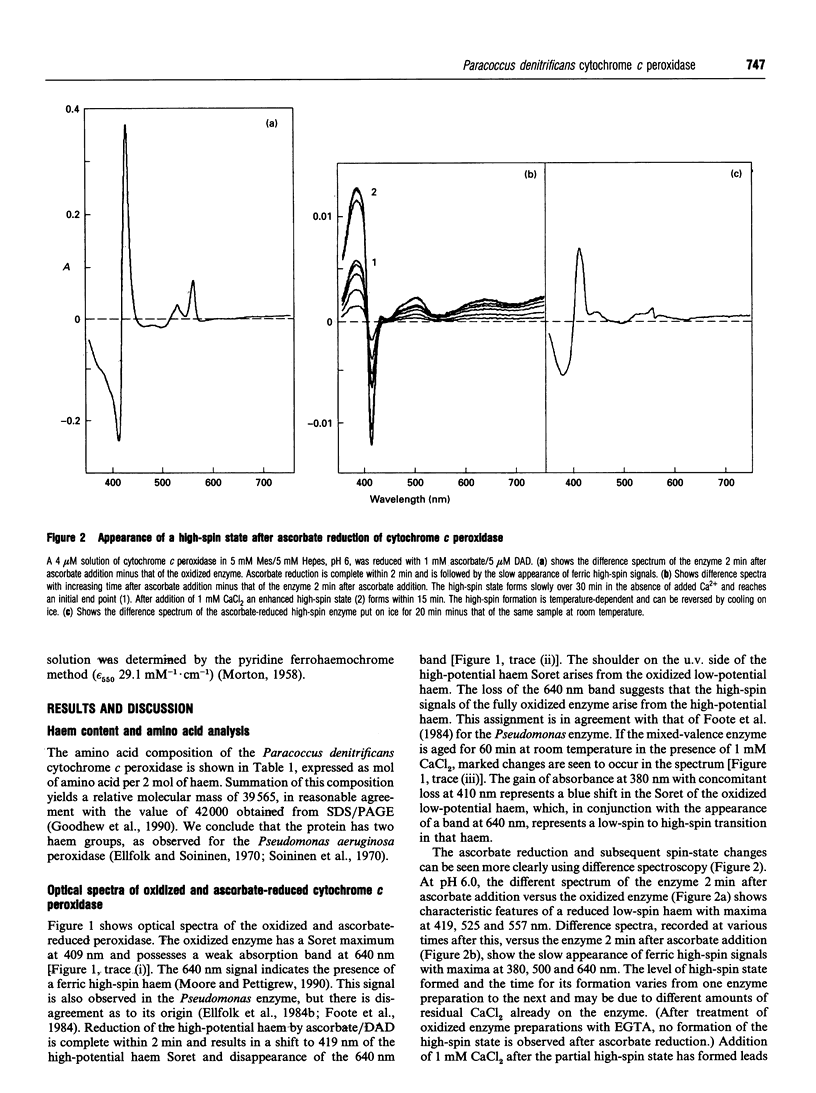
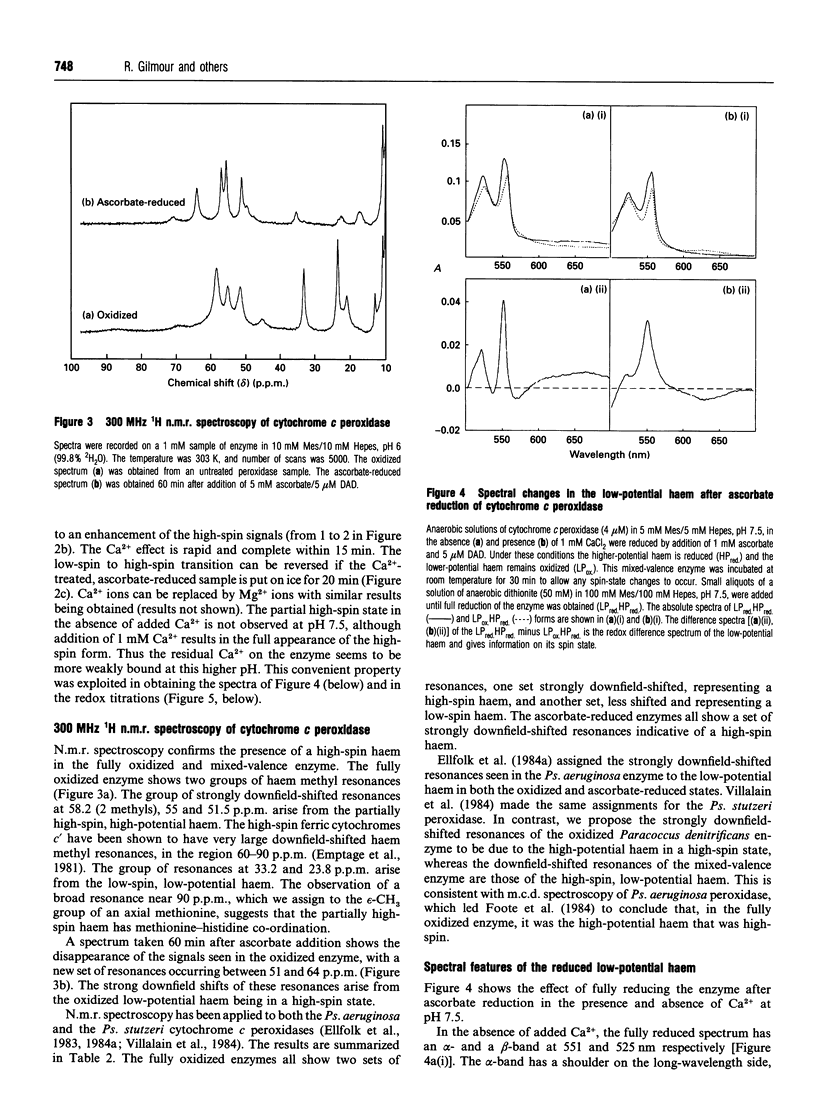
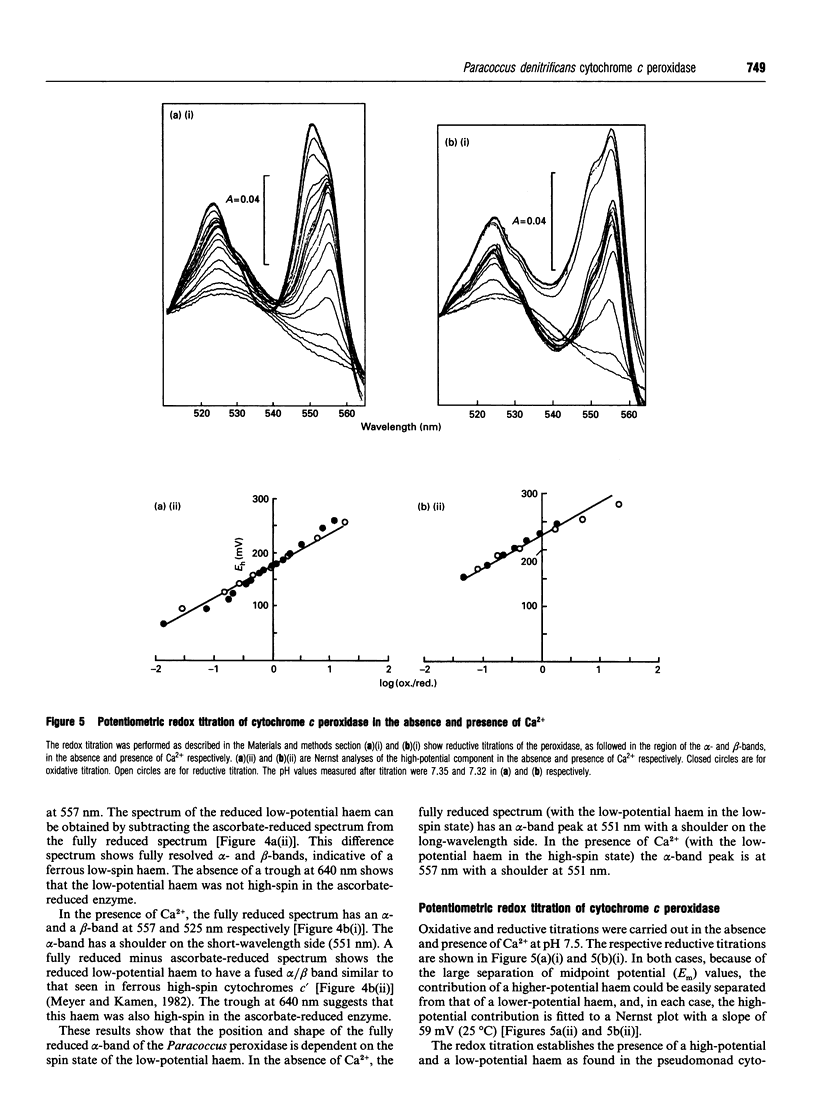
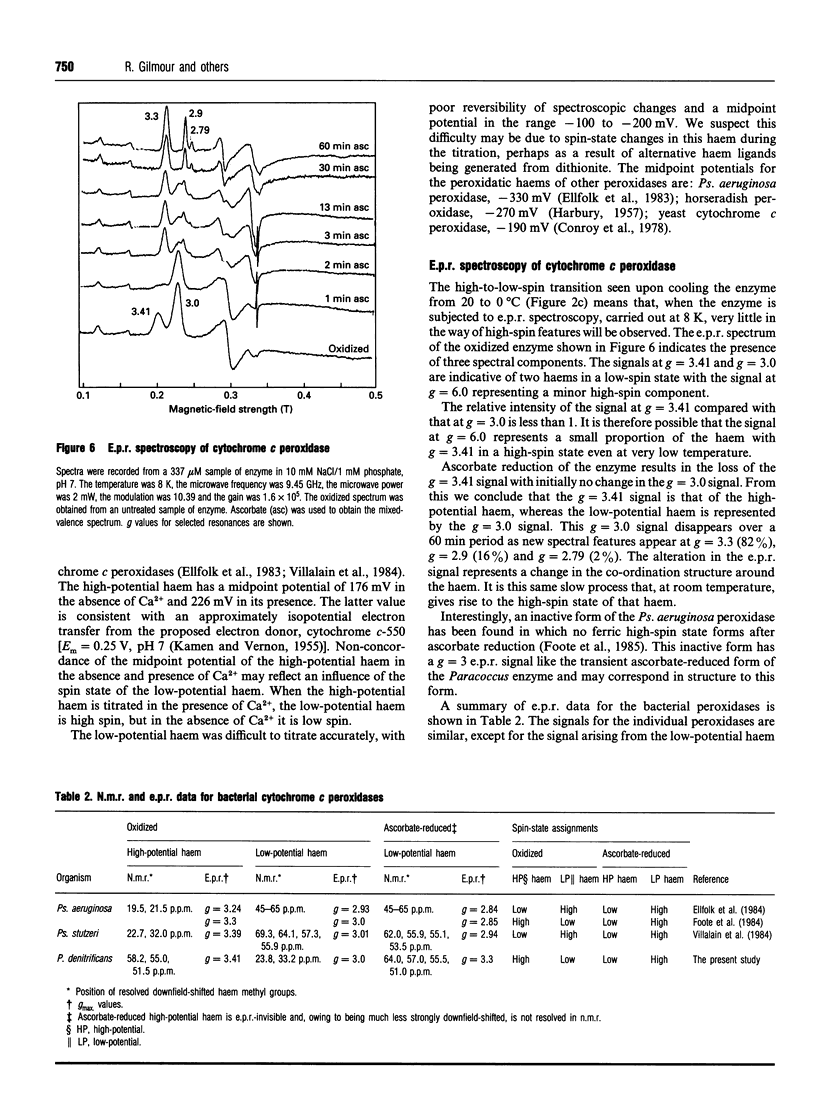
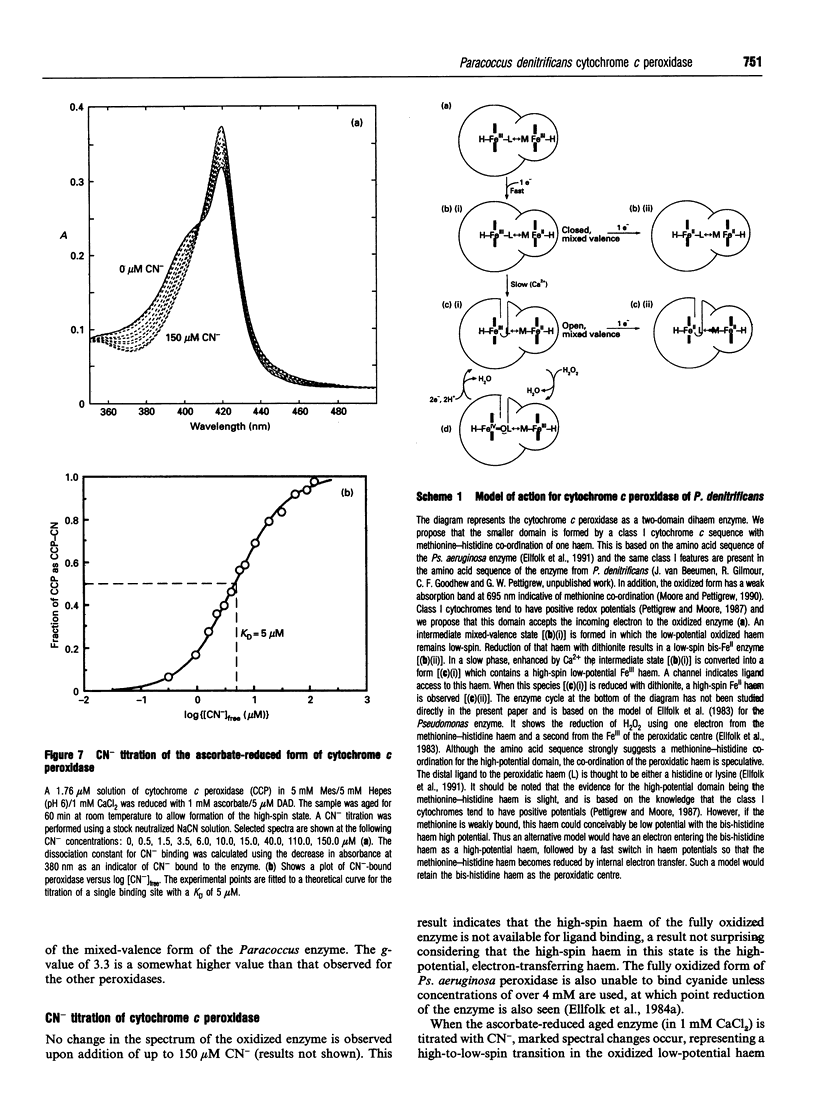
The cytochrome c peroxidase of Paracoccus denitrificans is similar to the well-studied enzyme from Pseudomonas aeruginosa. Like the Pseudomonas enzyme, the Paracoccus peroxidase contains two haem c groups, one high potential and one low potential. The high-potential haem acts as a source of the second electron for H2O2 reduction, and the low-potential haem acts as a peroxidatic centre. Reduction with ascorbate of the high-potential haem of the Paracoccus enzyme results in a switch of the low-potential haem to a high-spin state, as shown by visible and n.m.r. spectroscopy. This high-spin haem of the mixed-valence enzyme is accessible to ligands and binds CN- with a KD of 5 microM. The Paracoccus enzyme is significantly different from that from Pseudomonas in the time course of high-spin formation after reduction of the high-potential haem, and in the requirement for bivalent cations. Reduction with 1 mM ascorbate at pH 6 is complete within 2 min, and this is followed by a slow appearance of the high-spin state with a half-time of 10 min. Thus the process of reduction and spin state change can be easily separated in time and