Abstract
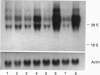
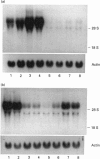
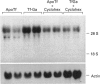
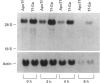
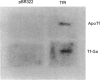
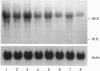
Gallium binds to the iron transport protein transferrin (Tf), is incorporated into cells through transferrin receptors (TfR) and inhibits iron-dependent DNA synthesis. Since cellular TfR expression is tightly regulated by the availability of iron, we investigated the effects of transferrin-gallium (Tf-Ga) on TfR mRNA levels in myeloid HL60 and lymphoid CCRF-CEM cells. In HL60 cells, Tf-Ga increased TfR mRNA levels in a dose-dependent fashion. This increase in TfR mRNA was blocked by Tf-Fe and by cycloheximide. Analysis of the rate of mRNA decay in the presence of actinomycin D revealed that the half-life of TfR mRNA was increased in HL60 cells incubated with Tf-Ga. The rate of transcription of TfR mRNA was not increased by Tf-Ga. In contrast with HL60 cells, CCRF-CEM cells displayed a decrease in the level of TfR mRNA after incubation with Tf-Ga. Tf-Ga inhibited iron uptake in both HL60 and CCRF-CEM cells but increased the level of TfR mRNA only in HL60 cells, suggesting that the Tf-Ga induction of TfR mRNA was not solely due to inhibition of cellular iron uptake. At growth-inhibitory concentrations, Tf-Ga increased the TfR mRNA level in HL60 cells but decreased it in CCRF-CEM cells. Our studies suggest that in HL60 cells, gallium regulates TfR expression at the post-transcriptional level by mechanisms which require de novo protein synthesis and involve interaction with iron. The divergent effects of Tf-Ga on TfR mRNA in myeloid HL60 and lymphoid CCRF-CEM cells suggest that differences exist in the regulation of TfR expression between these two cell types.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridges K. R., Cudkowicz A. Effect of iron chelators on the transferrin receptor in K562 cells. J Biol Chem. 1984 Nov 10;259(21):12970–12977. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chitambar C. R., Massey E. J., Seligman P. A. Regulation of transferrin receptor expression on human leukemic cells during proliferation and induction of differentiation. Effects of gallium and dimethylsulfoxide. J Clin Invest. 1983 Oct;72(4):1314–1325. doi: 10.1172/JCI111087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitambar C. R., Matthaeus W. G., Antholine W. E., Graff K., O'Brien W. J. Inhibition of leukemic HL60 cell growth by transferrin-gallium: effects on ribonucleotide reductase and demonstration of drug synergy with hydroxyurea. Blood. 1988 Dec;72(6):1930–1936. [PubMed] [Google Scholar]
- Chitambar C. R., Narasimhan J., Guy J., Sem D. S., O'Brien W. J. Inhibition of ribonucleotide reductase by gallium in murine leukemic L1210 cells. Cancer Res. 1991 Nov 15;51(22):6199–6201. [PubMed] [Google Scholar]
- Chitambar C. R., Sax D. Regulatory effects of gallium on transferrin-independent iron uptake by human leukemic HL60 cells. Blood. 1992 Jul 15;80(2):505–511. [PubMed] [Google Scholar]
- Chitambar C. R., Seligman P. A. Effects of different transferrin forms on transferrin receptor expression, iron uptake, and cellular proliferation of human leukemic HL60 cells. Mechanisms responsible for the specific cytotoxicity of transferrin-gallium. J Clin Invest. 1986 Dec;78(6):1538–1546. doi: 10.1172/JCI112746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitambar C. R., Zivkovic Z. Inhibition of hemoglobin production by transferrin-gallium. Blood. 1987 Jan;69(1):144–149. [PubMed] [Google Scholar]
- Chitambar C. R., Zivkovic Z. Uptake of gallium-67 by human leukemic cells: demonstration of transferrin receptor-dependent and transferrin-independent mechanisms. Cancer Res. 1987 Aug 1;47(15):3929–3934. [PubMed] [Google Scholar]
- Forsbeck K., Nilsson K. The dynamic morphology of the transferrin-transferrin receptor system in human leukaemia/lymphoma cell lines and its relation to iron metabolism and cell proliferation. Scand J Haematol. 1985 Aug;35(2):145–154. doi: 10.1111/j.1600-0609.1985.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Tang C. K., Chin J., Harford J. B., Klausner R. D. Reciprocal control of RNA-binding and aconitase activity in the regulation of the iron-responsive element binding protein: role of the iron-sulfur cluster. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7536–7540. doi: 10.1073/pnas.89.16.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris W. R., Pecoraro V. L. Thermodynamic binding constants for gallium transferrin. Biochemistry. 1983 Jan 18;22(2):292–299. doi: 10.1021/bi00271a010. [DOI] [PubMed] [Google Scholar]
- Kaptain S., Downey W. E., Tang C., Philpott C., Haile D., Orloff D. G., Harford J. B., Rouault T. A., Klausner R. D. A regulated RNA binding protein also possesses aconitase activity. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10109–10113. doi: 10.1073/pnas.88.22.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Harford J. B. cis-trans models for post-transcriptional gene regulation. Science. 1989 Nov 17;246(4932):870–872. doi: 10.1126/science.2683086. [DOI] [PubMed] [Google Scholar]
- Larrick J. W., Cresswell P. Modulation of cell surface iron transferrin receptors by cellular density and state of activation. J Supramol Struct. 1979;11(4):579–586. doi: 10.1002/jss.400110415. [DOI] [PubMed] [Google Scholar]
- Larson S. M., Rasey J. S., Allen D. R., Nelson N. J., Grunbaum Z., Harp G. D., Williams D. L. Common pathway for tumor cell uptake of gallium-67 and iron-59 via a transferrin receptor. J Natl Cancer Inst. 1980 Jan;64(1):41–53. [PubMed] [Google Scholar]
- Rao K. K., Shapiro D., Mattia E., Bridges K., Klausner R. Effects of alterations in cellular iron on biosynthesis of the transferrin receptor in K562 cells. Mol Cell Biol. 1985 Apr;5(4):595–600. doi: 10.1128/mcb.5.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman P. A., Crawford E. D. Treatment of advanced transitional cell carcinoma of the bladder with continuous-infusion gallium nitrate. J Natl Cancer Inst. 1991 Nov 6;83(21):1582–1584. doi: 10.1093/jnci/83.21.1582. [DOI] [PubMed] [Google Scholar]
- Taetle R., Ralph S., Smedsrud S., Trowbridge I. Regulation of transferrin receptor expression in myeloid leukemia cells. Blood. 1987 Sep;70(3):852–859. [PubMed] [Google Scholar]
- Taetle R. The role of transferrin receptors in hemopoietic cell growth. Exp Hematol. 1990 May;18(4):360–365. [PubMed] [Google Scholar]
- Theil E. C. Regulation of ferritin and transferrin receptor mRNAs. J Biol Chem. 1990 Mar 25;265(9):4771–4774. [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci U S A. 1981 May;78(5):3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrell R. P., Jr Clinical trials of gallium nitrate in patients with cancer-related hypercalcemia. Semin Oncol. 1991 Aug;18(4 Suppl 5):26–31. [PubMed] [Google Scholar]
- Warrell R. P., Jr, Coonley C. J., Straus D. J., Young C. W. Treatment of patients with advanced malignant lymphoma using gallium nitrate administered as a seven-day continuous infusion. Cancer. 1983 Jun 1;51(11):1982–1987. doi: 10.1002/1097-0142(19830601)51:11<1982::aid-cncr2820511104>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]