Abstract
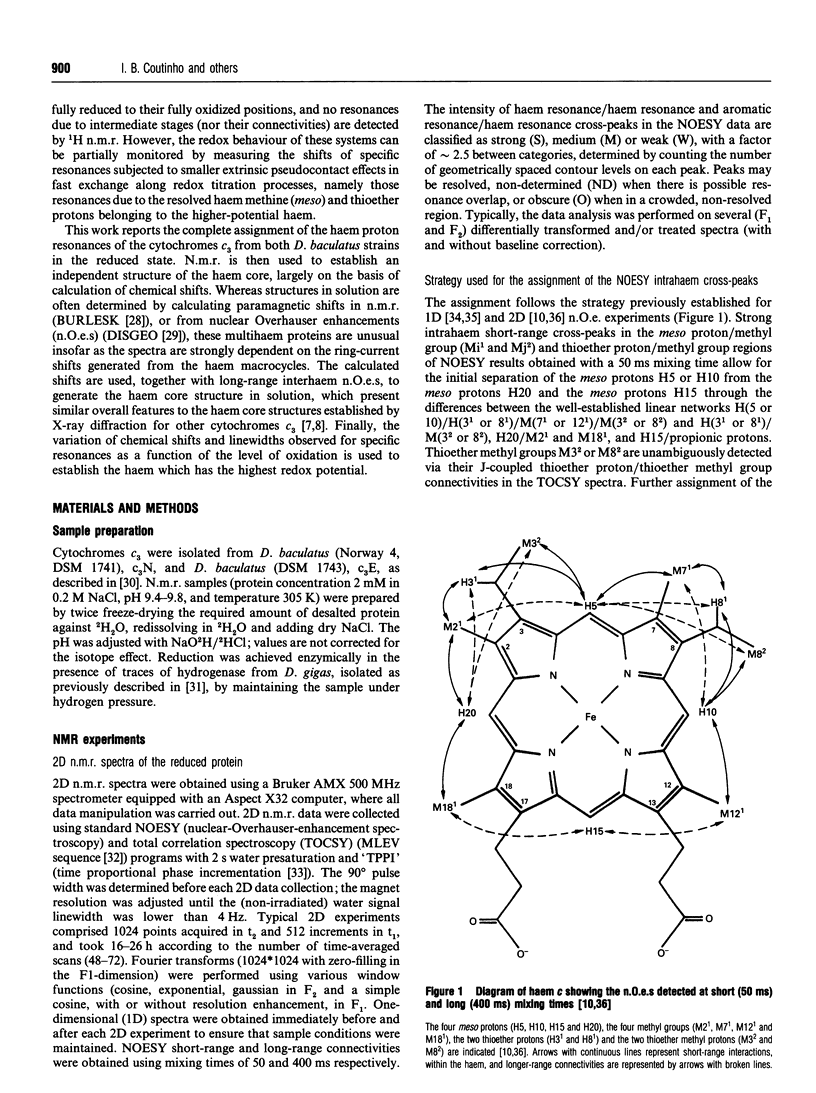
