Abstract
Large-bore aspiration thrombectomy is emerging as a promising alternative for thrombus removal in acute pulmonary embolism (PE). In this article, we report a successful case with the newly approved AlphaVac F1885 device in a 76-year-old patient presenting with an intermediate- to high-risk acute PE. Preoperative imaging demonstrated bilateral PE with a modified miller index of 30 and an right ventricle-to-left ventricle ratio of 2.1. Mechanical thrombectomy was performed under local anesthesia with mild sedation. The pulmonary artery pressures decreased from 85/27 to 46/13 immediately after thrombectomy. The postoperative course was notable for marked symptom improvement and repeat imaging showed a 63% decrease in clot burden with a decrease in right ventricle-to-left ventricle ratio to 1.3.
Keywords: Pulmonary embolism, Mechanical thrombectomy, Large-bore aspiration thrombectomy, Catheter-directed therapies, Right heart strain, Right ventricle dysfunction
With an estimated annual incidence of 121 per 100,000 inhabitants and a 7% in-hospital mortality rate, acute pulmonary embolism (PE) is the third leading cause of cardiovascular death in the United States.1 The primary therapy is anticoagulation, with systemic thrombolysis being reserved for more severe forms. However, there is increasing evidence supporting catheter-directed therapies for intermediate-to-high risk PE.2,3 Among these is large-bore aspiration thrombectomy (LBAT), which has shown promising outcomes with reported in-hospital mortality being as low as 0.3% in some series.4 The Alpha Vac F1885 (Angiodynamics Inc, Latham, NY) is the latest LBAT device approved by the US Food and Drug Administration for acute PE indication.5 Although originally limited to venous and atrial indications,6 its safety and effectiveness in acute PE have been the focus of the recent APEX-AV trial (Acute Pulmonary Embolism Extraction Trial with the AlphaVac System), whose results are yet to be published.6,7
We herein present our successful experience with the Alpha Vac System (AVS) for the treatment of intermediate- to high-risk PE in a septuagenarian. The patient provided informed consent for publication.
Case report
Clinical evaluation and investigations
A 76-year-old woman with a history of diabetes mellitus, arterial hypertension, multiple myeloma with spinal osteolytic lesions, gastroesophageal reflux, gastrointestinal hemorrhage, and left tibial venous thrombosis presented to the emergency department with worsening shortness of breath over the past 2 weeks. She also reported generalized weakness and difficulty completing daily tasks, but denied chest pain, cough, or wheezing.
Upon admission, she was found to be morbidly obese with a body mass index of 43. Her vital signs were notable for a heart rate of 120 bpm, blood pressure of 153/111 mm Hg, respiratory rate of 18 cycles/min, and pulse oximetry of 94% on room air. Physical examination revealed increased work of breathing and difficulty completing sentences. Electrocardiogram showed sinus tachycardia with anterior T wave inversion, and chest x-ray revealed mild bibasilar atelectasis. Laboratory findings included elevated troponin, brain natriuretic peptide, and lactic acid at 18 ng/dL, 3938 pg/L, and 2.9 mmol/L respectively.
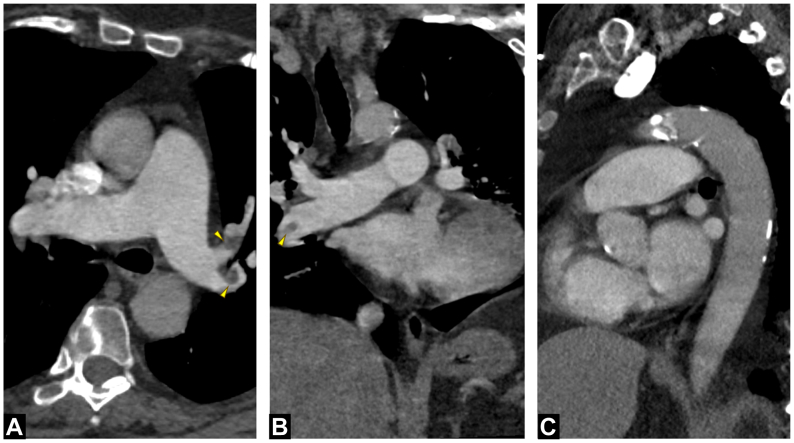
A chest computed tomographic angiography revealed bilateral pulmonary emboli (Fig 1) with severe right heart dysfunction (RHD) indicated by a right ventricle-to-left ventricle ratio of 2.1. The pulmonary artery diameter measured 40 mm, and the Modified Miller Index was 30/32 (94%), indicating a significant clot burden. Venous duplex ultrasound examination from the external iliac to the calf vessels was negative for deep venous thrombosis.
