Abstract
Malnutrition is classified into marasmus and kwashiorkor in children. However, the clinical significance of these aspects is unclear in adult patients with heart failure (HF). We divided 2308 adult patients with HF into four groups according to marasmus type (body mass index < 18.5 kg/m2) and kwashiorkor type (serum albumin < 3.4 g/dL) malnutrition: Group C (no malnutrition, n = 1511, 65.5%), Group M (marasmus type malnutrition, n = 133, 5.8%), Group K (kwashiorkor type malnutrition, n = 554, 24.0%) and Group MK (marasmic-kwashiorkor type malnutrition, n = 110, 4.8%). Group M showed the lowest blood pressure. Groups K and MK showed higher levels of B-type natriuretic peptide. Right atrial pressure was lowest in Groups M and MK. Kaplan-Meir analysis demonstrated that Group MK had the lowest event-free rate of all-cause death and cardiac death. In the multivariable Cox proportional hazard analysis, Groups M, K, and MK were associated with all-cause death (hazard ratio 1.790, 1.657 and 2.313, respectively) and cardiac death (hazard ratio 2.053, 1.855 and 3.001, respectively) compared to Group C as a reference. Marasmus type and kwashiorkor type malnutrition are associated with distinct profiles and high mortality, and marasmic-kwashiorkor type malnutrition has the poorest prognosis.
Subject terms: Cardiovascular biology, Cardiovascular diseases, Nutrition disorders
Introduction
Malnutrition is one of the most common comorbidities of patients with heart failure (HF)1. Malnutrition in patients with HF is associated with metabolic alteration characterized by increased inflammation and decreased protein synthesis2–4. As malnutrition worsens, mortality progressively increases in these population independently of left ventricular systolic function3,5,6. Patients at risk of malnutrition should be identified by validated screening tools7, and several criteria for malnutrition have been reported8–11. The components of criteria for malnutrition screening vary depending on the screening tools8–11. In patients with HF, both Mini-Nutritional Assessment Short-Form (MNA-SF), which includes body mass index (BMI)9, and Geriatric Nutritional Risk Index, which is based on serum albumin levels and body weight8, are associated with all-cause mortality3,12. However, these screening tools may reflect different heterogeneous pathophysiological conditions, such as dietary intake, weight loss, and inflammation, due to the different factors that are included in each set of criteria13. HF, especially HF with preserved ejection fraction, is a heterogeneous syndrome, and understanding the clinical phenotypes of HF may be beneficial with regard to different targeted intervention strategies14.
Pathophysiological characteristics of malnutrition have been well categorized in children, namely marasmus, kwashiorkor, and a mixture (marasmic-kwashiorkor)15. Marasmus is clinically characterized by loss of subcutaneous fat and muscle wasting, whereas kwashiorkor presents with edema and abdominal distention15,16. However, the clinical implications of these phenotypes of malnutrition remain unclear in adult patients with HF. In the present study, we aimed to compare and elucidate the characteristics and prognosis of adult HF patients with/without marasmus and/or kwashiorkor type malnutrition.
Results
Among the total population, the median age was 70.0 (60.0–79.0) years, with 59.7% males. Comparisons of patient characteristics are shown in Table 1. Group MK had the oldest age and lowest proportion of male sex. Systolic blood pressure was lowest in Group M compared to the other groups. Heart rate was lowest in Group C, followed by Groups M, MK, and K, in order. The prevalence of malignant tumor was highest in Group MK, followed by Group M. Regarding medication, the usage of loop diuretics was the highest in Group MK, followed by Groups K, M, and C, in that order. In laboratory data, levels of B-type natriuretic peptide (BNP) increased and hemoglobin decreased in Groups C, M, K, and MK. According to right heart catheterization, Group K showed higher mean pulmonary artery wedge pressure, systolic/diastolic/mean pulmonary artery pressure, and mean right atrial pressure compared to Groups C and M.
Table 1.
Patient characteristics (n = 2308).
| Group C (n = 1511) | Group M (n = 133) | Group K (n = 554) | Group MK (n = 110) | P value | |
|---|---|---|---|---|---|
| Age, years | 69.0 (59.0–77.0)† | 68.0 (60.0–78.0)† | 73.5 (63.0–82.0)* | 75.0 (67.0–84.0)* | < 0.001 |
| Male sex | 951 (62.9) | 61 (45.9) | 316 (57.0) | 50 (45.5) | < 0.001 |
| Body mass index, kg/m2 | 23.5 (21.4–26.1)*† | 17.4 (16.7–18.0)† | 22.9 (21.1–25.3)* | 17.2 (16.5–17.8)† | < 0.001 |
