Graphical abstract

Keywords: PPARγ, Ferroptosis, PM2.5, Epithelial–mesenchymal transition, Tubular injury
Highlights
-
•
Aberrant PPARγ expression results in ferroptotic susceptibility and promotes tubular EMT in response to PM2.5 exposure.
-
•
PPARγ overexpression protect against EMT by mitigating the inhibition of glutathione peroxidase 4 (GPX4) induced by PM2.5.
-
•
Activation of PPARγ could serve as a preventive strategy for PM2.5-induced renal injury by reducing ferroptosis.
Abstract
Exposure to fine particulate matter (PM2.5) has been associated with the development and progression of renal disease. Peroxisome proliferator-activated receptor gamma (PPARγ), a key transcription factor involved in inflammation as well as lipid and glucose metabolism, helps maintain the integrity of tubular epithelial cells. However, the precise role of PPARγ in PM2.5-induced tubular injury remains unclear. In this study, we investigated the regulatory effects of PPARγ on PM2.5-induced ferroptotic stress and epithelial–mesenchymal transition (EMT) in tubular (HK-2) cells. We found that downregulation of PPARγ expression was correlated with EMT in PM2.5-exposed cells. Pretreatment with the PPARγ agonist 15d-PGJ2 protected the cells from EMT by reducing ferroptotic stress, whereas that with the PPARγ antagonist GW9662 promoted EMT. Furthermore, pretreatment with ferrostatin-1 (Fer-1) significantly prevented PM2.5-induced EMT and downregulation of PPARγ expression. Notably, overexpression of PPARγ blocked PM2.5-induced downregulation of E-cadherin and GPX4 expression and upregulation of α-SMA expression. This study highlights the complex associations of PPARγ with ferroptosis and EMT in PM2.5-exposed tubular cells. Our findings suggest that PPARγ activation confers protection against PM2.5-induced renal injury.
1. Introduction
Epidemiological studies have demonstrated that ambient air pollution is closely associated with morbidity and mortality worldwide, particularly in developing countries (Boogaard et al., 2019, Cromar et al., 2022). Fine particulate matter (PM2.5) is a mixture of air pollutants with a diameter of < 2.5 µm that can reach the alveoli, enter the bloodstream, and exert adverse systemic effects. PM2.5 exposure can cause various diseases, such as respiratory disorders, cardiovascular disease, and cancer (Li and Wang, 2023, Su et al., 2020, Wang et al., 2021a). Approximately 72.8 % of all cases of chronic kidney disease (CKD) worldwide are attributable to PM2.5 (Bowe et al., 2020). A 10 μg/m3 increase in the concentration of ambient PM2.5 results in a 1.28-fold increase in the risk of CKD (Li et al., 2021). Exposure to PM2.5 can cause CKD and end-stage renal disease by inducing oxidative stress, inflammatory responses, and cytotoxicity; thus, PM2.5 exposure is a key risk factor for renal function decline (Wen et al., 2023, Xu et al., 2023, Zhang et al., 2023).
Peroxisome proliferator-activated receptor gamma (PPARγ) is a ligand-activated nuclear receptor that plays an essential role in several cellular processes, including the maintenance of glucose levels, management of lipid metabolism, regulation of inflammation, and control of extracellular matrix remodeling (Kokeny et al., 2021, Menendez-Gutierrez et al., 2012). PPARγ activation protects renal cells from inflammation and oxidative stress—the hallmarks of acute kidney injury and contributors to CKD progression (Liu et al., 2020, Maquigussa et al., 2018).
Given that PM2.5 induces ferroptosis, understanding the role of PM2.5-induced ferroptotic stress in the pathogenesis and treatment of PM2.5-related diseases is imperative (Wang et al., 2021b). Tubular epithelial cells are susceptible to inflammation and ferroptosis induced by various stimuli (Giuliani et al., 2022, Ide et al., 2021). Interventions targeting ferroptotic stress have been demonstrated to prevent the occurrence of epithelial–mesenchymal transition (EMT) in tubular cells and decelerate the progression of renal diseases (Feng et al., 2022, Zhou et al., 2022). In this study, we investigated the regulatory effects of PPARγ on ferroptotic stress and EMT in PM2.5-exposed tubular cells.
2. Materials and methods
2.1. Cell culture
The human proximal tubular epithelial cell line HK-2 was purchased from the American Type Culture Collection center and cultured in Dulbecco’s Modified Eagle Medium/F12 supplemented with 10 % fetal bovine serum. The cells were cultured at 37 °C in a humidified environment with 5 % CO2. After 24 h of incubation, the cells were subjected to serum starvation and subsequently treated with PM2.5.