Fig 1.
Axial (A), coronal (B) and sagittal (C) views of preoperative computed tomographic angiography demonstrating bilateral pulmonary emboli with significant clot burden.
Preoperative planning
Intravenous heparin and oxygen therapy were initiated, and, owing to RHD, interventional thrombus removal options were discussed. Her concomitant multiple myeloma with spinal lesions was considered a relative contraindication for thrombolytic therapy owing to the risk of bleeding. The patient opted to undergo mechanical thrombectomy, but her severe RHD and resting tachycardia raised concerns for potential acute intraoperative decompensation. A cardiac surgery consult was therefore obtained to have extracorporeal membrane oxygenation (ECMO) on standby in the operating room for timely intervention in case of acute deterioration.
Endovascular access
The intervention was performed under local anesthesia with mild sedation. A left femoral arterial line was placed for blood pressure monitoring and to provide readily available access in case of ECMO cannulation. The right femoral vein was accessed under ultrasound guidance, 6F sheath placed and a 6F angled pigtail catheter and 0.035 J-Wire were used to select the main pulmonary artery. Pulmonary artery (PA) pressures were obtained, measuring 85/27 mm Hg. Given the extremely high PA systolic pressure, we paused to ensure all ECMO components and personnel were available before proceeding. Although our PE treatment algorithm would typically involve catheter-directed thrombolysis (CDT) for severe pulmonary hypertension (PA systolic pressure >70), we considered this procedure contraindicated owing to the spinal lesions and proceeded with LBAT using the AVS.
Device description
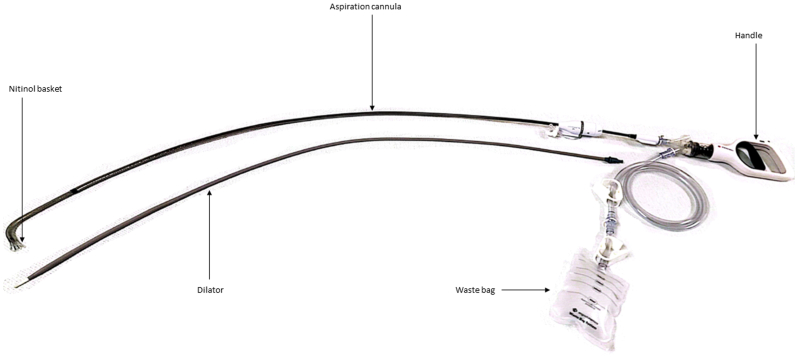
The AVS comprises a flexible 18F × 105-cm cannula with a self-expandable, nitinol reinforced angled (∼85°) funnel-shaped basket at its distal tip (Fig 2).8 The cannula is attached to a handle that serves as the vacuum source for aspiration and is equipped with a volume limiter switch to control the rate of suction. The device comes with a 22F sheath and a waste bag for collecting aspirate.
Fig 2.
Illustration of the AlphaVac F1885 Multipurpose Mechanical Aspiration device.
