Abstract
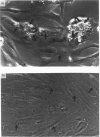
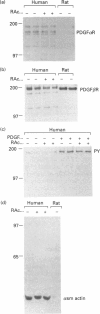
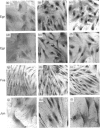
Activated Ito-cell-like myofibroblasts proliferate in vivo during human liver injury and subsequent fibrogenesis. To examine the associated regulatory mechanisms, human liver myofibroblasts were characterized after culture purification from mixed liver-cell isolates obtained from perfused normal human livers. The cells resembled rat Ito-cell-derived myofibroblasts expressing desmin and alpha-smooth-muscle actin filaments as well as the interstitial collagens type I and III. [3H]Thymidine incorporation was inducible with platelet-derived growth factor (PDGF) and was suppressible with retinoic acid (RAc) in a concentration-dependent fashion. RAc suppression did not alter PDGF alpha- or beta-receptor abundance or activation. In addition, RAc functioned via a pathway distal or independent of cytoplasmic raf activation (i.e. phosphorylation, kinase function and perinuclear translocation) and nuclear fos, jun and egr expression, as these steps were similarly unaffected by RAc treatment. Since normal Ito cells contain abundant amounts of vitamin A which is lost during activation, these data suggest that retinoids could contribute to the maintenance of the quiescent non-proliferative state by suppressing mitogenesis at a post-cytokine receptor step distal from or independent of fos/jun/egr [e.g. via changes in activator protein-1 (AP-1) binding].
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amédée-Manesme O., Furr H. C., Alvarez F., Hadchouel M., Alagille D., Olson J. A. Biochemical indicators of vitamin A depletion in children with cholestasis. Hepatology. 1985 Nov-Dec;5(6):1143–1148. doi: 10.1002/hep.1840050614. [DOI] [PubMed] [Google Scholar]
- Bachem M. G., Meyer D., Melchior R., Sell K. M., Gressner A. M. Activation of rat liver perisinusoidal lipocytes by transforming growth factors derived from myofibroblastlike cells. A potential mechanism of self perpetuation in liver fibrogenesis. J Clin Invest. 1992 Jan;89(1):19–27. doi: 10.1172/JCI115561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beno D. W., Espinal R., Edelstein B. M., Davis B. H. Administration of prostaglandin E1 analog reduces rat hepatic and Ito cell collagen gene expression and collagen accumulation after bile duct ligation injury. Hepatology. 1993 Apr;17(4):707–714. doi: 10.1002/hep.1840170427. [DOI] [PubMed] [Google Scholar]
- Cao X. M., Koski R. A., Gashler A., McKiernan M., Morris C. F., Gaffney R., Hay R. V., Sukhatme V. P. Identification and characterization of the Egr-1 gene product, a DNA-binding zinc finger protein induced by differentiation and growth signals. Mol Cell Biol. 1990 May;10(5):1931–1939. doi: 10.1128/mcb.10.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaja M. J., Weiner F. R., Flanders K. C., Giambrone M. A., Wind R., Biempica L., Zern M. A. In vitro and in vivo association of transforming growth factor-beta 1 with hepatic fibrosis. J Cell Biol. 1989 Jun;108(6):2477–2482. doi: 10.1083/jcb.108.6.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. H., Kramer R. T., Davidson N. O. Retinoic acid modulates rat Ito cell proliferation, collagen, and transforming growth factor beta production. J Clin Invest. 1990 Dec;86(6):2062–2070. doi: 10.1172/JCI114943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. H., Madri J. A. Type I and type III procollagen peptides during hepatic fibrogenesis. An immunohistochemical and ELISA serum study in the CCl4 rat model. Am J Pathol. 1987 Jan;126(1):137–147. [PMC free article] [PubMed] [Google Scholar]