2.2. PM2.5 and chemicals
PM2.5 was purchased from Sigma–Aldrich Chemical Reagent (St. Louis, MO, USA) and stored as a 10-mg/mL stock solution after ultrasonication. The viability of the HK-2 cells after PM2.5 exposure was examined. Ultimately, a PM2.5 concentration of 50 µg/mL was used in subsequent experiments. The PPARγ agonist 15d-PGJ2 and the PPARγ antagonist GW9662 were purchased from Cayman Chemicals (Ann Arbor, MI, USA). The ferroptosis inhibitor ferrostatin-1 (Fer-1) was obtained from Santa Cruz Biotechnology (Paso Robles, CA, USA).
2.3. Measurement of iron content
The iron content in the PM2.5-exposed HK-2 cells was evaluated using a colorimetric iron assay kit (Abcam, Cambridge, UK) in accordance with the manufacturer’s protocol. Absorbance was measured at 593 nm by using a microplate reader.
2.4. Reduced and oxidized glutathione assay
The levels of reduced glutathione (GSH) and oxidized glutathione (GSSG) in the HK-2 cells were measured using the GSH/GSSG Ratio Detection Assay Kit (Abcam) in accordance with the manufacturer’s instructions. The GSH/GSSG ratio was determined through linear regression of values obtained from the standard curve.
2.5. Measurement of malondialdehyde (MDA) levels
The level of MDA, a representative end product of lipid peroxidation, in the HK-2 cells was evaluated through a colorimetric assay of lipid reactive oxygen species (ROS) production. The assay was performed using a lipid peroxidation (MDA) assay kit (Sigma–Aldrich) in accordance with the manufacturer’s instructions.
2.6. Assessment of lipid peroxidation
Lipid peroxidation in the live HK-2 cells was studied using the Image‐iT Lipid Peroxidation Kit (Invitrogen, Eugene, USA) in accordance with the manufacturer’s protocol. The fluorescence density of lipid peroxidation products was detected using the BODIPY 581/591 reagent, a fluorescent sensor that shifts from red to green fluorescence upon oxidation. Images were taken using a fluorescence microscope (Leica DM 4000B).
2.7. Cell migration assay
The migration of the HK-2 cells was assessed using 24‑well Transwell plates with polycarbonate filter membranes (pore size: 8.0 µm). The cells were seeded at a density of 50,000 cells per well and incubated for 24 h. Subsequently, the cells were treated with PM2.5 under the appropriate conditions. After removal of nonmigratory cells, the cells on the lower surface of the membrane were fixed with methanol for 10 min and then stained with 0.5 % crystal violet for 30 min. The total count of stained cells in five randomly selected fields was determined.
2.8. Quantitative real-time polymerase chain reaction
Total RNA was extracted from the HK-2 cells by using TRIzol (Invitrogen) and was reverse-transcribed into complementary DNA by using the PrimeScript RT reagent kit in accordance with the manufacturer’s instructions. Next, the levels of target gene expression were measured through quantitative real-time polymerase chain reaction (PCR), which was performed using the SYBR Green Master Mix and the ABI PRISM 7900 system (Applied Biosystems, Foster City, CA, USA). The mRNA levels of the target genes were normalized to the mRNA level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
The sequences of the primers used in the PCR were as follows.
GAPDH: 5′-ACGGGAAGCTCACTGGCATGG-3′ (forward) and 5′-GGTCCACCACCCTGTTGCTGTA-3′ (reverse).
PPARγ: 5′-TCGCTGATGCACTGCCTATG-3′ (forward) and 5′-GAGAGGTCCACAGAGCTGATT-3′ (reverse).
E-cadherin: 5′-TGCCCAGAAAATGAAAAAGG-3′ (forward) and 5′-GTGTATGTGGCAATGCGTTC-3′ (reverse).
α-smooth muscle actin (α-SMA): 5′-CTATGCCTCTGGACGCACAACT-3′ (forward) and 5′-CAGATCCAGACGCATGATGGCA-3′ (reverse).
2.9. Western blotting
Protein extracts from the HK-2 cells were separated on a 10 % sodium dodecyl sulfate polyacrylamide gel. The resultant bands were transferred onto a polyvinylidene fluoride membrane. The membrane was incubated with primary antibodies—rabbit polyclonal anti-PPARγ (Abcam), rabbit monoclonal anti-glutathione peroxidase 4 (GPX4) antibodies (Abcam), rabbit polyclonal anti-E-cadherin (Sigma), mouse monoclonal anti-α-SMA (Sigma), and anti-GAPDH (loading control). After washing, the membrane was incubated with corresponding enzyme-linked secondary antibodies. Immunoreactivity was detected using an enhanced chemiluminescence detection system.