| Systolic blood pressure, mmHg | 123.0 (109.0–140.0)* | 111.0 (98.0–126.0)† | 125.5 (108.0–149.0)* | 121.5 (106.0–144.0)* | < 0.001 |
| Heart rate, bpm | 72.0 (62.0–87.0)*† | 76.0 (65.0–86.0)† | 86.0 (70.0–101.0)* | 85.0 (71.0–95.0)*† | < 0.001 |
| NYHA class 3 or 4, n (%) | 63 (4.2) | 5 (3.8) | 41 (7.4) | 16 (14.5) | < 0.001 |
| Atrial fibrillation, n (%) | 589 (39.0) | 52 (39.1) | 215 (38.8) | 42 (38.2) | 0.998 |
| CAD, n (%) | 453 (30.0) | 23 (17.3) | 208 (37.5) | 27 (24.5) | < 0.001 |
| Stroke, n (%) | 250 (16.5) | 26 (19.5) | 127 (23.0) | 21 (19.1) | 0.010 |
| COPD, n (%) | 386 (29.6) | 35 (30.7) | 113 (27.9) | 24 (27.9) | 0.888 |
| Malignant tumor, n (%) | 236 (15.9) | 29 (22.0) | 113 (20.5) | 31 (28.2) | 0.001 |
| RASIs, n (%) | 1087 (71.9) | 75 (56.4) | 387 (69.9) | 67 (60.9) | < 0.001 |
| Beta blockers, n (%) | 1093 (72.3) | 98 (73.7) | 393 (70.9) | 76 (69.1) | 0.793 |
| MRAs, n (%) | 563 (37.3) | 58 (43.6) | 236 (42.6) | 54 (49.1) | 0.014 |
| Loop diuretics, n (%) | 923 (61.1) | 91 (68.4) | 423 (76.4) | 92 (83.6) | < 0.001 |
| Statins, n (%) | 652 (43.2) | 29 (21.8) | 180 (32.5) | 30 (27.3) | < 0.001 |
| Antihyperuricemic agents, n (%) | 365 (24.2) | 27 (20.3) | 108 (19.5) | 16 (14.5) | 0.022 |
| ICD or CRT, n (%) | 240 (15.9) | 26 (19.5) | 54 (9.7) | 8 (7.3) | < 0.001 |
| BNP, pg/mL | 179.6 (63.5–439.9)*† | 374.4 (100.3–595.8)† | 543.9 (225.3–1048.0)* | 598.6 (357.9–1097.1)*† | < 0.001 |
| Hemoglobin, g/dL | 13.3 (12.0–14.7)*† | 12.7 (11.5–13.9)† | 11.3 (9.7–13.1)* | 10.5 (8.9–11.8)*† | < 0.001 |
| Lymphocytes, % | 24.0 (17.0–31.0)*† | 22.0 (15.0–30.0)† | 15.0 (9.0–21.0)* | 13.5 (9.0–20.0)*† | < 0.001 |
| Albumin, g/dL | 4.0 (3.7–4.3)† | 3.9 (3.6–4.2)† | 3.0 (2.7–3.2)* | 2.9 (2.6–3.1)*† | < 0.001 |
| eGFR, mL/min/1.73m2 | 60.3 (47.1–72.9)*† | 59.3 (44.1–79.8)† | 51.7 (35.5–68.5)* | 58.3 (37.4–75.7)*† | < 0.001 |
| Sodium, mmol/L | 140.0 (138.0–142.0)*† | 139.0 (137.0–141.0)† | 139.0 (136.0–141.0)* | 138.0 (134.5–141.0)*† | < 0.001 |
| Uric acid, mg/dL | 6.1 (5.1–7.4) | 6.1 (4.6–7.6) | 6.3 (4.8–8.0) | 5.7 (4.5–7.2) | 0.140 |
| C-reactive protein, mg/dL | 0.12 (0.05–0.38)*† | 0.10 (0.05–0.28)† | 1.54 (0.39–5.33)* | 1.11 (0.28–4.53)*† | < 0.001 |
| Total cholesterol, mg/dL | 182.0 (157.0–210.0)*† | 189.5 (167.0–211.0)† | 154.0 (128.5–181.5)* | 152.0 (127.0–189.0)*† | < 0.001 |
| HbA1c, % | 5.8 (5.5–6.3)* | 5.7 (5.3–6.1)† | 5.9 (5.4–6.5)* | 5.7 (5.3–6.2)† | < 0.001 |
| LVEF, % | 55.0 (40.4–64.2) | 57.0 (36.1–65.4) | 54.0 (39.2–62.6) | 56.5 (42.6–63.0) | 0.427 |
| Mean PAWP, mmHg§ | 12.0 (9.0–18.0)† | 11.0 (8.0–15.5)† | 15.0 (9.0–22.0)* | 12.0 (6.0–17.5)† | < 0.001 |
| Systolic PAP, mmHg§ | 32.0 (25.0–41.0)† | 29.0 (23.0–39.0)† | 34.0 (28.0–44.0)* | 34.0 (25.5–44.0) | 0.002 |
| Diastolic PAP, mmHg§ | 14.0 (10.0–20.0)† | 12.0 (8.0–16.0)† | 17.0 (12.0–23.0)* | 15.0 (9.5–20.0) | < 0.001 |
| Mean PAP, mmHg§ | 21.0 (16.0–28.0)† | 19.0 (15.0–26.0)† | 24.0 (18.0–31.0)* | 22.5 (16.0–29.0) | < 0.001 |
| Mean RAP, mmHg§ | 6.0 (4.0–9.0)*† | 5.0 (2.0–8.0)† | 8.0 (5.0–12.0)* | 5.0 (3.0–8.5)† | < 0.001 |
| Cardiac index, L/min/m2§ | 2.5 (2.2–3.0)† | 2.5 (2.1–3.0) | 2.4 (2.0–2.9) | 2.4 (2.0–3.0) | 0.026 |
*P < 0.05 vs. Group M and †P < 0.05 vs. Group K.
§Right heart catheterization was performed in patients selected by attending physicians (n = 1303).
NYHA, New York Heart Association; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; RASI, renin-angiotensin system inhibitor; MRA, mineralocorticoid receptor antagonist; ICD, implantable cardioverter-defibrillator; CRT, cardiac resynchronization therapy; BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; LVEF, left ventricular ejection fraction; PAWP, pulmonary artery wedge pressure; PAP, pulmonary artery pressure; RAP, right atrial pressure.
Tables 2 and 3 show the results of the logistic regression analysis evaluating factors associated with marasmus type and kwashiorkor type malnutrition, respectively. According to the multivariable analysis, systolic blood pressure, use of renin-angiotensin system inhibitors and statins, and levels of hemoglobin, sodium, and glycated hemoglobin were negatively associated with marasmus type malnutrition, while levels of log-transformed BNP and estimated glomerular filtration rate were positively associated. On the other hand, levels of hemoglobin, lymphocytes, sodium, and total cholesterol were negatively associated with kwashiorkor type malnutrition, while levels of BNP and C-reactive protein were positively associated.
Table 2.
Logistic regression analysis for marasmus type malnutrition (n = 243/2,308 patients).