the intermediate form obtained. This separation is also evident in e.p.r. spectra, although the slow change involves an alteration in the low-spin ligation at this temperature rather than a change in spin state. The separation is even more striking at pH 7.5, where no high-spin form is obtained until 1 mM Ca2+ is added to the mixed-valence enzyme. The spin-state switch of the low-potential haem shifts the midpoint redox potential of the high-potential haem by 50 mV, a further indication of haem-haem interaction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araiso T., Rönnberg M., Dunford H. B., Ellfolk N. The formation of the primary compound from hydrogen peroxide and Pseudomonas cytochrome c peroxidase. FEBS Lett. 1980 Aug 25;118(1):99–102. doi: 10.1016/0014-5793(80)81227-0. [DOI] [PubMed] [Google Scholar]
- Conroy C. W., Tyma P., Daum P. H., Erman J. E. Oxidation-reduction potential measurements of cytochrome c peroxidase and pH dependent spectral transitions in the ferrous enzyme. Biochim Biophys Acta. 1978 Nov 20;537(1):62–69. doi: 10.1016/0005-2795(78)90602-5. [DOI] [PubMed] [Google Scholar]
- Ellfolk N., Rönnberg M., Aasa R., Andréasson L. E., Vänngård T. Anion binding to resting and half-reduced Pseudomonas cytochrome c peroxidase. Biochim Biophys Acta. 1984 Jan 18;784(1):62–67. doi: 10.1016/0167-4838(84)90173-0. [DOI] [PubMed] [Google Scholar]