2.10. Transfection
A pcDNA3.1-PPARγ plasmid (Thermo Fisher, Waltham, MA, USA) was used for the overexpression of PPARγ. PPARγ was amplified through PCR and inserted into the expression vector, which was transfected into E. coli DH5a cells by using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions. Then, the cells were streaked onto Luria–Bertini agar plates containing 100 mg/mL ampicillin and incubated overnight at 37 °C. A single colony from a plate was selected for PCR and validated through sequencing.
2.11. Statistical analysis
Data were normalized to the data obtained from control cells and are presented in terms of mean ± standard deviation values of at least three independent experiments. The experimental and control groups were compared using the one-way and two-way analysis of variance tests. A p value of < 0.05 indicated significance.
3. Results
3.1. PM2.5 exposure inhibits expression of PPARγ in HK-2 cells
The effect of PM2.5 on the expression of PPARγ in HK-2 cells was evaluated through quantitative PCR and Western blotting. After 24 h of PM2.5 (50 µg/mL) exposure, a significant reduction was noted in the mRNA level of PPARγ compared with the level in control cells (Fig. 1A). Such a reduction was also observed in the protein level of PPARγ and occurred in a dose-dependent manner (25, 50, and 100 µg/mL; Fig. 1B, C). These findings strongly suggest that PM2.5 downregulates the expression of PPARγ in HK-2 cells.
Fig. 1.
PPARγ expression in HK-2 cells exposed to different concentrations of PM2.5. The mRNA and protein levels of PPARγ were measured through quantitative real-time polymerase chain reaction and Western blotting. (A) Level of PPARγ expression in PM2.5-exposed HK-2 cells. (B) Immunoblots depicting PPARγ levels after PM2.5 exposure. (C) Densitometric data on the protein level of PPARγ. Data are presented in terms of mean ± standard deviation values. *p < 0.05 and **p < 0.01, compared with the respective controls.
3.2. PPARγ agonist and antagonist modulate the effects of PM2.5 on HK-2 cells
PM2.5-induced changes in the phenotype of HK-2 cells toward EMT were blocked by pretreatment with 15d-PGJ2 but enhanced by that with GW9662 (Fig. 2A). PM2.5 increased the level of HK-2 cell migration; this change was prevented by pretreatment with 15d-PGJ2 and enhanced by that with GW9662 (Fig. 2B). As presented in Fig. 2C, 15d-PGJ2 significantly blocked PM2.5-induced downregulation of PPARγ expression, whereas GW9662 partially exacerbated PM2.5-induced downregulation of PPARγ expression. In addition, the PM2.5-induced increase in the mRNA level of α-SMA and reduction in the mRNA level of E-cadherin were blocked by pretreatment with 15d-PGJ2 but enhanced by that with GW9662. Therefore, aberrant PPARγ expression may contribute to this pathological process.
Fig. 2.
