Abstract
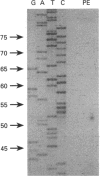
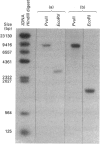
Towards the goal of gaining a better understanding of the molecular mechanisms controlling branched-chain-amino-acid biosynthesis in plants, we have isolated, sequenced and characterized a gene encoding acetohydroxy acid isomero-reductase (ketol-acid reductoisomerase) from Arabidopsis thaliana (thale cress). Comparison between the acetohydroxy acid isomeroreductase cDNA and the genomic sequence has allowed us to determine the exon structure of the coding region. The isolated acetohydroxy acid isomeroreductase gene is distributed over approx. 4.5 kbp and contains nine introns (79-347 bp). The transcriptional start site was found to be 52 bp upstream of the translational initiation site. Southern-blot analysis of A. thaliana genomic DNA shows that the acetohydroxy acid isomeroreductase is encoded by a single-copy gene.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar O. M., Grasso D. H. The product of the Rhizobium meliloti ilvC gene is required for isoleucine and valine synthesis and nodulation of alfalfa. J Bacteriol. 1991 Dec;173(24):7756–7764. doi: 10.1128/jb.173.24.7756-7764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfin S. M., Umbarger H. E. Purification and properties of the acetohydroxy acid isomeroreductase of Salmonella typhimurium. J Biol Chem. 1969 Mar 10;244(5):1118–1127. [PubMed] [Google Scholar]
- Aulabaugh A., Schloss J. V. Oxalyl hydroxamates as reaction-intermediate analogues for ketol-acid reductoisomerase. Biochemistry. 1990 Mar 20;29(11):2824–2830. doi: 10.1021/bi00463a027. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazey D. L., Burns R. O. Regulation of Salmonella typhimurium ilvYC genes. J Bacteriol. 1984 Sep;159(3):951–957. doi: 10.1128/jb.159.3.951-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Cavener D. R., Ray S. C. Eukaryotic start and stop translation sites. Nucleic Acids Res. 1991 Jun 25;19(12):3185–3192. doi: 10.1093/nar/19.12.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chunduru S. K., Mrachko G. T., Calvo K. C. Mechanism of ketol acid reductoisomerase--steady-state analysis and metal ion requirement. Biochemistry. 1989 Jan 24;28(2):486–493. doi: 10.1021/bi00428a012. [DOI] [PubMed] [Google Scholar]
- Curien G., Dumas R., Douce R. Nucleotide sequence and characterization of a cDNA encoding the acetohydroxy acid isomeroreductase from Arabidopsis thaliana. Plant Mol Biol. 1993 Feb;21(4):717–722. doi: 10.1007/BF00014556. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas R., Job D., Ortholand J. Y., Emeric G., Greiner A., Douce R. Isolation and kinetic properties of acetohydroxy acid isomeroreductase from spinach (Spinacia oleracea) chloroplasts overexpressed in Escherichia coli. Biochem J. 1992 Dec 15;288(Pt 3):865–874. doi: 10.1042/bj2880865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas R., Joyard J., Douce R. Purification and characterization of acetohydroxyacid reductoisomerase from spinach chloroplasts. Biochem J. 1989 Sep 15;262(3):971–976. doi: 10.1042/bj2620971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas R., Lebrun M., Douce R. Isolation, characterization and sequence analysis of a full-length cDNA clone encoding acetohydroxy acid reductoisomerase from spinach chloroplasts. Biochem J. 1991 Jul 15;277(Pt 2):469–475. doi: 10.1042/bj2770469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friden P., Schimmel P. LEU3 of Saccharomyces cerevisiae activates multiple genes for branched-chain amino acid biosynthesis by binding to a common decanucleotide core sequence. Mol Cell Biol. 1988 Jul;8(7):2690–2697. doi: 10.1128/mcb.8.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godon J. J., Chopin M. C., Ehrlich S. D. Branched-chain amino acid biosynthesis genes in Lactococcus lactis subsp. lactis. J Bacteriol. 1992 Oct;174(20):6580–6589. doi: 10.1128/jb.174.20.6580-6589.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofler J. G., Decedue C. J., Luginbuhl G. H., Reynolds J. A., Burns R. O. The subunit structure of alpha-acetohydroxyacid isomeroreductase from Salmonella typhimurium. J Biol Chem. 1975 Feb 10;250(3):877–882. [PubMed] [Google Scholar]