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 1.004 (0.995–1.014) | 0.343 | 1.006 (0.992–1.019) | 0.423 |
| Male sex | 0.530 (0.405–0.692) | < 0.001 | 0.779 (0.539–1.125) | 0.182 |
| Systolic blood pressure | 0.992 (0.987–0.997) | 0.001 | 0.990 (0.983–0.998) | 0.013 |
| Heart rate | 1.003 (0.998–1.008) | 0.238 | NS | |
| NYHA class 3 or 4 | 1.783 (1.093–2.907) | 0.020 | 1.064 (0.543–2.085) | 0.857 |
| Atrial fibrillation | 0.989 (0.753–1.300) | 0.939 | NS | |
| Coronary artery disease | 0.550 (0.398–0.761) | < 0.001 | 0.802 (0.493–1.305) | 0.374 |
| Stroke | 1.073 (0.766–1.504) | 0.682 | NS | |
| COPD | 1.014 (0.735–1.398) | 0.933 | NS | |
| Malignant tumor | 1.593 (1.164–2.179) | 0.004 | 1.438 (0.938–2.204) | 0.096 |
| RASIs | 0.564 (0.429–0.740) | < 0.001 | 0.555 (0.386–0.799) | 0.002 |
| Beta blockers | 0.983 (0.732–1.320) | 0.907 | NS | |
| MRAs | 1.355 (1.037–1.770) | 0.026 | 1.017 (0.676–1.530) | 0.935 |
| Loop diuretics | 1.629 (1.201–2.211) | 0.002 | 1.321 (0.830–2.104) | 0.241 |
| Statins | 0.475 (0.350–0.645) | < 0.001 | 0.645 (0.419–0.993) | 0.047 |
| Antihyperuricemic agents | 0.724 (0.512–1.022) | 0.066 | NS | |
| ICD or CRT | 0.980 (0.668–1.437) | 0.917 | NS | |
| Log-BNP | 2.051 (1.565–2.687) | < 0.001 | 1.648 (1.152–2.358) | 0.006 |
| Hemoglobin | 0.820 (0.772–0.871) | < 0.001 | 0.871 (0.796–0.953) | 0.003 |
| Lymphocytes | 0.982 (0.967–0.997) | 0.019 | 0.998 (0.979–1.018) | 0.873 |
| eGFR | 1.007 (1.001–1.012) | 0.023 | 1.010 (1.002–1.019) | 0.014 |
| Sodium | 0.920 (0.889–0.951) | < 0.001 | 0.931 (0.890–0.974) | 0.002 |
| Uric acid | 0.916 (0.848–0.989) | 0.025 | 0.977 (0.885–1.079) | 0.647 |
| C-reactive protein | 1.021 (0.985–1.060) | 0.225 | NS | |
| Total cholesterol | 1.000 (0.995–1.004) | 0.859 | NS | |
| HbA1c | 0.710 (0.595–0.847) | < 0.001 | 0.724 (0.564–0.930) | 0.011 |
| LVEF | 1.000 (0.989–1.010) | 0.933 | NS | |
Age, male sex, and variables with P values of < 0.05 were entered into multivariable model.
OR, odds ratio; CI, confidence interval; NYHA, New York Heart Association; COPD, chronic obstructive pulmonary disease; RASI, renin-angiotensin system inhibitor; MRA, mineralocorticoid receptor antagonist; ICD, implantable cardioverter-defibrillator; CRT, cardiac resynchronization therapy; Log-BNP, log-transformed B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; LVEF, left ventricular ejection fraction; NS, not selected.
Age, systolic blood pressure, heart rate, log-BNP, hemoglobin, lymphocytes, eGFR, sodium, uric acid, C-reactive protein, total cholesterol, HbA1c, and LVEF were analyzed as continuous variables.
Table 3.
Logistic regression analysis for kwashiorkor type malnutrition (n = 664/2308 patients).
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 1.017 (1.011–1.024) | < 0.001 | 1.000 (0.985–1.015) | 0.969 |
| Male sex | 0.767 (0.639–0.920) | 0.004 | 0.754 (0.493–1.153) | 0.192 |
| Systolic blood pressure | 1.003 (1.000–1.006) | 0.031 | 1.005 (0.998–1.012) | 0.126 |
| Heart rate | 1.017 (1.013–1.021) | < 0.001 | 1.003 (0.995–1.011) | 0.504 |
| NYHA class 3 or 4 | 2.175 (1.511–3.131) | < 0.001 | 0.497 (0.189–1.308) | 0.157 |
| Atrial fibrillation | 0.988 (0.821–1.189) | 0.899 | NS | |
| Coronary artery disease | 1.344 (1.110–1.628) | 0.002 | 1.209 (0.745–1.963) | 0.443 |
| Stroke | 1.424 (1.139–1.782) | 0.002 | 1.184 (0.738–1.901) | 0.484 |
| COPD | 0.916 (0.729–1.150) | 0.448 | NS | |
| Malignant tumor | 1.420 (1.132–1.782) | 0.002 | 0.755 (0.463–1.232) | 0.261 |
| RASIs | 0.897 (0.738–1.090) | 0.273 | NS | |
| Betablockers | 0.915 (0.750–1.116) | 0.380 | NS | |
| MRAs | 1.277 (1.064–1.534) | 0.009 | 0.968 (0.636–1.474) | 0.880 |
| Loop diuretics | 2.147 (1.745–2.643) | < 0.001 | 1.168 (0.722–1.891) | 0.527 |
| Statins | 0.654 (0.541–0.792) | < 0.001 | 0.683 (0.443–1.053) | 0.084 |
| Antihyperuricemic agents | 0.733 (0.585–0.919) | 0.007 | 0.853 (0.539–1.350) | 0.497 |
| ICD or CRT | 0.534 (0.398–0.715) | < 0.001 | 0.774 (0.453–1.323) | 0.349 |
| Log-BNP | 4.869 (3.903–6.073) | < 0.001 | 3.595 (2.356–5.487) | < 0.001 |
| Hemoglobin | 0.658 (0.626–0.693) | < 0.001 | 0.797 (0.721–0.882) | < 0.001 |
| Lymphocytes | 0.910 (0.898–0.922) | < 0.001 | 0.955 (0.932–0.977) | < 0.001 |
| eGFR | 0.989 (0.985–0.994) | < 0.001 | 1.006 (0.997–1.016) | 0.189 |
| Sodium | 0.893 (0.870–0.916) | < 0.001 | 0.932 (0.884–0.983) | 0.009 |
| Uric acid | 1.023 (0.972–1.076) | 0.384 | NS | |
| C-reactive protein | 1.446 (1.371–1.525) | < 0.001 | 1.361 (1.229–1.506) | < 0.001 |
| Total cholesterol | 0.981 (0.977–0.985) | < 0.001 | 0.991 (0.986–0.997) | < 0.001 |
| HbA1c | 1.108 (1.013–1.213) | 0.025 | 1.075 (0.881–1.311) | 0.475 |
| LVEF | 0.995 (0.988–1.002) | 0.144 | NS | |
Age, male sex, and variables with P values of < 0.05 were entered into multivariable model.
OR, odds ratio; CI, confidence interval; NYHA, New York Heart Association; COPD, chronic obstructive pulmonary disease; RASI, renin-angiotensin system inhibitor; MRA, mineralocorticoid receptor antagonist; ICD, implantable cardioverter-defibrillator; CRT, cardiac resynchronization therapy; Log-BNP, log-transformed B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; LVEF, left ventricular ejection fraction; NS, not selected.
Age, systolic blood pressure, heart rate, log-BNP, hemoglobin, lymphocytes, eGFR, sodium, uric acid, C-reactive protein, total cholesterol, HbA1c, and LVEF were analyzed as continuous variables.