Pretreatments with 15d-PGJ2 and GW9662 altered the EMT-related changes in PM2.5-exposed HK-2 cells. HK-2 cells were treated with 15d-PGJ2 or GW9662 24 h before PM2.5 exposure. (A) Cell morphology observed under a microscope (magnification, 100 × ). (B) Cell migration was assayed using the Transwell migration assay. (C) PM2.5-induced changes in the mRNA levels of EMT-related markers were mitigated or exacerbated by 15d-PGJ2 and GW9662, respectively. Data are presented in terms of mean ± standard deviation values. *p < 0.05, compared with the control group; #p < 0.05, compared with the PM2.5-exposed group.
3.3. PPARγ regulates ferroptosis susceptibility of PM2.5-exposed HK-2 cells
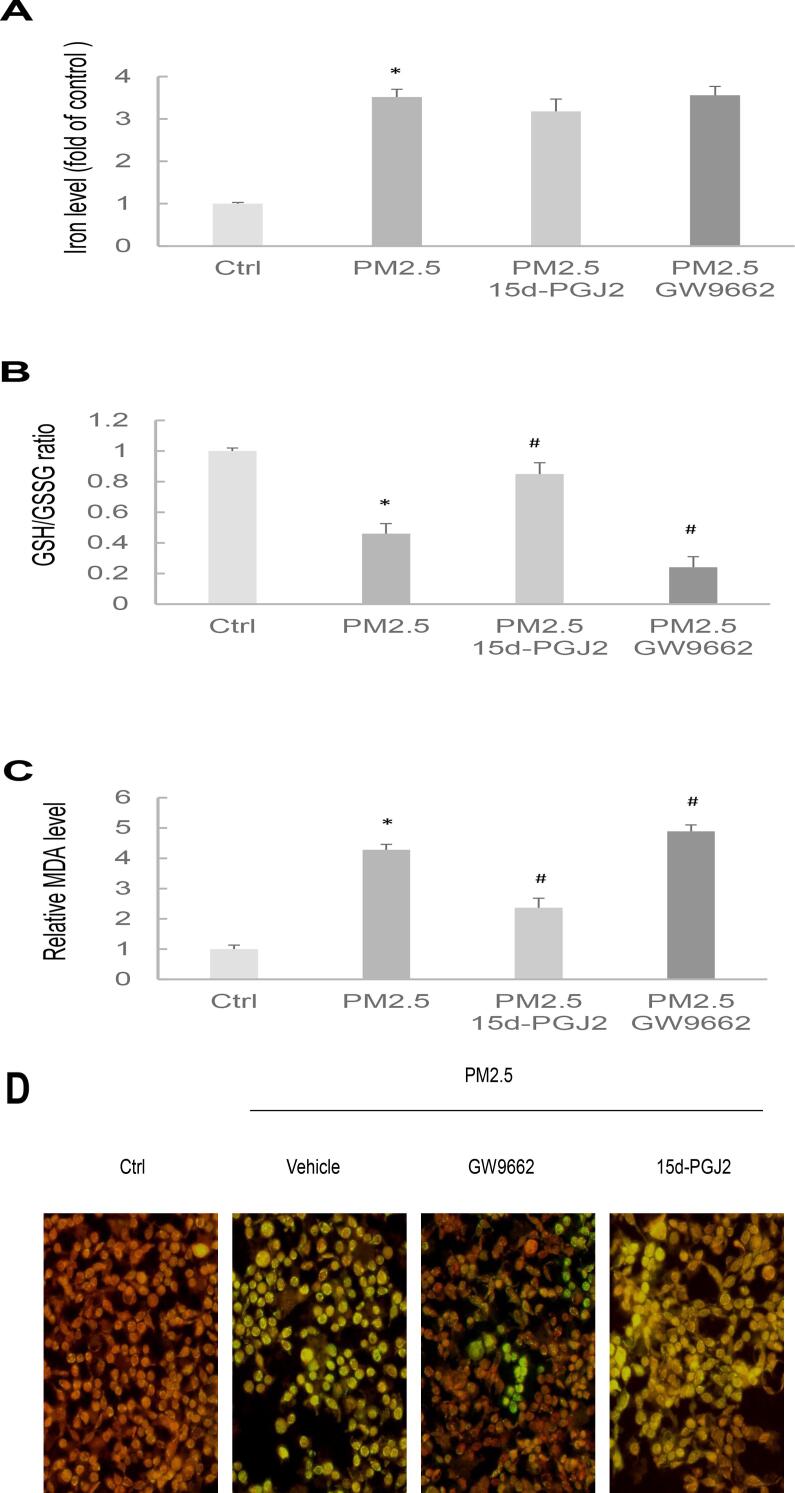
Ferroptotic stress is pivotal for various physiological and pathological responses, and its association with PM2.5-induced cellular toxicity has been noted. We investigated the potential role of PPARγ in regulating PM2.5-induced ferroptotic stress. This was examined by assessing iron levels, the ratio of GSH to GSSG, MDA levels, and the fluorescence of lipid peroxidation in cells subjected to 15d-PGJ2 or GW9662 pretreatment followed by PM2.5 exposure. PM2.5 markedly increased the iron production in the HK-2 cells, and pretreatment with 15d-PGJ2 or GW9662 did not significantly change the iron status (Fig. 3A). Furthermore, PM2.5 reduced the GSH/GSSG ratio and increased the MDA level in the HK-2 cells; these effects were blocked by pretreatment with 15d-PGJ2 and exacerbated and enhanced, respectively, by pretreatment with GW9662 (Fig. 3B,C). In addition, PM2.5 exposure increased the level of lipid ROS; this effect was blocked by pretreatment with 15d-PGJ2 and exacerbated by pretreatment with GW9662 (Fig. 3D). These findings indicate that induction of ferroptotic stress is associated with modulation of PPARγ expression in PM2.5-exposed HK-2 cells.
Fig. 3.
Effects of 15d-PGJ2 and GW966 on the ferroptosis susceptibility of PM2.5-exposed HK-2 cells. HK-2 cells were treated with 15d-PGJ2 or GW9662 24 h before PM2.5 exposure. (A) Iron contents. (B) Reduced glutathione level. (C) MDA level. (D) The detection of lipid peroxidation in live cells.
3.4. Alleviation of ferroptotic stress blocks PM2.5-induced EMT and downregulation of PPARγ expression in HK-2 cells
To elucidate the associations between ferroptotic stress, PPARγ activity, and EMT, HK-2 cells were treated with the ferroptosis inhibitor fer-1 4 h before being exposed to PM2.5. As indicated in Fig. 4A, fer-1 significantly mitigated PM2.5-induced downregulation of PPARγ expression as well as concurrent upregulation and downregulation of α-SMA and E-cadherin expression, respectively. Moreover, fer-1 effectively prevented PM2.5-induced activation of EMT (Fig. 4B). These results were corroborated by those of the cell migration assay, confirming that fer-1 reduces PM2.5-induced migration of HK-2 cells (Fig. 4C). Thus, the inhibitory effect of fer-1 on ferroptotic stress effectively mitigates PM2.5-induced downregulation of PPARγ and alteration of the cellular phenotype in HK-2 cells.
