Abstract
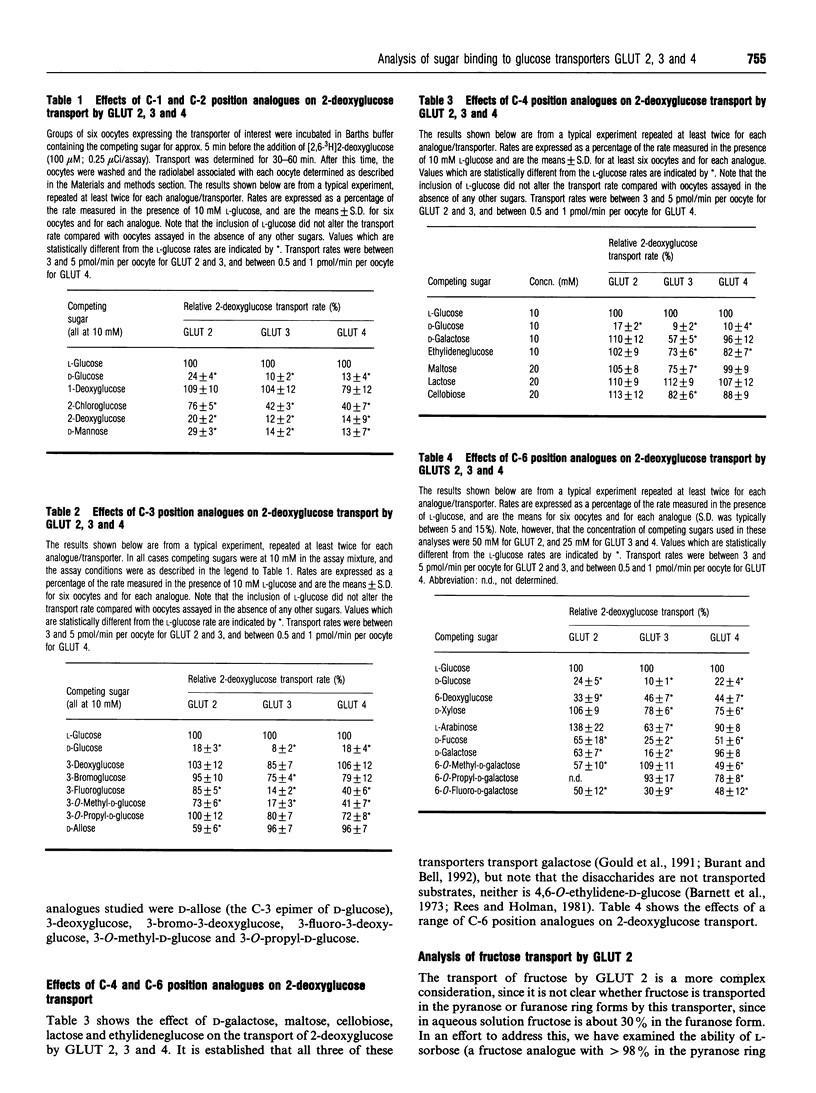
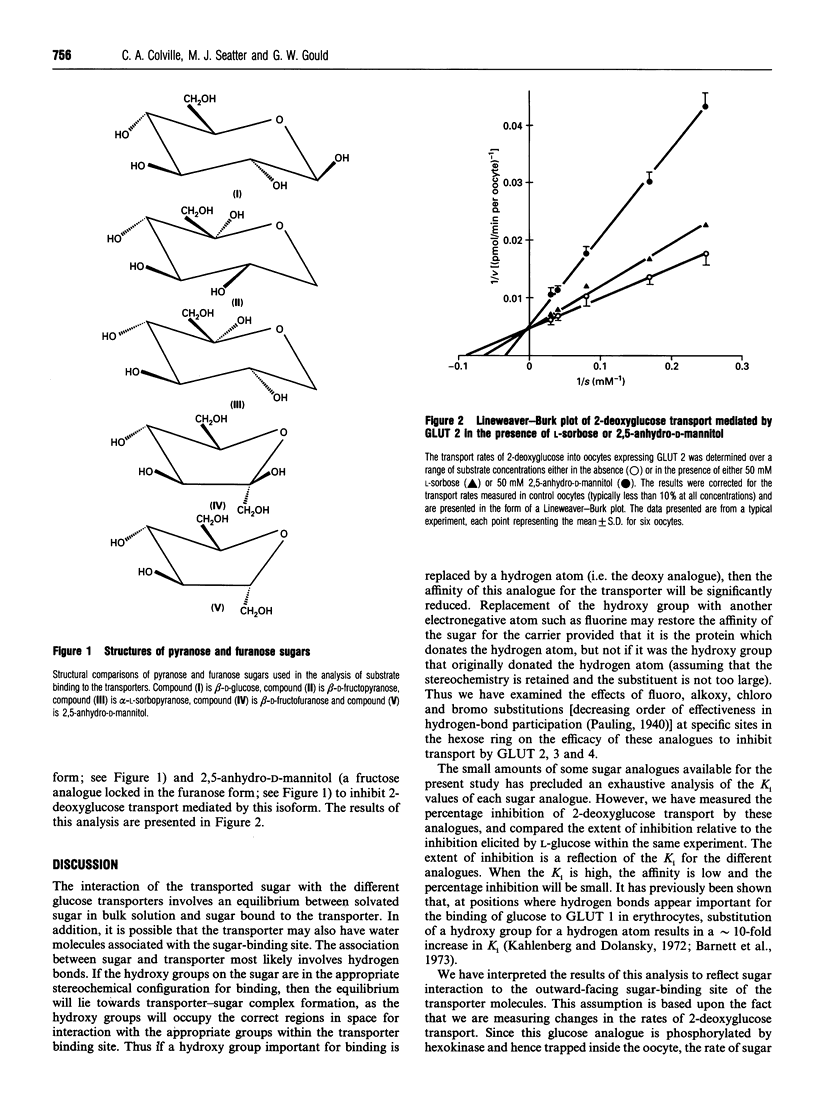
We have expressed the liver (GLUT 2), brain (GLUT 3) and insulin-responsive (GLUT 4) glucose transporters in oocytes from Xenopus laevis by microinjection of in vitro-transcribed mRNA. Using a range of halogeno- and deoxy-glucose analogues, and other hexoses, we have studied the structural basis of sugar binding to these different isoforms. We show that a hydrogen bond to the C-3 position is involved in sugar binding for all three isoforms, but that the direction of this hydrogen bond is different in GLUT 2 from either GLUT 1, 3 or 4. Hydrogen-bonding at the C-4 position is also involved in sugar recognition by all three isoforms, but we propose that in GLUT 3 this hydrogen bond plays a less significant role than in GLUT 2 and 4. In all transporters we propose that the C-4 position is directed out of the sugar-binding pocket. The role of the C-6 position is also discussed. In addition, we have analysed the ability of fructopyranose and fructofuranose analogues to inhibit the transport mediated by GLUT2. We show that fructofuranose analogues, but not fructopyranose analogues, are efficient inhibitors of transport mediated by GLUT 2, and therefore suggest that GLUT 2 accommodates D-glucose as a pyranose ring, but D-fructose as a furanose ring. Models for the binding sites of GLUT 2, 3 and 4 are presented.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett J. E., Holman G. D., Chalkley R. A., Munday K. A. Evidence for two asymmetric conformational states in the human erythrocyte sugar-transport system. Biochem J. 1975 Mar;145(3):417–429. doi: 10.1042/bj1450417a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett J. E., Holman G. D., Munday K. A. Structural requirements for binding to the sugar-transport system of the human erythrocyte. Biochem J. 1973 Feb;131(2):211–221. doi: 10.1042/bj1310211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett J. E., Ralph A., Munday K. A. Structural requirements for active intestinal transport. The nature of the carrier-sugar bonding at C-2 and the ring oxygen of the sugar. Biochem J. 1970 Aug;118(5):843–850. doi: 10.1042/bj1180843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Kayano T., Buse J. B., Burant C. F., Takeda J., Lin D., Fukumoto H., Seino S. Molecular biology of mammalian glucose transporters. Diabetes Care. 1990 Mar;13(3):198–208. doi: 10.2337/diacare.13.3.198. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum M. J. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989 Apr 21;57(2):305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Bell G. I. Mammalian facilitative glucose transporters: evidence for similar substrate recognition sites in functionally monomeric proteins. Biochemistry. 1992 Oct 27;31(42):10414–10420. doi: 10.1021/bi00157a032. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Takeda J., Brot-Laroche E., Bell G. I., Davidson N. O. Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem. 1992 Jul 25;267(21):14523–14526. [PubMed] [Google Scholar]
- Charron M. J., Brosius F. C., 3rd, Alper S. L., Lodish H. F. A glucose transport protein expressed predominately in insulin-responsive tissues. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2535–2539. doi: 10.1073/pnas.86.8.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colville C. A., Seatter M. J., Jess T. J., Gould G. W., Thomas H. M. Kinetic analysis of the liver-type (GLUT2) and brain-type (GLUT3) glucose transporters in Xenopus oocytes: substrate specificities and effects of transport inhibitors. Biochem J. 1993 Mar 15;290(Pt 3):701–706. doi: 10.1042/bj2900701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier J. S., Mueckler M. M., Usher P., Lodish H. F. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987 Mar 20;235(4795):1492–1495. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- Fukumoto H., Kayano T., Buse J. B., Edwards Y., Pilch P. F., Bell G. I., Seino S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989 May 15;264(14):7776–7779. [PubMed] [Google Scholar]
- Fukumoto H., Seino S., Imura H., Seino Y., Eddy R. L., Fukushima Y., Byers M. G., Shows T. B., Bell G. I. Sequence, tissue distribution, and chromosomal localization of mRNA encoding a human glucose transporter-like protein. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5434–5438. doi: 10.1073/pnas.85.15.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould G. W., Bell G. I. Facilitative glucose transporters: an expanding family. Trends Biochem Sci. 1990 Jan;15(1):18–23. doi: 10.1016/0968-0004(90)90125-u. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Brant A. M., Kahn B. B., Shepherd P. R., McCoid S. C., Gibbs E. M. Expression of the brain-type glucose transporter is restricted to brain and neuronal cells in mice. Diabetologia. 1992 Apr;35(4):304–309. doi: 10.1007/BF00401196. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Lienhard G. E. Expression of a functional glucose transporter in Xenopus oocytes. Biochemistry. 1989 Nov 28;28(24):9447–9452. doi: 10.1021/bi00450a030. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Thomas H. M., Jess T. J., Bell G. I. Expression of human glucose transporters in Xenopus oocytes: kinetic characterization and substrate specificities of the erythrocyte, liver, and brain isoforms. Biochemistry. 1991 May 28;30(21):5139–5145. doi: 10.1021/bi00235a004. [DOI] [PubMed] [Google Scholar]
- Holman G. D., Pierce E. J., Rees W. D. Spatial requirements for insulin-sensitive sugar transport in rat adipocytes. Biochim Biophys Acta. 1981 Sep 7;646(3):382–388. doi: 10.1016/0005-2736(81)90306-0. [DOI] [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Kaestner K. H., Christy R. J., McLenithan J. C., Braiterman L. T., Cornelius P., Pekala P. H., Lane M. D. Sequence, tissue distribution, and differential expression of mRNA for a putative insulin-responsive glucose transporter in mouse 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1989 May;86(9):3150–3154. doi: 10.1073/pnas.86.9.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayano T., Burant C. F., Fukumoto H., Gould G. W., Fan Y. S., Eddy R. L., Byers M. G., Shows T. B., Seino S., Bell G. I. Human facilitative glucose transporters. Isolation, functional characterization, and gene localization of cDNAs encoding an isoform (GLUT5) expressed in small intestine, kidney, muscle, and adipose tissue and an unusual glucose transporter pseudogene-like sequence (GLUT6). J Biol Chem. 1990 Aug 5;265(22):13276–13282. [PubMed] [Google Scholar]
- Kayano T., Fukumoto H., Eddy R. L., Fan Y. S., Byers M. G., Shows T. B., Bell G. I. Evidence for a family of human glucose transporter-like proteins. Sequence and gene localization of a protein expressed in fetal skeletal muscle and other tissues. J Biol Chem. 1988 Oct 25;263(30):15245–15248. [PubMed] [Google Scholar]
- Keller K., Strube M., Mueckler M. Functional expression of the human HepG2 and rat adipocyte glucose transporters in Xenopus oocytes. Comparison of kinetic parameters. J Biol Chem. 1989 Nov 15;264(32):18884–18889. [PubMed] [Google Scholar]
- Krupka R. M. Inhibition of sugar transport in erythrocytes by fluorodinitrobenzene. Biochemistry. 1971 Mar 30;10(7):1148–1153. doi: 10.1021/bi00783a008. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Mueckler M. Family of glucose-transporter genes. Implications for glucose homeostasis and diabetes. Diabetes. 1990 Jan;39(1):6–11. doi: 10.2337/diacare.39.1.6. [DOI] [PubMed] [Google Scholar]