Mechanical thrombectomy
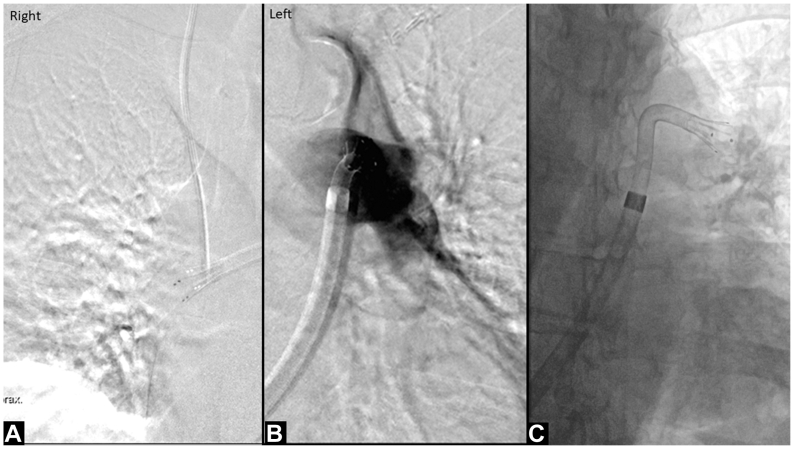
We upsized our femoral sheath to a 22F Gore Dryseal sheath (Gore Medical, Newark, DE) over an Amplatz wire. The AVS sheath was then positioned in the main PA, and the dilator and wire were withdrawn. Next, the handle and waste bag were connected to the cannula and primed. The aspiration cannula was then advanced to the tip of the sheath and angiography performed, revealing opacification defects (Fig 3). The nitinol basket was then advanced out of the sheath to engage the base of the thrombus, while pulling the sheath back. Suction thrombectomy was activated by pulling the handle back, and multiple passes were made until no more clots were aspirated. We proceeded in similar fashion in all the PA branches bilaterally, and retrieved clots of mixed ages successfully. Selection of bilateral pulmonary arteries with upper and lower branches was done without a wire, achievable because of the precurved shape of the catheter and soft funnel tip. A completion angiogram showed brisk flow into all branches (Fig 4) and repeat pressure measurements showed a decrease to 46/13 mm Hg. A haemonetics sq40 filter (Haemonetics, Braintree, MA) was used, off-label, to return aspirated blood to the patient throughout the procedure. The estimated net blood loss upon completion was 50 mL.
Fig 3.
Intraoperative images before thrombus removal. Angiography demonstrated significant opacification defects in the right (A) and left (B) pulmonary arteries. The suction catheter was advanced out of the sheath without wire access to engage the base of the thrombus with the nitinol basket (C).
Fig 4.
Intraoperative images demonstrating successful removal of clots of mixed ages (A). Completion angiogram demonstrated brisk flow of blood into both lungs (B and C).
Postoperative course
The postoperative course showed marked symptom improvement. Repeat computed tomographic angiography demonstrated a Modified Miller Index of 11, representing a 63% clot decrease (Fig 5), and a decrease in the right ventricle-to-left ventricle ratio to 1.3. The patient was referred to pulmonology for workup on a possible underlying chronic pulmonary hypertension. She was switched from heparin to apixaban (Eliquis) and discharged in stable condition on postoperative day 2. At the 30-day follow-up, she had improved remarkably and presented no shortness of breath.
Fig 5.
Axial (A), coronal (B), and sagittal (C) views of postoperative computerized tomographic angiography demonstrating significant reduction in clot burden.
Discussion
This paper demonstrates the use of a novel LBAT device to clear pulmonary emboli with a satisfactory outcome. The benefit of LBAT in acute PE hinges on the ability to provide immediate symptom relief without the need for thrombolytics, thus extending interventional therapy to patients with contraindications to thrombolytics such as in our patient, who had an active neoplasm with bleeding risk.
The Alpha Vac device presents certain distinguishing features, the most prominent of which is the nitinol basket. Although the size of the catheter is smaller than the 24F devices available from other manufacturers, the funnel tip expands to a diameter of 11 mm—the equivalent of 33F—which helps to engage a large amount of clot without the need to upsize the catheter.8 Previous studies have hypothesized that severe RHD may worsen with large catheters, potentially leading to acute intraoperative decompensation.9 The ability to clear a large amount of clot with this relatively smaller cannula may, therefore, be an advantage that warrants further investigation. Another advantageous feature of this device is that it does not require continued wire access. The wire is removed after the outer sheath is placed in the main PA and the aspiration catheter is advanced selectively without a wire.8 This is achievable because of the precurved shape of the aspiration catheter, which forms as it advances out of the sheath, and the soft funnel tip. Therefore, all procedural focus can be on thrombus aspiration, rather than maintaining distal wire access. Last, the volume control switch helps to limit blood loss and the attached waste bag helps to capture aspirates without disengaging the vacuum source, thus improving the workflow.6
A potential shortcoming of this device is difficult navigation into the right main PA because it is done without a wire. However, we have not had this problem in our limited experience. This technical difficulty may be mitigated potentially by selecting the right PA first to position the cannula and beginning suction thrombectomy from there. Another possible limitation is the inability to go into smaller branches owing to the size of the funnel. Further studies and more experience are required to ascertain if these are real issues.