Fig. 4.
Fer-1 mitigates downregulation of PPARγ expression and alteration of the cellular phenotype in PM2.5-exposed HK-2 cells. HK-2 cells were treated with fer-1 (10 μg/mL) 4 h before PM2.5 exposure. (A) Expression levels of PPARγ, α-SMA, and E-cadherin. (B) Morphology of control and experimental cells. (C) Cell migration assessed through Transwell migration assay. Data are presented in terms of mean ± standard deviation values. *p < 0.05, compared with the control group; #p < 0.05, compared with the PM2.5-exposed group.
3.5. PPARγ overexpression restores GPX4 expression and suppresses EMT in PM2.5-exposed HK-2 cells
To understand the role of PPARγ in PM2.5-induced alterations in the epithelial cell phenotype, we designed a pcDNA3.1-PPARγ plasmid that selectively expresses PPARγ. HK-2 cells were transfected with either the pcDNA3.1-PPARγ plasmid or an empty vector. The expression of PPARγ was evaluated 48 h after transfection. Real-time PCR revealed an approximately 3.3-fold increase in the mRNA level of PPARγ in the cells transfected with the pcDNA3.1-PPARγ plasmid compared with the level in those transfected with the empty vector (Fig. 5A). Additionally, Western blotting revealed a significant increase in the protein level of PPARγ in the cells transfected with the pcDNA3.1-PPARγ plasmid (Fig. 5B,C), indicating successful overexpression. Next, we measured the protein levels of E-cadherin, α-SMA, and GPX4 in PM2.5-exposed cells with or without PPARγ overexpression (Fig. 5D,E). A substantial increase and reduction were noted in the levels of E-cadherin and α-SMA, respectively, in the cells transfected with the pcDNA3.1-PPARγ plasmid compared with the levels in those transfected with the empty vector. Notably, PPARγ overexpression blocked PM2.5-induced downregulation of GPX4 expression in the HK-2 cells, perhaps by alleviating PM-2.5-induced ferroptotic stress. These results indicate that PPARγ confers protection against PM2.5-induced EMT and ferroptotic stress in tubular cells. Thus, PPARγ activation may emerge as a potential strategy for alleviating PM2.5-induced tubular injury.
Fig. 5.
PPARγ overexpression blocked the PM2.5-induced changes in EMT and GPX4 expression in HK-2 cells. (A) Expression level of PPARγ was estimated as the ratio of the control. Data are presented in terms of mean ± standard deviation values. **p < 0.01, compared with the vector group. (B) Levels of PPARγ in control cells, cells transfected with an empty vector, and cells transfected with the pcDNA3.1-PPARγ plasmid. (C) Densitometric ratios of PPARγ were normalized. **p < 0.01, compared with the vector group. (D) Levels of E-cadherin, α-SMA, and GPX4 in cells transfected with the empty vector or the pcDNA3.1-PPARγ plasmid and then exposed to PM2.5. (E) Densitometric ratios of E-cadherin, α-SMA, or GPX4 were normalized. Data are presented in terms of mean ± standard deviation values. *p < 0.05, compared with the control group; #p < 0.05, compared with the PM2.5-exposed group.
4. Discussion
Ambient air pollution is a major risk factor for poor health worldwide. PM2.5 negatively affects human health by causing various diseases (Liao et al., 2023, Thangavel et al., 2022), and exposure to PM2.5 increases the risks of renal disease and CKD progression (Chang et al., 2022, Politis et al., 2024, Wathanavasin et al., 2024). PM2.5 exposure enhances inflammation and oxidative stress, thereby reducing cells’ antioxidant capacity and increasing lipid peroxidation; PM2.5 exposure also influences some signaling pathways related to EMT and organ fibrosis (Fu et al., 2019, Lee et al., 2020, Lin et al., 2021).
In the present study, PM2.5 exposure reduced the mRNA and protein levels of PPARγ and altered the cellular phenotype toward EMT in HK-2 cells. The trend toward EMT was associated with PM2.5-induced downregulation of PPARγ expression. Exposure to PM2.5 results in aberrant PPARγ activity, which increases the risks of lung and hepatic fibroses (Jeong et al., 2021, Zheng et al., 2015). We found that PM2.5 exposure inhibited PPARγ expression, causing tubular injury and inducing ferroptotic stress, as evidenced by the elevated iron content, MDA level, and lipid ROS production and the reduced GSH/GSSG ratio. The alterations in PPARγ expression by its agonist and antagonist might explain the different susceptibility levels of HK-2 cells to PM2.5-induced ferroptosis. EMT-related changes were observed in PM2.5-exposed cells: the cells lost cell–cell adhesion ability and exhibited enhanced motility; in addition, the expression of α-SMA was upregulated, whereas that of E-cadherin was downregulated. However, pretreatment with 15d-PGJ2 or GW9662 suppressed or promoted EMT in these cells. Fer-1 can prevent downregulation of PPARγ, block changes in α-SMA and E-cadherin levels, and suppress phenotypic switching in PM2.5-exposed cells. These findings highlight the associations among ferroptotic stress, aberrant PPARγ activity, and EMT in PM2.5-exposed cells.