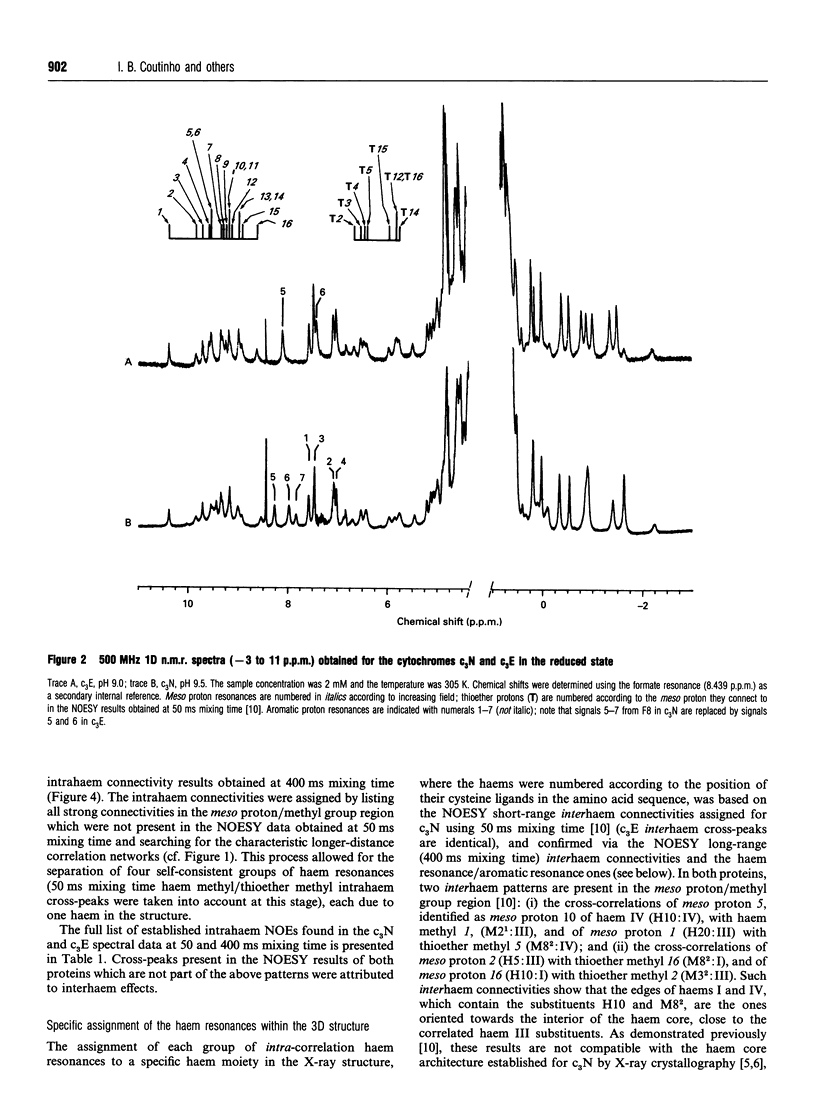
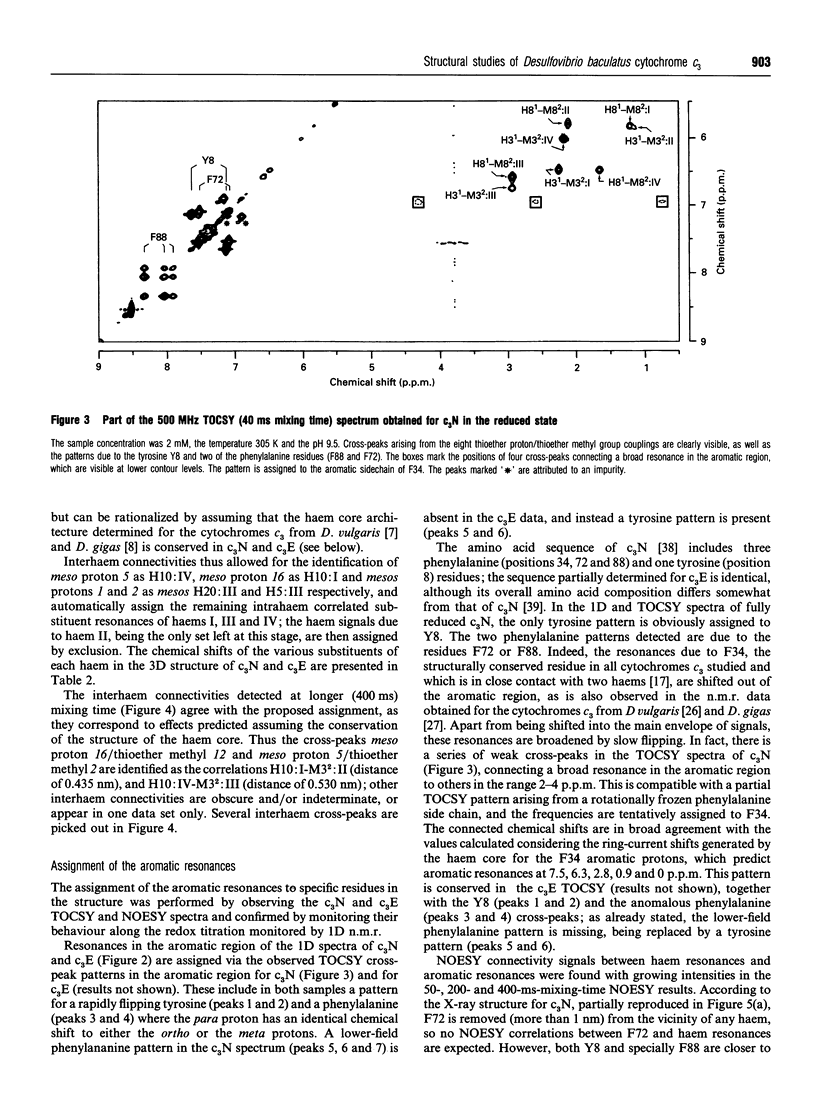
