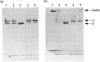
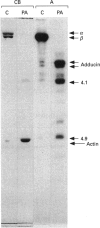
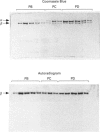
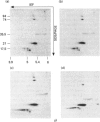
Abstract
Human erythrocyte spectrin is an alpha beta heterodimer which forms tetramers by self-association. This association involves the N-terminal region of the alpha chain and the C-terminal region of the beta chain. The latter contains a cluster of four phosphorylation sites (one phosphothreonine and three phosphoserine residues). The role of this phosphorylation is as yet unknown. We show in this paper that the spectrin beta chain occurs in the cell in subpopulations differing in the degree of occupancy of their phosphorylation sites: 32P peptide maps obtained by 2-nitro-5-thiocyanobenzoic acid (NTCB) cleavage revealed the presence of six components with apparent molecular masses of 17.5 kDa, differing in their isoelectric points; this is most simply interpreted as reflecting the presence of six exchangeable phosphorylation sites in the spectrin beta chain, rather than four as had been supposed. When the alpha beta dimers were partly dissociated by urea, the most highly phosphorylated fraction of the beta chain was found in the undissociated dimers. This high specific activity in the undissociated dimer reflected multiple phosphorylated sites, as revealed by NTCB cleavage. The dephosphorylation or the hyperphosphorylation of spectrin beta chains did not modify the equilibrium between dissociated and undissociated spectrin dimers in the presence of urea. However, the data revealed the existence of two spectrin dimer populations in respect to phosphate turnover and spectrin dimer dissociation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Tyler J. M. State of spectrin phosphorylation does not affect erythrocyte shape or spectrin binding to erythrocyte membranes. J Biol Chem. 1980 Feb 25;255(4):1259–1265. [PubMed] [Google Scholar]
- Bennett V. Spectrin-based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. Physiol Rev. 1990 Oct;70(4):1029–1065. doi: 10.1152/physrev.1990.70.4.1029. [DOI] [PubMed] [Google Scholar]
- Boivin P. Role of the phosphorylation of red blood cell membrane proteins. Biochem J. 1988 Dec 15;256(3):689–695. doi: 10.1042/bj2560689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert R., Bennett P., Gratzer W. Properties and structural role of the subunits of human spectrin. Eur J Biochem. 1980 Jun;107(2):355–361. doi: 10.1111/j.1432-1033.1980.tb06036.x. [DOI] [PubMed] [Google Scholar]
- Chao T. S., Tao M. Modulation of protein 4.1 binding to inside-out membrane vesicles by phosphorylation. Biochemistry. 1991 Oct 29;30(43):10529–10535. doi: 10.1021/bi00107a023. [DOI] [PubMed] [Google Scholar]
- Cianci C. D., Giorgi M., Morrow J. S. Phosphorylation of ankyrin down-regulates its cooperative interaction with spectrin and protein 3. J Cell Biochem. 1988 Jul;37(3):301–315. doi: 10.1002/jcb.240370305. [DOI] [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Colbran R. J., Schworer C. M., Hashimoto Y., Fong Y. L., Rich D. P., Smith M. K., Soderling T. R. Calcium/calmodulin-dependent protein kinase II. Biochem J. 1989 Mar 1;258(2):313–325. doi: 10.1042/bj2580313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Danilov Y. N., Fennell R., Ling E., Cohen C. M. Selective modulation of band 4.1 binding to erythrocyte membranes by protein kinase C. J Biol Chem. 1990 Feb 15;265(5):2556–2562. [PubMed] [Google Scholar]
- Eder P. S., Soong C. J., Tao M. Phosphorylation reduces the affinity of protein 4.1 for spectrin. Biochemistry. 1986 Apr 8;25(7):1764–1770. doi: 10.1021/bi00355a047. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Harris H. W., Jr, Levin N., Lux S. E. Comparison of the phosphorylation of human erythrocyte spectrin in the intact red cell and in various cell-free systems. J Biol Chem. 1980 Dec 10;255(23):11521–11525. [PubMed] [Google Scholar]