Ferroptotic stress, which plays a crucial role in pathological responses, has been implicated in PM2.5-induced cellular toxicity (Gu et al., 2023, Gu et al., 2022, Yan et al., 2022). We found that expression PPARγ was negatively correlated with that of ferroptosis markers in PM2.5-exposed cells. The activation of PPARγ reduced the cells’ ferroptotic susceptibility and thus suppressed EMT. Furthermore, overexpression of PPARγ prevented inhibition of GPX4 expression and blocked alterations in E-cadherin and α-SMA expression in PM2.5-exposed cells. PPARγ exerts anti-inflammatory effects and can indirectly alleviate oxidative stress and ROS production (Anderson et al., 2014, Croasdell et al., 2015, Fan et al., 2022). We found that PPARγ upregulates expression of GPX4, a key antioxidant enzyme essential for protecting cells from the negative effects of lipid peroxidation and ferroptosis. These findings support the notion that the upregulation of PPARγ expression could potentially mitigate PM2.5-induced tubular injury by suppressing ferroptosis and EMT.
5. Conclusion
This study revealed that PM2.5-induced impairment of PPARγ activity results in ferroptotic stress and EMT in tubular cells. PPARγ activation holds potential as a preventive strategy for PM2.5-induced renal injury.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
Funding
National Science and Technology Council - Grant numbers: 107-2314-B-075-036, 108-2314-B-075-043-MY2, 110-2314-B-075-017, 111-2314-B-075-046-MY2, 111-2314-B-075-048, 113-2314-B-075-070. Taipei Veterans General Hospital - Grant numbers: V111C-177, V112C-177, V113C-062. Yen Tjing Ling Medical Foundation Grant numbers: CI-111- 21, CI-113- 24. Taipei Institute of Pathology - Grant numbers: TIP-112-004, TIP-113-002.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Contributor Information
Chien-Hung Lin, Email: chlin5@vghtpe.gov.tw.
Hsin-Hui Wang, Email: hhwang@vghtpe.gov.tw.
Data availability
Data will be made available on request.
References
- Anderson E.J., Thayne K.A., Harris M., Shaikh S.R., Darden T.M., Lark D.S., Williams J.M., Chitwood W.R., Kypson A.P., Rodriguez E. Do fish oil omega-3 fatty acids enhance antioxidant capacity and mitochondrial fatty acid oxidation in human atrial myocardium via PPARgamma activation? Antioxid. Redox Signal. 2014;21:1156–1163. doi: 10.1089/ars.2014.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boogaard H., Walker K., Cohen A.J. Air pollution: the emergence of a major global health risk factor. Int. Health. 2019;11:417–421. doi: 10.1093/inthealth/ihz078. [DOI] [PubMed] [Google Scholar]
- Bowe B., Artimovich E., Xie Y., Yan Y., Cai M., Al-Aly Z. The global and national burden of chronic kidney disease attributable to ambient fine particulate matter air pollution: a modelling study. BMJ Glob. Health. 2020;5:e002063. doi: 10.1136/bmjgh-2019-002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P.Y., Li Y.L., Chuang T.W., Chen S.Y., Lin L.Y., Lin Y.F., Chiou H.Y. Exposure to ambient air pollutants with kidney function decline in chronic kidney disease patients. Environ. Res. 2022;215 doi: 10.1016/j.envres.2022.114289. [DOI] [PubMed] [Google Scholar]
- Croasdell A., Duffney P.F., Kim N., Lacy S.H., Sime P.J., Phipps R.P. PPARgamma and the Innate Immune System Mediate the Resolution of Inflammation. PPAR Res. 2015;2015 doi: 10.1155/2015/549691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromar K.R., Gladson L.A., Hicks E.A., Marsh B., Ewart G. Excess Morbidity and Mortality Associated with Air Pollution above American Thoracic Society Recommended Standards, 2017–2019. Ann. Am. Thorac. Soc. 2022;19:603–613. doi: 10.1513/AnnalsATS.202107-860OC. [DOI] [PubMed] [Google Scholar]