- Ellfolk N., Rönnberg M., Aasa R., Andréasson L. E., Vänngård T. Properties and function of the two hemes in Pseudomonas cytochrome c peroxidase. Biochim Biophys Acta. 1983 Feb 28;743(1):23–30. doi: 10.1016/0167-4838(83)90413-2. [DOI] [PubMed] [Google Scholar]
- Ellfolk N., Rönnberg M., Osterlund K. Structural and functional features of Pseudomonas cytochrome c peroxidase. Biochim Biophys Acta. 1991 Oct 11;1080(1):68–77. doi: 10.1016/0167-4838(91)90113-e. [DOI] [PubMed] [Google Scholar]
- Ellfolk N., Soininen R. Pseudomonas cytochrome c peroxidase. I. Purification procedure. Acta Chem Scand. 1970;24(6):2126–2136. doi: 10.3891/acta.chem.scand.24-2126. [DOI] [PubMed] [Google Scholar]
- Emptage M. H., Xavier A. V., Wood J. M., Alsaadi B. M., Moore G. R., Pitt R. C., Williams R. J., Ambler R. P., Bartsch R. G. Nuclear magnetic resonance studies of Rhodospirillum rubrum cytochrome c'. Biochemistry. 1981 Jan 6;20(1):58–64. doi: 10.1021/bi00504a010. [DOI] [PubMed] [Google Scholar]
- Foote N., Peterson J., Gadsby P. M., Greenwood C., Thomson A. J. A study of the oxidized form of Pseudomonas aeruginosa cytochrome c-551 peroxidase with the use of magnetic circular dichroism. Biochem J. 1984 Oct 15;223(2):369–378. doi: 10.1042/bj2230369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote N., Peterson