- Davis B. H., Rapp U. R., Davidson N. O. Retinoic acid and transforming growth factor beta differentially inhibit platelet-derived-growth-factor-induced Ito-cell activation. Biochem J. 1991 Aug 15;278(Pt 1):43–47. doi: 10.1042/bj2780043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. H. Transforming growth factor beta responsiveness is modulated by the extracellular collagen matrix during hepatic ito cell culture. J Cell Physiol. 1988 Sep;136(3):547–553. doi: 10.1002/jcp.1041360323. [DOI] [PubMed] [Google Scholar]
- Davis B. H., Vucic A. The effect of retinol on Ito cell proliferation in vitro. Hepatology. 1988 Jul-Aug;8(4):788–793. doi: 10.1002/hep.1840080416. [DOI] [PubMed] [Google Scholar]
- Diliberto P. A., Gordon G. W., Yu C. L., Earp H. S., Herman B. Platelet-derived growth factor (PDGF) alpha receptor activation modulates the calcium mobilizing activity of the PDGF beta receptor in Balb/c3T3 fibroblasts. J Biol Chem. 1992 Jun 15;267(17):11888–11897. [PubMed] [Google Scholar]
- Diliberto P. A., Gordon G., Herman B. Regional and mechanistic differences in platelet-derived growth factor-isoform-induced alterations in cytosolic free calcium in porcine vascular smooth muscle cells. J Biol Chem. 1991 Jul 5;266(19):12612–12617. [PubMed] [Google Scholar]
- Friedman S. L., Arthur M. J. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989 Dec;84(6):1780–1785. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. L., Rockey D. C., McGuire R. F., Maher J. J., Boyles J. K., Yamasaki G. Isolated hepatic lipocytes and Kupffer cells from normal human liver: morphological and functional characteristics in primary culture. Hepatology. 1992 Feb;15(2):234–243. doi: 10.1002/hep.1840150211. [DOI] [PubMed] [Google Scholar]
- Friedman S. L., Roll F. J., Boyles J., Arenson D. M., Bissell D. M. Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J Biol Chem. 1989 Jun 25;264(18):10756–10762. [PubMed] [Google Scholar]
- Gressner A. M., Lotfi S., Gressner G., Lahme B. Identification and partial characterization of a hepatocyte-derived factor promoting proliferation of cultured fat-storing cells (parasinusoidal lipocytes). Hepatology. 1992 Nov;16(5):1250–1266. [PubMed] [Google Scholar]
- Gronwald R. G., Seifert R. A., Bowen-Pope D. F. Differential regulation of expression of two platelet-derived growth factor receptor subunits by transforming growth factor-beta. J Biol Chem. 1989 May 15;264(14):8120–8125. [PubMed] [Google Scholar]
- Jaffey P., Chan L. N., Shao J., Schneider-Schaulies J., Chan T. S. Retinoic acid inhibition of serum-induced c-fos transcription in a fibrosarcoma cell line. Cancer Res. 1992 May 1;52(9):2384–2388. [PubMed] [Google Scholar]
- Kolch W., Weissinger E., Mischak H., Troppmair J., Showalter S. D., Lloyd P., Heidecker G., Rapp U. R. Probing structure and function of the raf protein kinase domain with monoclonal antibodies. Oncogene. 1990 May;5(5):713–720. [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Carey K. D., Estlack L. E., Smith G. C., Hay R. V. Analysis of plasma protein and lipoprotein synthesis in long-term primary cultures of baboon hepatocytes maintained in serum-free medium. In Vitro Cell Dev Biol. 1989 Feb;25(2):174–182. doi: 10.1007/BF02626175. [DOI] [PubMed] [Google Scholar]
- Laskin D. L. Nonparenchymal cells and hepatotoxicity. Semin Liver Dis. 1990 Nov;10(4):293–304. doi: 10.1055/s-2008-1040485. [DOI] [PubMed] [Google Scholar]
- Leo M. A., Lowe N., Lieber C. S. Decreased hepatic vitamin A after drug administration in men and in rats. Am J Clin Nutr. 1984 Dec;40(6):1131–1136. doi: 10.1093/ajcn/40.6.1131. [DOI] [PubMed] [Google Scholar]