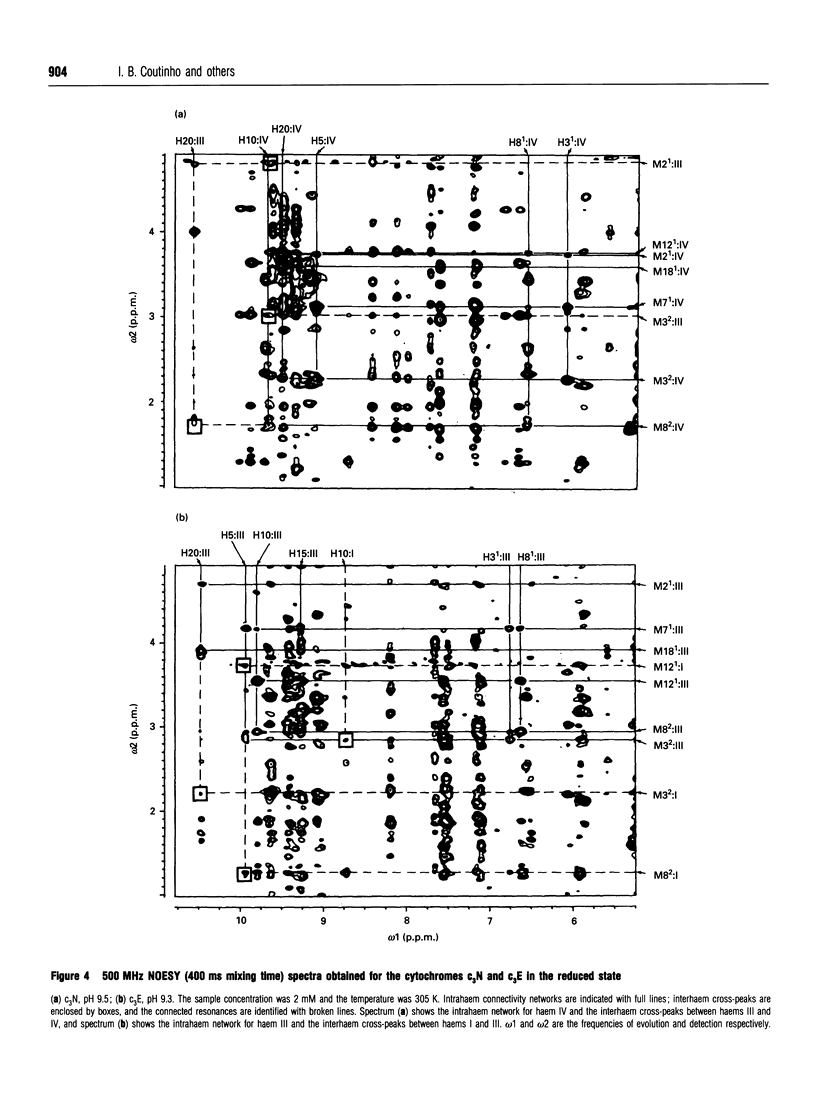