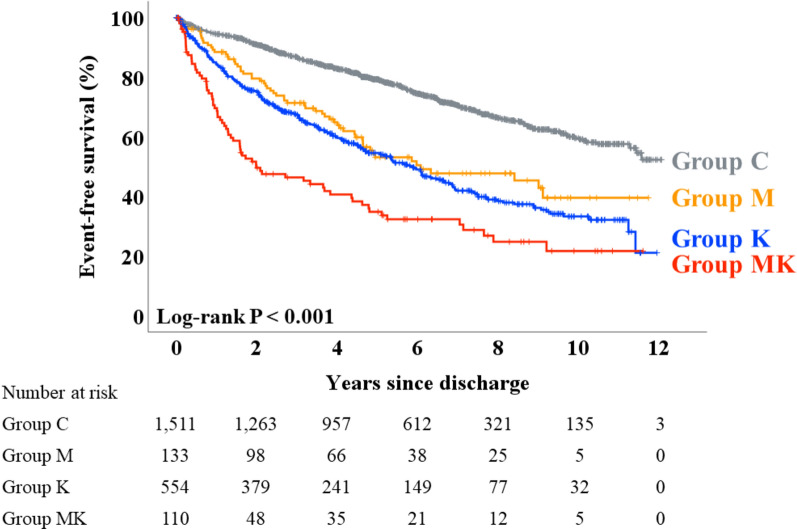
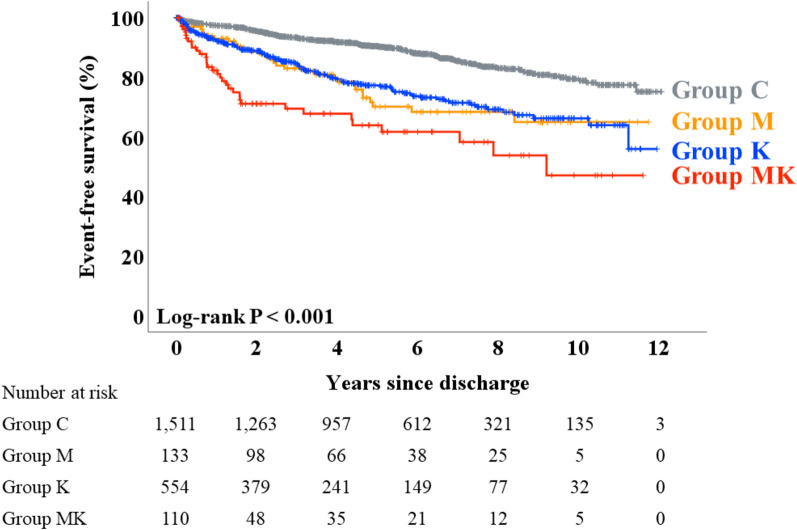
During the post-discharge follow-up period (median 1679 days), a total of 809 deaths from any cause, including 362 cardiac deaths, occurred. Kaplan–Meier analysis revealed that event-free survival from death from any cause was the lowest in Group MK, while Group C had the highest rate of survival (Fig. 1, log-rank P < 0.001). Regarding cardiac death, Group MK showed the lowest and Group C showed the highest event-free survival, while Groups M and K demonstrated intermediate survival (Fig. 2, log-rank P < 0.001). Table 4 summarizes the results of the Cox proportional hazard analysis performed to assess the association between malnutrition and event-free survival time, adjusting for the potential confounding factors. In the univariable analysis, Groups M, K, and MK were associated with both death from any cause and cardiac death, compared to Group C as a reference, respectively. After adjustment for prespecified covariables, Groups M (hazard ratio 1.790, P = 0.011), K (hazard ratio 1.657, P = 0.004), and MK (hazard ratio 2.313, P = 0.002) were associated with the primary endpoint, respectively (Table 4A). Similarly, Groups M (hazard ratio 2.053, P = 0.029), K (hazard ratio 1.855, P = 0.021), and MK (hazard ratio 3.001, P = 0.004) were also associated with the secondary endpoint, respectively (Table 4B).
Figure 1.
Primary outcome: Kaplan–Meier analysis. Kaplan–Meier analysis demonstrated that event-free survival of death from any cause was lowest in Group MK while Group C showed the best prognosis.
Figure 2.
Secondary outcome: Kaplan–Meier analysis. Shown are the results of the Kaplan–Meier analysis which revealed that event-free survival of cardiac death was the lowest in Group MK, whereas Groups M and K showed intermediate prognosis.
Table 4.
Cox proportional hazard analysis for the primary and secondary outcomes (n = 2308).
| (A) Death from any cause (event n = 809) | Hazard ratio (95% CI) | P value |
|---|---|---|
| Group C | Reference | – |
| Group M (unadjusted) | 2.053 (1.568–2.688) | < 0.001 |
| Group M (model 1) | 2.287 (1.743–2.999) | < 0.001 |
| Group M (model 2) | 1.790 (1.141–2.810) | 0.011 |
| Group K (unadjusted) | 2.451 (2.102–2.857) | < 0.001 |
| Group K (model 1) | 2.251 (1.928–2.628) | < 0.001 |
| Group K (model 2) | 1.657 (1.172–2.344) | 0.004 |
| Group MK (unadjusted) | 4.112 (3.193–5.296) | < 0.001 |
| Group MK (model 1) | 3.718 (2.879–4.801) | < 0.001 |
| Group MK (model 2) | 2.313 (1.375–3.893) | 0.002 |
| (B) Cardiac death (event n = 362) | Hazard ratio (95% CI) | P value |
|---|---|---|
| Group C | Reference | – |
| Group M (unadjusted) | 2.363 (1.622–3.443) | < 0.001 |
| Group M (model 1) | 2.627 (1.798–3.838) | < 0.001 |
| Group M (model 2) | 2.053 (1.074–3.922) | 0.029 |
| Group K (unadjusted) | 2.236 (1.770–2.826) | < 0.001 |
| Group K (model 1) | 2.102 (1.661–2.661) | < 0.001 |
| Group K (model 2) | 1.855 (1.096–3.139) | 0.021 |
| Group MK (unadjusted) | 4.282 (2.965–6.183) | < 0.001 |
| Group MK (model 1) | 4.030 (2.780–5.843) | < 0.001 |
| Group MK (model 2) | 3.001 (1.419–6.345) | 0.004 |
CI, confidence interval.
Model 1: adjusted for age and sex.
Model 2: adjusted for age, sex, New York Heart Association class 3 or 4, left ventricular ejection fraction, systolic blood pressure, coronary artery disease, use of renin-angiotensin system inhibitors, beta blockers, mineralocorticoid receptor antagonists, statins, and antihyperuricemic agents, implantable cardioverter-defibrillator and/or cardiac resynchronization therapy, and levels of log-transformed B-type natriuretic peptide, estimated glomerular filtration rate, hemoglobin, lymphocytes, uric acid, total cholesterol, and sodium.