- Fan X., Xu M., Ren Q., Fan Y., Liu B., Chen J., Wang Z., Sun X. Downregulation of fatty acid binding protein 4 alleviates lipid peroxidation and oxidative stress in diabetic retinopathy by regulating peroxisome proliferator-activated receptor gamma-mediated ferroptosis. Bioengineered. 2022;13:10540–10551. doi: 10.1080/21655979.2022.2062533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q., Yu X., Qiao Y., Pan S., Wang R., Zheng B., Wang H., Ren K.D., Liu H., Yang Y. Ferroptosis and Acute Kidney Injury (AKI): Molecular Mechanisms and Therapeutic Potentials. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.858676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Lu R., Cui J., Sun H., Yang H., Meng Q., Wu S., Aschner M., Li X., Chen R. Inhibition of ATP citrate lyase (ACLY) protects airway epithelia from PM2.5-induced epithelial-mesenchymal transition. Ecotoxicol. Environ. Saf. 2019;167:309–316. doi: 10.1016/j.ecoenv.2018.10.033. [DOI] [PubMed] [Google Scholar]
- Giuliani K.T.K., Grivei A., Nag P., Wang X., Rist M., Kildey K., Law B., Ng M.S., Wilkinson R., Ungerer J., Forbes J.M., Healy H., Kassianos A.J. Hypoxic human proximal tubular epithelial cells undergo ferroptosis and elicit an NLRP3 inflammasome response in CD1c(+) dendritic cells. Cell Death Dis. 2022;13:739. doi: 10.1038/s41419-022-05191-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Hao S., Liu K., Gao M., Lu B., Sheng F., Zhang L., Xu Y., Wu D., Han Y., Chen S., Zhao W., Lou X., Wang X., Li P., Chen Z., Yao K., Fu Q. Airborne fine particulate matter (PM2.5) damages the inner blood-retinal barrier by inducing inflammation and ferroptosis in retinal vascular endothelial cells. Sci. Total Environ. 2022;838 doi: 10.1016/j.scitotenv.2022.156563. [DOI] [PubMed] [Google Scholar]
- Gu W., Hou T., Zhou H., Zhu L., Zhu W., Wang Y. Ferroptosis is involved in PM2.5-induced acute nasal epithelial injury via AMPK-mediated autophagy. Int. Immunopharmacol. 2023;115 doi: 10.1016/j.intimp.2022.109658. [DOI] [PubMed] [Google Scholar]
- Ide S., Kobayashi Y., Ide K., Strausser S.A., Abe K., Herbek S., O'Brien L.L., Crowley S.D., Barisoni L., Tata A., Tata P.R., Souma T. Ferroptotic stress promotes the accumulation of pro-inflammatory proximal tubular cells in maladaptive renal repair. Elife. 2021;10 doi: 10.7554/eLife.68603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J., Bae S.Y., Choi J. Identification of toxicity pathway of diesel particulate matter using AOP of PPARgamma inactivation leading to pulmonary fibrosis. Environ. Int. 2021;147 doi: 10.1016/j.envint.2020.106339. [DOI] [PubMed] [Google Scholar]
- Kokeny G., Calvier L., Hansmann G. PPARgamma and TGFbeta-Major Regulators of Metabolism, Inflammation, and Fibrosis in the Lungs and Kidneys. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms221910431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Hwang-Bo H., Ji S.Y., Kim M.Y., Kim S.Y., Park C., Hong S.H., Kim G.Y., Song K.S., Hyun J.W., Choi Y.H. Diesel particulate matter2.5 promotes epithelial-mesenchymal transition of human retinal pigment epithelial cells via generation of reactive oxygen species. Environ. Pollut. 2020;262 doi: 10.1016/j.envpol.2020.114301. [DOI] [PubMed] [Google Scholar]
- Li G., Huang J., Wang J., Zhao M., Liu Y., Guo X., Wu S., Zhang L. Long-Term Exposure to Ambient PM(2.5) and Increased Risk of CKD Prevalence in China. J Am Soc Nephrol. 2021;32:448–458. doi: 10.1681/ASN.2020040517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Wang W. Causal effects of exposure to ambient air pollution on cancer risk: Insights from genetic evidence. Sci. Total Environ. 2023;912 doi: 10.1016/j.scitotenv.2023.168843. [DOI] [PubMed] [Google Scholar]
- Liao Q., Li Z., Li Y., Dai X., Kang N., Niu Y., Tao Y. Specific analysis of PM(2.5)-attributed disease burden in typical areas of Northwest China. Front. Public Health. 2023;11:1338305. doi: 10.3389/fpubh.2023.1338305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.H., Wan C., Liu W.S., Wang H.H. PM2.5 Induces Early Epithelial Mesenchymal Transition in Human Proximal Tubular Epithelial Cells through Activation of IL-6/STAT3 Pathway. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222312734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zhao N., Shi G., Wang H. Geniposide ameliorated sepsis-induced acute kidney injury by activating PPARgamma. Aging (Albany NY) 2020;12:22744–22758. doi: 10.18632/aging.103902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquigussa E., Paterno J.C., de Oliveira Pokorny G.H., da Silva Perez M., Varela V.A., da Silva Novaes A., Schor N., Boim M.A. Klotho and PPAR Gamma Activation Mediate the Renoprotective Effect of Losartan in the 5/6 Nephrectomy Model. Front. Physiol. 2018;9:1033. doi: 10.3389/fphys.2018.01033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez-Gutierrez M.P., Roszer T., Ricote M. Biology and therapeutic applications of peroxisome proliferator- activated receptors. Curr. Top. Med. Chem. 2012;12:548–584. doi: 10.2174/156802612799436669. [DOI] [PubMed] [Google Scholar]
- Politis M.D., Gutierrez-Avila I., Just A., Pizano-Zarate M.L., Tamayo-Ortiz M., Greenberg J.H., Tellez-Rojo M.M., Sanders A.P., Rosa M.J. Recent ambient temperature and fine particulate matter (PM(2.5)) exposure is associated with urinary kidney injury biomarkers in children. Sci. Total Environ. 2024;907 doi: 10.1016/j.scitotenv.2023.168119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X., Tian J., Li B., Zhou L., Kang H., Pei Z., Zhang M., Li C., Wu M., Wang Q., Han B., Chu C., Pang Y., Ning J., Zhang B., Niu Y., Zhang R. Ambient PM2.5 caused cardiac dysfunction through FoxO1-targeted cardiac hypertrophy and macrophage-activated fibrosis in mice. Chemosphere. 2020;247 doi: 10.1016/j.chemosphere.2020.125881. [DOI] [PubMed] [Google Scholar]
- Thangavel P., Park D., Lee Y.C. Recent Insights into Particulate Matter (PM(2.5))-Mediated Toxicity in Humans: An Overview. Int. J. Environ. Res. Public Health. 2022;19 doi: 10.3390/ijerph19127511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Chen T., Chang Q., Kao Y.W., Li J., Chen M., Li Y., Shia B.C. Respiratory diseases are positively associated with PM2.5 concentrations in different areas of Taiwan. PLoS One. 2021;16:e0249694. doi: 10.1371/journal.pone.0249694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhong Y., Liao J., Wang G. PM2.5-related cell death patterns. Int. J. Med. Sci. 2021;18:1024–1029. doi: 10.7150/ijms.46421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathanavasin W., Banjongjit A., Phannajit J., Eiam-Ong S., Susantitaphong P. Association of fine particulate matter (PM(2.5)) exposure and chronic kidney disease outcomes: a systematic review and meta-analysis. Sci. Rep. 2024;14:1048. doi: 10.1038/s41598-024-51554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen F., Xie Y., Li B., Li P., Qi H., Zhang F., Sun Y., Zhang L. Combined effects of ambient air pollution and PM(2.5) components on renal function and the potential mediation effects of metabolic risk factors in China. Ecotoxicol. Environ. Saf. 2023;259 doi: 10.1016/j.ecoenv.2023.115039. [DOI] [PubMed] [Google Scholar]
- Xu C., Zhang Q., Huang G., Huang J., Zhang H. The impact of PM2.5 on kidney. J. Appl. Toxicol. 2023;43:107–121. doi: 10.1002/jat.4356. [DOI] [PubMed] [Google Scholar]
- Yan K., Hou T., Zhu L., Ci X., Peng L. PM2.5 inhibits system Xc- activity to induce ferroptosis by activating the AMPK-Beclin1 pathway in acute lung injury. Ecotoxicol. Environ. Saf. 2022;245 doi: 10.1016/j.ecoenv.2022.114083. [DOI] [PubMed] [Google Scholar]
- Zhang X., Tao J., Lei F., Sun T., Lin L., Huang X., Zhang P., Ji Y.X., Cai J., Zhang X.J., Li H. Association of the components of ambient fine particulate matter (PM(2.5)) and chronic kidney disease prevalence in China. J. Environ. Manage. 2023;339 doi: 10.1016/j.jenvman.2023.117885. [DOI] [PubMed] [Google Scholar]
- Zheng Z., Zhang X., Wang J., Dandekar A., Kim H., Qiu Y., Xu X., Cui Y., Wang A., Chen L.C., Rajagopalan S., Sun Q., Zhang K. Exposure to fine airborne particulate matters induces hepatic fibrosis in murine models. J. Hepatol. 2015;63:1397–1404. doi: 10.1016/j.jhep.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Xue X., Hou Q., Dai C. Targeting ferroptosis attenuates interstitial inflammation and kidney fibrosis. Kidney Dis (basel). 2022;8:57–71. doi: 10.1159/000517723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.