- Harris H. W., Jr, Lux S. E. Structural characterization of the phosphorylation sites of human erythrocyte spectrin. J Biol Chem. 1980 Dec 10;255(23):11512–11520. [PubMed] [Google Scholar]
- Husain-Chishti A., Levin A., Branton D. Abolition of actin-bundling by phosphorylation of human erythrocyte protein 4.9. Nature. 1988 Aug 25;334(6184):718–721. doi: 10.1038/334718a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lecomte M. C., Gautero H., Bournier O., Galand C., Lahary A., Vannier J. P., Garbarz M., Delaunay J., Tchernia G., Boivin P. Elliptocytosis-associated spectrin Rouen (beta 220/218) has a truncated but still phosphorylatable beta chain. Br J Haematol. 1992 Feb;80(2):242–250. doi: 10.1111/j.1365-2141.1992.tb08907.x. [DOI] [PubMed] [Google Scholar]
- Lecomte M. C., Gautero H., Garbarz M., Boivin P., Dhermy D. Abnormal tryptic peptide from the spectrin alpha-chain resulting from alpha- or beta-chain mutations: two genetically distinct forms of the Sp alpha I/74 variant. Br J Haematol. 1990 Nov;76(3):406–413. doi: 10.1111/j.1365-2141.1990.tb06376.x. [DOI] [PubMed] [Google Scholar]
- Ling E., Danilov Y. N., Cohen C. M. Modulation of red cell band 4.1 function by cAMP-dependent kinase and protein kinase C phosphorylation. J Biol Chem. 1988 Feb 15;263(5):2209–2216. [PubMed] [Google Scholar]
- Lu P. W., Soong C. J., Tao M. Phosphorylation of ankyrin decreases its affinity for spectrin tetramer. J Biol Chem. 1985 Dec 5;260(28):14958–14964. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Soong C. J., Lu P. W., Tao M. Analysis of band 3 cytoplasmic domain phosphorylation and association with ankyrin. Arch Biochem Biophys. 1987 May 1;254(2):509–517. doi: 10.1016/0003-9861(87)90131-7. [DOI] [PubMed] [Google Scholar]
- Speicher D. W., Morrow J. S., Knowles W. J., Marchesi V. T. A structural model of human erythrocyte spectrin. Alignment of chemical and functional domains. J Biol Chem. 1982 Aug 10;257(15):9093–9101. [PubMed] [Google Scholar]
- Swaney J. B., O'Brien K. Cross-linking studies of the self-association properties of apo-A-I and apo-A-II from human high density lipoprotein. J Biol Chem. 1978 Oct 10;253(19):7069–7077. [PubMed] [Google Scholar]
- Tse W. T., Gallagher P. G., Pothier B., Costa F. F., Scarpa A., Delaunay J., Forget B. G. An insertional frameshift mutation of the beta-spectrin gene associated with elliptocytosis in spectrin nice (beta 220/216). Blood. 1991 Jul 15;78(2):517–523. [PubMed] [Google Scholar]
- Tuazon P. T., Traugh J. A. Casein kinase I and II--multipotential serine protein kinases: structure, function, and regulation. Adv Second Messenger Phosphoprotein Res. 1991;23:123–164. [PubMed] [Google Scholar]
- Ungewickell E., Gratzer W. Self-association of human spectrin. A thermodynamic and kinetic study. Eur J Biochem. 1978 Aug 1;88(2):379–385. doi: 10.1111/j.1432-1033.1978.tb12459.x. [DOI] [PubMed] [Google Scholar]
- Weaver D. C., Pasternack G. R., Marchesi V. T. The structural basis of ankyrin function. II. Identification of two functional domains. J Biol Chem. 1984 May 25;259(10):6170–6175. [PubMed] [Google Scholar]
- Winkelmann J. C., Chang J. G., Tse W. T., Scarpa A. L., Marchesi V. T., Forget B. G. Full-length sequence of the cDNA for human erythroid beta-spectrin. J Biol Chem. 1990 Jul 15;265(20):11827–11832. [PubMed] [Google Scholar]
- Yoshino H., Marchesi V. T. Isolation of spectrin subunits and reassociation in vitro. Analysis by fluorescence polarization. J Biol Chem. 1984 Apr 10;259(7):4496–4500. [PubMed] [Google Scholar]