J., Gadsby P. M., Greenwood C., Thomson A. J. Redox-linked spin-state changes in the di-haem cytochrome c-551 peroxidase from Pseudomonas aeruginosa. Biochem J. 1985 Aug 15;230(1):227–237. doi: 10.1042/bj2300227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodhew C. F., Wilson I. B., Hunter D. J., Pettigrew G. W. The cellular location and specificity of bacterial cytochrome c peroxidases. Biochem J. 1990 Nov 1;271(3):707–712. doi: 10.1042/bj2710707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARBURY H. A. Oxidation-reduction potentials of horseradish peroxidase. J Biol Chem. 1957 Apr;225(2):1009–1024. [PubMed] [Google Scholar]
- KAMEN M. D., VERNON L. P. Comparative studies on bacterial cytochromes. Biochim Biophys Acta. 1955 May;17(1):10–22. doi: 10.1016/0006-3002(55)90315-2. [DOI] [PubMed] [Google Scholar]
- KEILIN D., HARTREE E. F. Purification of horse-radish peroxidase and comparison of its properties with those of catalase and methaemoglobin. Biochem J. 1951 Jun;49(1):88–104. doi: 10.1042/bj0490088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. E., Kamen M. D. New perspectives on c-type cytochromes. Adv Protein Chem. 1982;35:105–212. doi: 10.1016/s0065-3233(08)60469-6. [DOI] [PubMed] [Google Scholar]
- Pettigrew G. W. The cytochrome c peroxidase of Paracoccus denitrificans. Biochim Biophys Acta. 1991 May 23;1058(1):25–27. doi: 10.1016/s0005-2728(05)80261-0. [DOI] [PubMed] [Google Scholar]