Numerous observational data have demonstrated the effectiveness of LBAT in acute PE,4,10,11 but there remains a lack of sufficient level A evidence to guide practice. The decision to perform LBAT in this patient conforms with current guidelines that recommend consideration of catheter-directed therapies in hemodynamically stable patients with evidence of RHD.12 In the absence of any robust randomized head-to-head comparisons between LBAT, CDT, and systemic thrombolysis, our institutional preference is to perform LBAT as a first-line treatment for intermediate- to high-risk patients. Additionally, we have incorporated preemptive ECMO into the treatment algorithm of patients who are clearly at the edge of acute collapse. For cases like this one, where the degree of RHD and initial high pulmonary pressures raise concerns for a possible intraoperative decompensation, we consider conversion to CDT and coordinate with cardiac surgery to prepare ECMO on standby because of experience with cardiovascular collapse during LBAT in this fragile patient population.9
Conclusions
The 18F Alpha Vac Multipurpose aspiration system is now an option approved by the US Food and Drug Administration for large volume thrombus removal in intermediate- to high-risk acute PE. Further studies are warranted to establish its safety and efficacy, and to guide patient selection.
Disclosures
E.K.P. is a consultant for Voyager Medical, Venostent and Humacyte Inc. All fees are retained by Houston Methodist.
Footnotes
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Martin K.A., Molsberry R., Cuttica M.J., Desai K.R., Schimmel D.R., Khan S.S. Time trends in pulmonary embolism mortality rates in the United States, 1999 to 2018. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piazza G. Advanced management of intermediate- and high-risk pulmonary embolism: JACC focus Seminar. J Am Coll Cardiol. 2020;76:2117–2127. doi: 10.1016/j.jacc.2020.05.028. [DOI] [PubMed] [Google Scholar]
- 3.Carroll B.J., Larnard E.A., Pinto D.S., Giri J., Secemsky E.A. Percutaneous management of high-risk pulmonary embolism. Circ Cardiovasc Interv. 2023;16 doi: 10.1161/CIRCINTERVENTIONS.122.012166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toma C., Jaber W.A., Weinberg M.D., et al. Acute outcomes for the full US cohort of the FLASH mechanical thrombectomy registry in pulmonary embolism. EuroIntervention. 2023;18:1201–1212. doi: 10.4244/EIJ-D-22-00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Endovascular T AngioDynamics’ AlphaVac F18⁸⁵ system Receives FDA clearance for treatment of PE. EVT. Endovascular Today Web site; 2024. https://evtoday.com/news/angiodynamics-completes-enrollment-in-APEX-AV-acute-PE-trial
- 6.Ranade M., Moriarty J.M. Case report: use of the AlphaVac system for the removal of right atrial thrombus. Endovascular Today. 2022;21:77–79. [Google Scholar]
- 7.Angiodynamics I Evaluating the safety and efficacy of the AlphaVac Multipurpose mechanical aspiration (MMA) F1885 PE for treatment of acute pulmonary embolism (APEX-AV); 2022. https://www.clinicaltrials.gov/study/NCT05318092?term=NCT05318092&rank=1
- 8.Angiodynamics I AlphaVac F22 Promotional Animation. YouTube; 2021. https://www.youtube.com/watch?v=NjL0jMoD0Wc
- 9.Benfor B., Haddad P., Bohle K., Atkins M.D., Lumsden A.B., Peden E.K. Cardiovascular collapse during mechanical thrombectomy for acute pulmonary embolism and the role of extracorporeal membrane oxygenation in patient rescue. J Vasc Surg Venous Lymphat Disord. 2023;11:978–985.e973. doi: 10.1016/j.jvsv.2023.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Tu T., Toma C., Tapson V.F., et al. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: the FLARE Study. JACC Cardiovasc Interv. 2019;12:859–869. doi: 10.1016/j.jcin.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Chandra V.M., Khaja M.S., Kryger M.C., et al. Mechanical aspiration thrombectomy for the treatment of pulmonary embolism: a systematic review and meta-analysis. Vasc Med. 2022;27:574–584. doi: 10.1177/1358863X221124681. [DOI] [PubMed] [Google Scholar]
- 12.Konstantinides S.V., Meyer G., Becattini C., et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) Eur Respir J. 2019;54 doi: 10.1183/13993003.01647-2019. [DOI] [PubMed] [Google Scholar]