- Maher J. J., Bissell D. M., Friedman S. L., Roll F. J. Collagen measured in primary cultures of normal rat hepatocytes derives from lipocytes within the monolayer. J Clin Invest. 1988 Aug;82(2):450–459. doi: 10.1172/JCI113618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak K. M., Leo M. A., Lieber C. S. Alcoholic liver injury in baboons: transformation of lipocytes to transitional cells. Gastroenterology. 1984 Jul;87(1):188–200. [PubMed] [Google Scholar]
- Matsui T., Pierce J. H., Fleming T. P., Greenberger J. S., LaRochelle W. J., Ruggiero M., Aaronson S. A. Independent expression of human alpha or beta platelet-derived growth factor receptor cDNAs in a naive hematopoietic cell leads to functional coupling with mitogenic and chemotactic signaling pathways. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8314–8318. doi: 10.1073/pnas.86.21.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. H., Jr, Moy D., Li A., Grippo J. F., Dmitrovsky E. Retinoic acid induces down-regulation of several growth factors and proto-oncogenes in a human embryonal cancer cell line. Oncogene. 1990 Apr;5(4):511–517. [PubMed] [Google Scholar]
- Minato Y., Hasumura Y., Takeuchi J. The role of fat-storing cells in Disse space fibrogenesis in alcoholic liver disease. Hepatology. 1983 Jul-Aug;3(4):559–566. doi: 10.1002/hep.1840030414. [DOI] [PubMed] [Google Scholar]
- Pinzani M., Failli P., Ruocco C., Casini A., Milani S., Baldi E., Giotti A., Gentilini P. Fat-storing cells as liver-specific pericytes. Spatial dynamics of agonist-stimulated intracellular calcium transients. J Clin Invest. 1992 Aug;90(2):642–646. doi: 10.1172/JCI115905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzani M., Gentilini P., Abboud H. E. Phenotypical modulation of liver fat-storing cells by retinoids. Influence on unstimulated and growth factor-induced cell proliferation. J Hepatol. 1992 Mar;14(2-3):211–220. doi: 10.1016/0168-8278(92)90160-q. [DOI] [PubMed] [Google Scholar]
- Pinzani M., Knauss T. C., Pierce G. F., Hsieh P., Kenney W., Dubyak G. R., Abboud H. E. Mitogenic signals for platelet-derived growth factor isoforms in liver fat-storing cells. Am J Physiol. 1991 Mar;260(3 Pt 1):C485–C491. doi: 10.1152/ajpcell.1991.260.3.C485. [DOI] [PubMed] [Google Scholar]
- Qureshi S. A., Rim M., Bruder J., Kolch W., Rapp U., Sukhatme V. P., Foster D. A. An inhibitory mutant of c-Raf-1 blocks v-Src-induced activation of the Egr-1 promoter. J Biol Chem. 1991 Nov 5;266(31):20594–20597. [PubMed] [Google Scholar]
- Rapp U. R. Role of Raf-1 serine/threonine protein kinase in growth factor signal transduction. Oncogene. 1991 Apr;6(4):495–500. [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Yang N., Kliewer S., Ransone L. J., Bolado J., Verma I. M., Evans R. M. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6092–6096. doi: 10.1073/pnas.88.14.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Schürch W., Seemayer T., Lagacé R., Montandon D., Pittet B., Gabbiani G. Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest. 1989 Feb;60(2):275–285. [PubMed] [Google Scholar]
- Smeal T., Binetruy B., Mercola D., Grover-Bardwick A., Heidecker G., Rapp U. R., Karin M. Oncoprotein-mediated signalling cascade stimulates c-Jun activity by phosphorylation of serines 63 and 73. Mol Cell Biol. 1992 Aug;12(8):3507–3513. doi: 10.1128/mcb.12.8.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T. Signal transduction by the platelet-derived growth factor receptor. Science. 1989 Mar 24;243(4898):1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]