- Rönnberg M., Kalkkinen N., Ellfolk N. The primary structure of Pseudomonas cytochrome c peroxidase. FEBS Lett. 1989 Jul 3;250(2):175–178. doi: 10.1016/0014-5793(89)80714-8. [DOI] [PubMed] [Google Scholar]
- Rönnberg M., Osterlund K., Ellfolk N. Resonance Raman spectra of Pseudomonas cytochrome c peroxidase. Biochim Biophys Acta. 1980 Nov 20;626(1):23–30. doi: 10.1016/0005-2795(80)90193-2. [DOI] [PubMed] [Google Scholar]
- Sievers G. Circular dichroism studies on cytochrome c peroxidase from baker's yeast (Saccharomyces cerevisiae). Biochim Biophys Acta. 1978 Sep 26;536(1):212–225. doi: 10.1016/0005-2795(78)90067-3. [DOI] [PubMed] [Google Scholar]
- Soininen R., Sojonen H., Ellfolk N. Pseudomonas cytochrome c peroxidase. II. Localization of cytochrome c peroxidase in Pseudomonas fluorescens. Acta Chem Scand. 1970;24(7):2314–2320. doi: 10.3891/acta.chem.scand.24-2314. [DOI] [PubMed] [Google Scholar]
- Villalaín J., Moura I., Liu M. C., Payne W. J., LeGall J., Xavier A. V., Moura J. J. NMR and electron-paramagnetic-resonance studies of a dihaem cytochrome from Pseudomonas stutzeri (ATCC 11607) (cytochrome c peroxidase). Eur J Biochem. 1984 Jun 1;141(2):305–312. doi: 10.1111/j.1432-1033.1984.tb08192.x. [DOI] [PubMed] [Google Scholar]