- KAKAR S. N., WAGNER R. P. GENETIC AND BIOCHEMICAL ANALYSIS OF ISOLEUCINE-VALINE MUTANTS OF YEAST. Genetics. 1964 Feb;49:213–222. doi: 10.1093/genetics/49.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Wek R. C., Lopes J. M., Pereira R., Taillon B. E., Hatfield G. W. The complete nucleotide sequence of the ilvGMEDA operon of Escherichia coli K-12. Nucleic Acids Res. 1987 Mar 11;15(5):2137–2155. doi: 10.1093/nar/15.5.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey C. J., Warburg R. J., Halvorson H. O., Zahler S. A. Genetic and physical analysis of the ilvBC-leu region in Bacillus subtilis. Gene. 1984 Dec;32(1-2):49–56. doi: 10.1016/0378-1119(84)90031-3. [DOI] [PubMed] [Google Scholar]
- Pattee P. A. Genetic linkage of chromosomal tetracycline resistance and pigmentation to a purine auxotrophic marker and the isoleucine-valine-leucine structural genes in Staphylococcus aureus. J Bacteriol. 1976 Sep;127(3):1167–1172. doi: 10.1128/jb.127.3.1167-1172.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J. G., Holmberg S. The ILV5 gene of Saccharomyces cerevisiae is highly expressed. Nucleic Acids Res. 1986 Dec 22;14(24):9631–9651. doi: 10.1093/nar/14.24.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieble S., Beale S. I. Structure and expression of a cyanobacterial ilvC gene encoding acetohydroxyacid isomeroreductase. J Bacteriol. 1992 Dec;174(24):7910–7918. doi: 10.1128/jb.174.24.7910-7918.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz A., Spönemann P., Köcher H., Wengenmayer F. The herbicidally active experimental compound Hoe 704 is a potent inhibitor of the enzyme acetolactate reductoisomerase. FEBS Lett. 1988 Oct 10;238(2):375–378. doi: 10.1016/0014-5793(88)80515-5. [DOI] [PubMed] [Google Scholar]
- Shematek E. M., Arfin S. M., Diven W. F. A kinetic study of -acetohydroxy acid isomeroreductase from Salmonella typhimurium. Arch Biochem Biophys. 1973 Sep;158(1):132–138. doi: 10.1016/0003-9861(73)90605-x. [DOI] [PubMed] [Google Scholar]
- Sista H., Bowman B. Characterization of the ilv-2 gene from Neurospora crassa encoding alpha-keto-beta-hydroxylacyl reductoisomerase. Gene. 1992 Oct 12;120(1):115–118. doi: 10.1016/0378-1119(92)90018-k. [DOI] [PubMed] [Google Scholar]
- Somers J. M., Amzallag A., Middleton R. B. Genetic fine structure of the leucine operon of Escherichia coli K-12. J Bacteriol. 1973 Mar;113(3):1268–1272. doi: 10.1128/jb.113.3.1268-1272.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Devereux J., Calvo J. M. Molecular structure of ilvIH and its evolutionary relationship to ilvG in Escherichia coli K12. Nucleic Acids Res. 1983 Aug 11;11(15):5299–5313. doi: 10.1093/nar/11.15.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze J. Y., Woontner M., Jaehning J. A., Kohlhaw G. B. In vitro transcriptional activation by a metabolic intermediate: activation by Leu3 depends on alpha-isopropylmalate. Science. 1992 Nov 13;258(5085):1143–1145. doi: 10.1126/science.1439822. [DOI] [PubMed] [Google Scholar]
- WAGNER R. P., BERGQUIST A., BARBEE T., KIRITANI K. GENETIC BLOCKS IN THE ISOLEUCINE-VALINE PATHWAY OF NEUROSPORA CRASSA. Genetics. 1964 May;49:865–882. doi: 10.1093/genetics/49.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek R. C., Hatfield G. W. Nucleotide sequence and in vivo expression of the ilvY and ilvC genes in Escherichia coli K12. Transcription from divergent overlapping promoters. J Biol Chem. 1986 Feb 15;261(5):2441–2450. [PubMed] [Google Scholar]
- Wek R. C., Hatfield G. W. Transcriptional activation at adjacent operators in the divergent-overlapping ilvY and ilvC promoters of Escherichia coli. J Mol Biol. 1988 Oct 5;203(3):643–663. doi: 10.1016/0022-2836(88)90199-4. [DOI] [PubMed] [Google Scholar]
- Wek R. C., Hauser C. A., Hatfield G. W. The nucleotide sequence of the ilvBN operon of Escherichia coli: sequence homologies of the acetohydroxy acid synthase isozymes. Nucleic Acids Res. 1985 Jun 11;13(11):3995–4010. doi: 10.1093/nar/13.11.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]