Age, left ventricular ejection fraction, systolic blood pressure, and levels of log-transformed B-type natriuretic peptide, estimated glomerular filtration rate, hemoglobin, lymphocytes, uric acid, total cholesterol, and sodium were analyzed as continuous variables.
Discussion
In the present study, we investigated the clinical characteristics and outcomes of a cohort of patients with HF, categorized into four distinct groups based on a combination of marasmus type and/or kwashiorkor type malnutrition. We revealed that approximately 35% of patients with HF had at least marasmus type malnutrition, kwashiorkor type malnutrition, or both. Although combined marasmic-kwashiorkor type malnutrition was rare in adult patients with HF, these patients had the worst prognosis.
In children, protein-energy malnutrition occurs as a result of inadequate protein and energy supply15,16. Marasmus, one of the two main extreme forms of protein-energy malnutrition, results from calorie and energy deficiency mainly in children younger than 5 years15,16. Children with marasmus present with loose skin due to the loss of subcutaneous fat, muscle wasting, hypotension, bradycardia, and absence of edema15. Although the present study defined marasmus type malnutrition simply based on low BMI, Group M showed some important characteristics that are similar to those in children with marasmus. Blood pressure was the lowest in Group M and heart rate was lower than in those with kwashiorkor type malnutrition. The lower use of loop diuretics and lower levels of BNP compared to those in Groups K and MK indicated that congestion was less severe in Group M. The results of right heart catheterization support this interpretation. The main features of the HF patients with marasmus type malnutrition included low BMI, lower blood pressure, and higher prevalence of malignant tumor as a comorbidity. These characteristics suggest greater significance of frailty, sarcopenia, and cachexia in those populations1,17–19. HF Patients with frailty sarcopenia, and/or cachexia are at high risk of application of implantable cardioverter-defibrillator and/or cardiac resynchronization therapy, impaired exercise capacity, and high mortality17,20–23. Considering these pathophysiologies, treatment for HF patients with marasmus type malnutrition should be multifactorial and include exercise training, nutritional supplementation, and treatment for comorbidities1,19. Although the causal relationship was unclear, the logistic regression analysis revealed several important factors that were associated with marasmus type malnutrition. Patients with lower systolic blood pressure were at risk of marasmus type malnutrition, suggesting that advanced HF is associated with marasmus type malnutrition1,19,24. The use of renin-angiotensin receptor inhibitors showed a protective effect on the presence of marasmus type malnutrition. Angiotensin II type 1 receptors are present in skeletal muscle fibers and increased renin-angiotensin system signaling promotes skeletal muscle wasting25. The results of the present study were consistent with those of previous studies reporting potential therapeutic effects of renin-angiotensin receptor inhibitors for sarcopenia26–30. There is a lack of evidence regarding the effectiveness of statins to prevent marasmus type malnutrition. The use of statins has been reported to be protective for sarcopenia31, but has not been proven to be effective for frailty and cachexia32,33. Since routine administration of statins in patients with HF without other indications is not recommended by the guidelines19, the priority of the use of statins seemed to be low in HF patients with marasmus type malnutrition, which showed lower prevalence of coronary artery disease and stroke.
Kwashiorkor is another extreme form of protein-energy malnutrition that mainly occurs in older infants and young children suffering from a diet with inadequate protein but reasonably normal caloric intake15,16. Children with kwashiorkor are characterized by edema, abdominal distension, hepatomegaly, dermatosis, and hypopigmented hair15. Low protein intake leads to a reduction in serum albumin and ferritin levels, causing hypoalbuminemia and anemia, respectively16. Hypoalbuminemia decreases oncotic pressure resulting in edema16, which occurs in adult patients with HF due to diminished intake of protein as in children, but several other mechanisms also exist34,35. Previous studies have reported that inflammation (reflected by increased levels of C-reactive protein)35, along with lower levels of lymphocytes35, hemoglobin36,37, sodium36,37, and total cholesterol36,37, are associated with hypoalbuminemia in patients with HF. BMI is not a determinant of hypoalbuminemia35–37. The results of logistic regression analysis in the present study were consistent with these reports. In addition, higher levels of BNP were also independently associated with kwashiorkor type malnutrition. Patients with this type of malnutrition in the present study showed a higher use of loop diuretics and higher levels of BNP, pulmonary artery wedge pressure, pulmonary artery pressure, and right atrial pressure, suggesting that their congestion was more severe. In those populations, congestion may lead to hypoalbuminemia by hemodilution and decreased liver synthesis due to congestive liver34,38,39. Lower levels of estimated glomerular filtration rate were derived from renal congestion in some patients with kwashiorkor type malnutrition40,41. Thus, more aggressive decongestion targeting levels of serum albumin should be considered in patients with kwashiorkor type malnutrition42–44.
The present study had some limitations. The definition of marasmus type malnutrition was based solely on BMI. Body composition (e.g., skeletal muscle and fat) should be assessed in further studies. Detailed information of malignant tumor was not recorded. Right heart catheterization was performed in selected patients based on the decision by their attending cardiologists. Patients with chronic liver disease and/or chronic kidney disease not requiring maintenance dialysis were not excluded.
Conclusion
HF patients with marasmus type malnutrition and those with kwashiorkor type malnutrition demonstrate distinct characteristics. Classifying the nutritional status based on these phenotypes is useful for understanding their underlying pathophysiology and determining the appropriate treatment. HF patients with marasmic-kwashiorkor type malnutrition are at the highest risk of death from any cause and cardiac death.