- Nagamatsu S., Kornhauser J. M., Burant C. F., Seino S., Mayo K. E., Bell G. I. Glucose transporter expression in brain. cDNA sequence of mouse GLUT3, the brain facilitative glucose transporter isoform, and identification of sites of expression by in situ hybridization. J Biol Chem. 1992 Jan 5;267(1):467–472. [PubMed] [Google Scholar]
- Orci L., Thorens B., Ravazzola M., Lodish H. F. Localization of the pancreatic beta cell glucose transporter to specific plasma membrane domains. Science. 1989 Jul 21;245(4915):295–297. doi: 10.1126/science.2665080. [DOI] [PubMed] [Google Scholar]
- Rees W. D., Holman G. D. Hydrogen bonding requirements for the insulin-sensitive sugar transport system of rat adipocytes. Biochim Biophys Acta. 1981 Aug 20;646(2):251–260. doi: 10.1016/0005-2736(81)90331-x. [DOI] [PubMed] [Google Scholar]
- Rees W. D., Holman G. D. Hydrogen bonding requirements for the insulin-sensitive sugar transport system of rat adipocytes. Biochim Biophys Acta. 1981 Aug 20;646(2):251–260. doi: 10.1016/0005-2736(81)90331-x. [DOI] [PubMed] [Google Scholar]
- Shepherd P. R., Gibbs E. M., Wesslau C., Gould G. W., Kahn B. B. Human small intestine facilitative fructose/glucose transporter (GLUT5) is also present in insulin-responsive tissues and brain. Investigation of biochemical characteristics and translocation. Diabetes. 1992 Oct;41(10):1360–1365. doi: 10.2337/diab.41.10.1360. [DOI] [PubMed] [Google Scholar]
- Thomas H. M., Takeda J., Gould G. W. Differential targeting of glucose transporter isoforms heterologously expressed in Xenopus oocytes. Biochem J. 1993 Mar 15;290(Pt 3):707–715. doi: 10.1042/bj2900707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Waddell I. D., Zomerschoe A. G., Voice M. W., Burchell A. Cloning and expression of a hepatic microsomal glucose transport protein. Comparison with liver plasma-membrane glucose-transport protein GLUT 2. Biochem J. 1992 Aug 15;286(Pt 1):173–177. doi: 10.1042/bj2860173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H., Seino Y., Inagaki N., Hinokio Y., Yamamoto T., Yasuda K., Masuda K., Someya Y., Imura H. Tissue distribution and species difference of the brain type glucose transporter (GLUT3). Biochem Biophys Res Commun. 1991 Jan 31;174(2):470–477. doi: 10.1016/0006-291x(91)91440-n. [DOI] [PubMed] [Google Scholar]