Methods
Study population
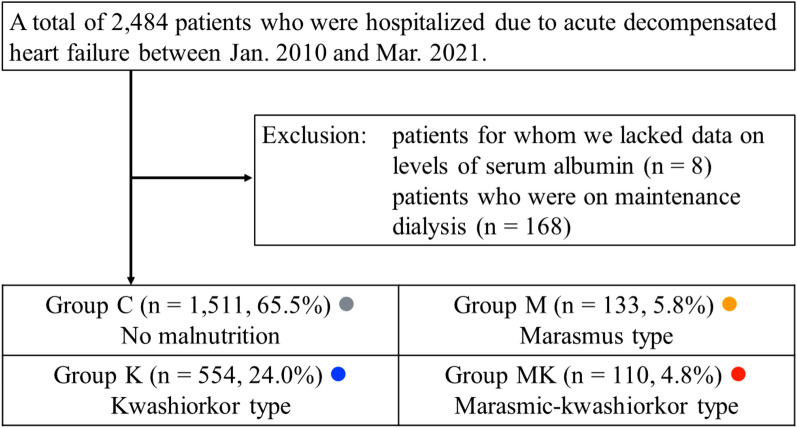
This was an observational study. The patient flow chart is shown in Fig. 3. We recruited a total of 2484 hospitalized patients with acute decompensated HF who were discharged alive between Jan. 2010 and Mar. 2021. Diagnosis of acute decompensated HF was confirmed by attending cardiologists based on the established HF guidelines19,24,45–48. Among them, patients for whom we lacked data on levels of serum albumin (n = 8) and those on maintenance dialysis (n = 168) were excluded. Finally, a total of 2308 patients were enrolled. In the present study, we defined marasmus type malnutrition as a BMI of < 18.5 kg/m2 and kwashiorkor type malnutrition as serum albumin of < 3.4 g/dL, based on previous studies35,37,49–51. The patients were divided into four groups according to the presence/absence of marasmus type and kwashiorkor type malnutrition: Group C (those with neither marasmus type nor kwashiorkor type malnutrition [control group], n = 1511, 65.5%), Group M (those with only marasmus type malnutrition, n = 133, 5.8%), Group K (those with only kwashiorkor type malnutrition, n = 554, 24.0%), and Group MK (those with both marasmus type and kwashiorkor type malnutrition [marasmic-kwashiorkor type malnutrition], n = 110, 4.8%). We compared the patients’ characteristics and prognosis after discharge. Demographic data, including BMI and medication, were obtained at discharge. BMI was calculated by dividing weight (kg) by height (m) squared49,52. Levels of serum albumin were measured using a bromocresol purple dye-binding method. Levels of plasma BNP were measured using a chemiluminescent immunoassay method. Levels of serum total cholesterol, C-reactive protein, uric acid, creatinine were measured using enzymic method, latex agglutination immunoassay method, uricase-peroxidase method, and enzymic method, respectively. Estimated glomerular filtration rate was determined by a 3-variable Japanese equation using levels of serum creatinine and age53. Laboratory tests were blindly performed by clinical laboratory technologists using Siemens Atellica CH at Fukushima Medical University Hospital. The results of laboratory data and echocardiography were based on data recorded within 1 week prior to discharge. We recruited data on right heart catheterization in some patients performed in a stable condition on medical judgement39. The decision to perform catheterization was left to the attending cardiologists. The primary and secondary outcomes were post-discharge death from any cause and cardiac death, respectively. We considered death as cardiac death if the attending physician judged the death was due to worsening HF, ventricular fibrillation, or acute coronary syndrome. The investigation conforms with the principles outlined in the Declaration of Helsinki54. The study protocol was approved by the ethical committee of Fukushima Medical University. All patients gave written informed consent to participate in this study.
Figure 3.
Patient flow chart.
Statistical analysis
All continuous variables were assessed for normality using the Shapiro–Wilk test and were determined to be non-normally distributed. Continuous variables were expressed as median (interquartile range) and compared using the Kruskal–Wallis test, followed by the Steel–Dwass post-hoc test if significant. Categorical variables were expressed as numbers (percentages) and compared using the chi-square test. Variables associated with the presence of marasmus type and kwashiorkor type malnutrition were evaluated by the logistic regression analysis. Age, sex, and other significant variables were further entered into multivariable model. Event-free survival after discharge was compared using Kaplan–Meier analysis with the log-rank test. The prognostic impact of marasmus type and kwashiorkor type malnutrition was evaluated using the Cox proportional hazard analysis with Group C as a reference. Hazard ratios were adjusted in two steps to account for the influence of confounding factors. Model 1 was adjusted for age and sex. Model 2, based on the Seattle Heart Failure Model55,56, was adjusted for age, sex, New York Heart Association class 3 or 4, left ventricular ejection fraction, systolic blood pressure, coronary artery disease, use of renin-angiotensin system inhibitors, beta blockers, mineralocorticoid receptor antagonists, statins, and antihyperuricemic agents, implantable cardioverter-defibrillator and/or cardiac resynchronization therapy, and levels of log-transformed BNP, estimated glomerular filtration rate, hemoglobin, lymphocytes, uric acid, total cholesterol, and sodium.
Acknowledgements
The authors thank Kumiko Watanabe, Yumi Yoshihisa, Shiori Togashi, and Tomiko Miura for their technical assistance. The acquisition of data was supported by Ayumi Haneda, Akito Endo, Mari Hoshi, Manami Akimoto, Mimori Itami, Shiori Urayama, and Yuuichi Yokoyama from the Office for Diversity and Inclusion, Fukushima Medical University, Fukushima, Japan. The present study was supported in part by grants-in-aid for Scientific Research (Nos. 20K07828 and 22K15670) from the Japan Society for the Promotion of Science.
Author contributions
Y.Sa., A.Y., Y.Su., T.M., T.S., T.K., M.O., A.K., T.Y., K.N., and Y.T. contributed to the conception or design of the work. Y.Sa., A.Y., Y.Su., T.M., T.S., T.K., M.O., A.K., T.Y., K.N., and Y.T. contributed to the acquisition, analysis, or interpretation of data for the work. Y.Sa., A.Y., and Y.T. drafted the manuscript. Y.Sa., A.Y., Y.Su., T.M., T.S., T.K., M.O., A.K., T.Y., K.N., and Y.T. critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Heidenreich, P. A. et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation145, e895–e1032. 10.1161/CIR.0000000000001063 (2022). 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 2.Esteban-Fernandez, A. et al. Diagnosis and management of malnutrition in patients with heart failure. J. Clin. Med.10.3390/jcm12093320 (2023). 10.3390/jcm12093320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshihisa, A. et al. Impact of nutritional indices on mortality in patients with heart failure. Open Heart5, e000730. 10.1136/openhrt-2017-000730 (2018). 10.1136/openhrt-2017-000730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandek, A. et al. Intestinal blood flow in patients with chronic heart failure: A link with bacterial growth, gastrointestinal symptoms, and cachexia. J. Am. Coll. Cardiol.64, 1092–1102. 10.1016/j.jacc.2014.06.1179 (2014). 10.1016/j.jacc.2014.06.1179 [DOI] [PubMed] [Google Scholar]
- 5.Sze, S. et al. Prevalence and prognostic significance of malnutrition using 3 scoring systems among outpatients with heart failure: A comparison with body mass index. JACC Heart Fail.6, 476–486. 10.1016/j.jchf.2018.02.018 (2018). 10.1016/j.jchf.2018.02.018 [DOI] [PubMed] [Google Scholar]
- 6.Lv, S. & Ru, S. The prevalence of malnutrition and its effects on the all-cause mortality among patients with heart failure: A systematic review and meta-analysis. PLoS ONE16, e0259300. 10.1371/journal.pone.0259300 (2021). 10.1371/journal.pone.0259300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cederholm, T. et al. Diagnostic criteria for malnutrition—An ESPEN consensus statement. Clin. Nutr.34, 335–340. 10.1016/j.clnu.2015.03.001 (2015). 10.1016/j.clnu.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 8.Bouillanne, O. et al. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr.82, 777–783. 10.1093/ajcn/82.4.777 (2005). 10.1093/ajcn/82.4.777 [DOI] [PubMed] [Google Scholar]
- 9.Rubenstein, L. Z., Harker, J. O., Salva, A., Guigoz, Y. & Vellas, B. Screening for undernutrition in geriatric practice: Developing the short-form mini-nutritional assessment (MNA-SF). J. Gerontol. A Biol. Sci. Med. Sci.56, M366-372. 10.1093/gerona/56.6.m366 (2001). 10.1093/gerona/56.6.m366 [DOI] [PubMed] [Google Scholar]
- 10.Kondrup, J., Rasmussen, H. H., Hamberg, O., Stanga, Z., Ad Hoc, E. W. G. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr.22, 321–336. 10.1016/s0261-5614(02)00214-5 (2003). 10.1016/s0261-5614(02)00214-5 [DOI] [PubMed] [Google Scholar]
- 11.Ignacio de Ulibarri, J. et al. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp.20, 38–45 (2005). [PubMed] [Google Scholar]
- 12.Martin-Sanchez, F. J. et al. Effect of risk of malnutrition on 30-day mortality among older patients with acute heart failure in Emergency Departments. Eur. J. Intern. Med.65, 69–77. 10.1016/j.ejim.2019.04.014 (2019). 10.1016/j.ejim.2019.04.014 [DOI] [PubMed] [Google Scholar]
- 13.Kinugasa, Y. et al. Diagnostic performance of nutritional indicators in patients with heart failure. ESC Heart Fail.9, 2096–2106. 10.1002/ehf2.13886 (2022). 10.1002/ehf2.13886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen, J. B. et al. Clinical phenogroups in heart failure with preserved ejection fraction: Detailed phenotypes, prognosis, and response to spironolactone. JACC Heart Fail.8, 172–184. 10.1016/j.jchf.2019.09.009 (2020). 10.1016/j.jchf.2019.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grover, Z. & Ee, L. C. Protein energy malnutrition. Pediatr. Clin. N. Am.56, 1055–1068. 10.1016/j.pcl.2009.07.001 (2009). 10.1016/j.pcl.2009.07.001 [DOI] [PubMed] [Google Scholar]
- 16.Michael, H., Amimo, J. O., Rajashekara, G., Saif, L. J. & Vlasova, A. N. Mechanisms of kwashiorkor-associated immune suppression: Insights from human, mouse, and pig studies. Front. Immunol.13, 826268. 10.3389/fimmu.2022.826268 (2022). 10.3389/fimmu.2022.826268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer, J. et al. Sarcopenia: A time for action. An SCWD position paper. J. Cachexia Sarcopenia Muscle10, 956–961. 10.1002/jcsm.12483 (2019). 10.1002/jcsm.12483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans, W. J. et al. Cachexia: A new definition. Clin. Nutr.27, 793–799. 10.1016/j.clnu.2008.06.013 (2008). 10.1016/j.clnu.2008.06.013 [DOI] [PubMed] [Google Scholar]
- 19.McDonagh, T. A. et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J.42, 3599–3726. 10.1093/eurheartj/ehab368 (2021). 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 20.Dewan, P. et al. The prevalence and importance of frailty in heart failure with reduced ejection fraction—An analysis of PARADIGM-HF and ATMOSPHERE. Eur. J. Heart Fail.22, 2123–2133. 10.1002/ejhf.1832 (2020). 10.1002/ejhf.1832 [DOI] [PubMed] [Google Scholar]
- 21.Vidan, M. T. et al. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur. J. Heart Fail.18, 869–875. 10.1002/ejhf.518 (2016). 10.1002/ejhf.518 [DOI] [PubMed] [Google Scholar]
- 22.Fulster, S. et al. Muscle wasting in patients with chronic heart failure: Results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur. Heart J.34, 512–519. 10.1093/eurheartj/ehs381 (2013). 10.1093/eurheartj/ehs381 [DOI] [PubMed] [Google Scholar]
- 23.Sato, Y. et al. Prognostic factors in heart failure patients with cardiac cachexia. J. Geriatr. Cardiol.17, 26–34. 10.11909/j.issn.1671-5411.2020.01.008 (2020). 10.11909/j.issn.1671-5411.2020.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsutsui, H. et al. JCS/JHFS 2021 guideline focused update on diagnosis and treatment of acute and chronic heart failure. Circ. J.85, 2252–2291. 10.1253/circj.CJ-21-0431 (2021). 10.1253/circj.CJ-21-0431 [DOI] [PubMed] [Google Scholar]
- 25.Deminice, R. et al. Human and rodent skeletal muscles express angiotensin II type 1 receptors. Cells10.3390/cells9071688 (2020). 10.3390/cells9071688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kingsley, J., Torimoto, K., Hashimoto, T. & Eguchi, S. Angiotensin II inhibition: A potential treatment to slow the progression of sarcopenia. Clin. Sci.135, 2503–2520. 10.1042/CS20210719 (2021). 10.1042/CS20210719 [DOI] [PubMed] [Google Scholar]
- 27.Cabello-Verrugio, C., Cordova, G. & Salas, J. D. Angiotensin II: Role in skeletal muscle atrophy. Curr. Protein Pept. Sci.13, 560–569. 10.2174/138920312803582933 (2012). 10.2174/138920312803582933 [DOI] [PubMed] [Google Scholar]
- 28.Cabello-Verrugio, C., Morales, M. G., Rivera, J. C., Cabrera, D. & Simon, F. Renin-angiotensin system: An old player with novel functions in skeletal muscle. Med. Res. Rev.35, 437–463. 10.1002/med.21343 (2015). 10.1002/med.21343 [DOI] [PubMed] [Google Scholar]
- 29.Powers, S. K., Morton, A. B., Hyatt, H. & Hinkley, M. J. The renin-angiotensin system and skeletal muscle. Exerc. Sport Sci. Rev.46, 205–214. 10.1249/JES.0000000000000158 (2018). 10.1249/JES.0000000000000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida, T. et al. Molecular mechanisms and signaling pathways of angiotensin II-induced muscle wasting: Potential therapeutic targets for cardiac cachexia. Int. J. Biochem. Cell Biol.45, 2322–2332. 10.1016/j.biocel.2013.05.035 (2013). 10.1016/j.biocel.2013.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valdiviesso, R. et al. Statins are associated with reduced likelihood of sarcopenia in a sample of heart failure outpatients: A cross-sectional study. BMC Cardiovasc. Disord.22, 356. 10.1186/s12872-022-02804-5 (2022). 10.1186/s12872-022-02804-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mondal, A. et al. Does statin use in frail patients provide survival benefits? Insights from a meta-analysis. Curr. Probl. Cardiol.49, 102038. 10.1016/j.cpcardiol.2023.102038 (2023). 10.1016/j.cpcardiol.2023.102038 [DOI] [PubMed] [Google Scholar]
- 33.Bielecka-Dabrowa, A. et al. Prosarcopenic effects of statins may limit their effectiveness in patients with heart failure. Trends Pharmacol. Sci.39, 331–353. 10.1016/j.tips.2018.02.003 (2018). 10.1016/j.tips.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 34.Arques, S. & Ambrosi, P. Human serum albumin in the clinical syndrome of heart failure. J. Card. Fail.17, 451–458. 10.1016/j.cardfail.2011.02.010 (2011). 10.1016/j.cardfail.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 35.Bonilla-Palomas, J. L. et al. Hypoalbuminemia in acute heart failure patients: Causes and its impact on hospital and long-term mortality. J. Card. Fail.20, 350–358. 10.1016/j.cardfail.2014.01.016 (2014). 10.1016/j.cardfail.2014.01.016 [DOI] [PubMed] [Google Scholar]
- 36.Horwich, T. B., Kalantar-Zadeh, K., MacLellan, R. W. & Fonarow, G. C. Albumin levels predict survival in patients with systolic heart failure. Am. Heart J.155, 883–889. 10.1016/j.ahj.2007.11.043 (2008). 10.1016/j.ahj.2007.11.043 [DOI] [PubMed] [Google Scholar]
- 37.Uthamalingam, S. et al. Serum albumin and mortality in acutely decompensated heart failure. Am. Heart J.160, 1149–1155. 10.1016/j.ahj.2010.09.004 (2010). 10.1016/j.ahj.2010.09.004 [DOI] [PubMed] [Google Scholar]
- 38.Harjola, V. P. et al. Organ dysfunction, injury and failure in acute heart failure: From pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail.19, 821–836. 10.1002/ejhf.872 (2017). 10.1002/ejhf.872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohara, H. et al. Hepatic venous stasis index reflects hepatic congestion and predicts adverse outcomes in patients with heart failure. J. Am .Heart Assoc.12, e029857. 10.1161/JAHA.122.029857 (2023). 10.1161/JAHA.122.029857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nohria, A. et al. Cardiorenal interactions: Insights from the ESCAPE trial. J. Am. Coll. Cardiol.51, 1268–1274. 10.1016/j.jacc.2007.08.072 (2008). 10.1016/j.jacc.2007.08.072 [DOI] [PubMed] [Google Scholar]
- 41.Kitani, T. et al. Kidney vascular congestion exacerbates acute kidney injury in mice. Kidney Int.101, 551–562. 10.1016/j.kint.2021.11.015 (2022). 10.1016/j.kint.2021.11.015 [DOI] [PubMed] [Google Scholar]
- 42.McCallum, W. et al. Acute kidney function declines in the context of decongestion in acute decompensated heart failure. JACC Heart Fail.8, 537–547. 10.1016/j.jchf.2020.03.009 (2020). 10.1016/j.jchf.2020.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCallum, W. et al. Rates of reversal of volume overload in hospitalized acute heart failure: Association with long-term kidney function. Am. J. Kidney Dis.80, 65–78. 10.1053/j.ajkd.2021.09.026 (2022). 10.1053/j.ajkd.2021.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Testani, J. M., Chen, J., McCauley, B. D., Kimmel, S. E. & Shannon, R. P. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation122, 265–272. 10.1161/CIRCULATIONAHA.109.933275 (2010). 10.1161/CIRCULATIONAHA.109.933275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsutsui, H. et al. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure-digest version. Circ. J.83, 2084–2184. 10.1253/circj.CJ-19-0342 (2019). 10.1253/circj.CJ-19-0342 [DOI] [PubMed] [Google Scholar]
- 46.Ponikowski, P. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J.37, 2129–2200. 10.1093/eurheartj/ehw128 (2016). 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 47.Yancy, C. W. et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol.62, e147-239. 10.1016/j.jacc.2013.05.019 (2013). 10.1016/j.jacc.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 48.Yancy, C. W. et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol.70, 776–803. 10.1016/j.jacc.2017.04.025 (2017). 10.1016/j.jacc.2017.04.025 [DOI] [PubMed] [Google Scholar]
- 49.Oreopoulos, A. et al. Body mass index and mortality in heart failure: A meta-analysis. Am. Heart J.156, 13–22. 10.1016/j.ahj.2008.02.014 (2008). 10.1016/j.ahj.2008.02.014 [DOI] [PubMed] [Google Scholar]
- 50.Takiguchi, M. et al. Impact of body mass index on mortality in heart failure patients. Eur. J. Clin. Investig.44, 1197–1205. 10.1111/eci.12354 (2014). 10.1111/eci.12354 [DOI] [PubMed] [Google Scholar]
- 51.Ancion, A. et al. Serum albumin level and hospital mortality in acute non-ischemic heart failure. ESC Heart Fail.4, 138–145. 10.1002/ehf2.12128 (2017). 10.1002/ehf2.12128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vergaro, G. et al. NT-proBNP for risk prediction in heart failure: Identification of optimal cutoffs across body mass index categories. JACC Heart Fail.9, 653–663. 10.1016/j.jchf.2021.05.014 (2021). 10.1016/j.jchf.2021.05.014 [DOI] [PubMed] [Google Scholar]
- 53.Matsuo, S. et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis.53, 982–992. 10.1053/j.ajkd.2008.12.034 (2009). 10.1053/j.ajkd.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 54.Rickham, P. P. Human experimentation. Code of Ethics of the World Medical Association. Declaration of Helsinki. Br. Med. J.2, 177. 10.1136/bmj.2.5402.177 (1964). 10.1136/bmj.2.5402.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levy, W. C. et al. The Seattle Heart Failure Model: Prediction of survival in heart failure. Circulation113, 1424–1433. 10.1161/CIRCULATIONAHA.105.584102 (2006). 10.1161/CIRCULATIONAHA.105.584102 [DOI] [PubMed] [Google Scholar]
- 56.Shiraishi, Y. et al. Validation and recalibration of Seattle Heart Failure Model in Japanese acute heart failure patients. J. Card. Fail.25, 561–567. 10.1016/j.cardfail.2018.07.463 (2019). 10.1016/j.cardfail.2018.07.463 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.