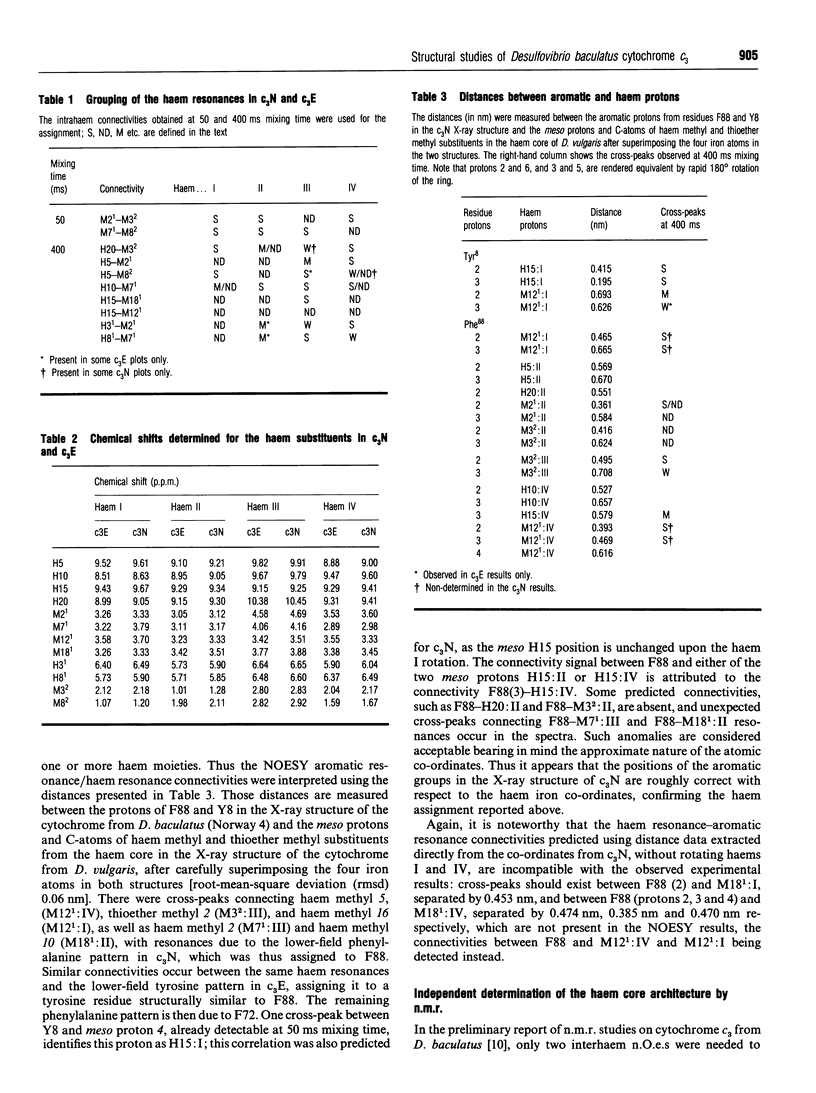
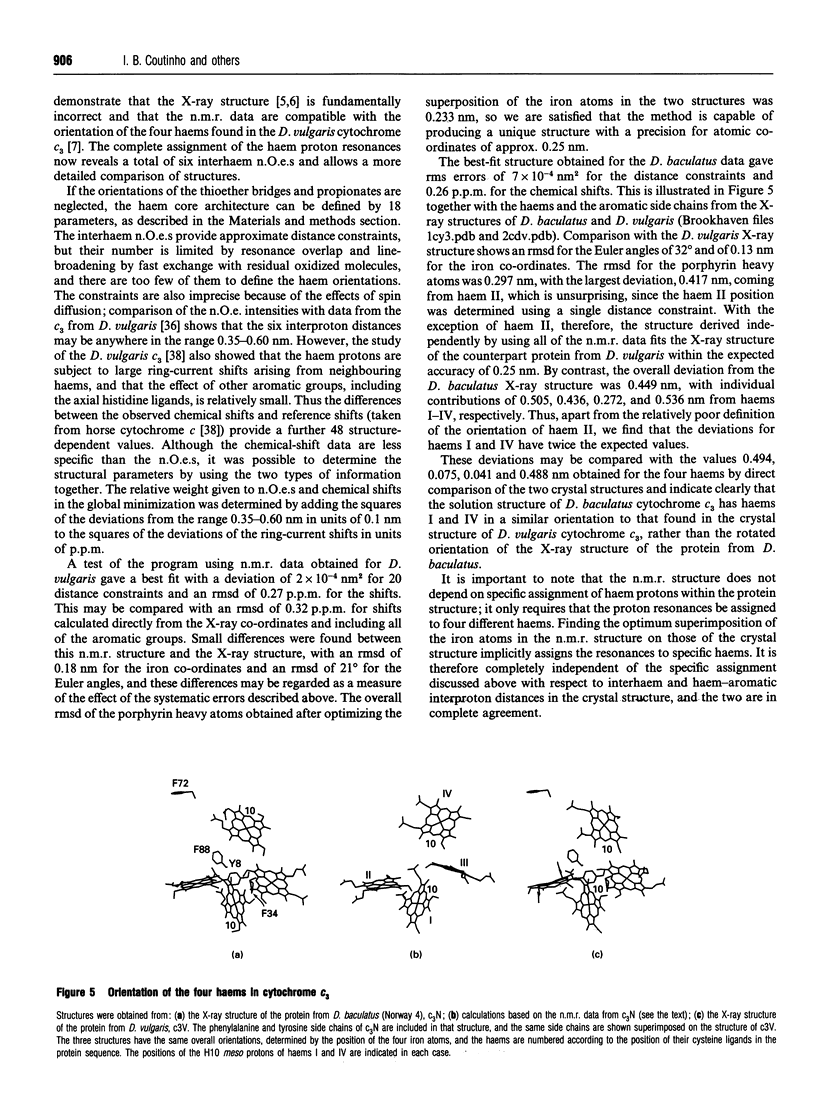
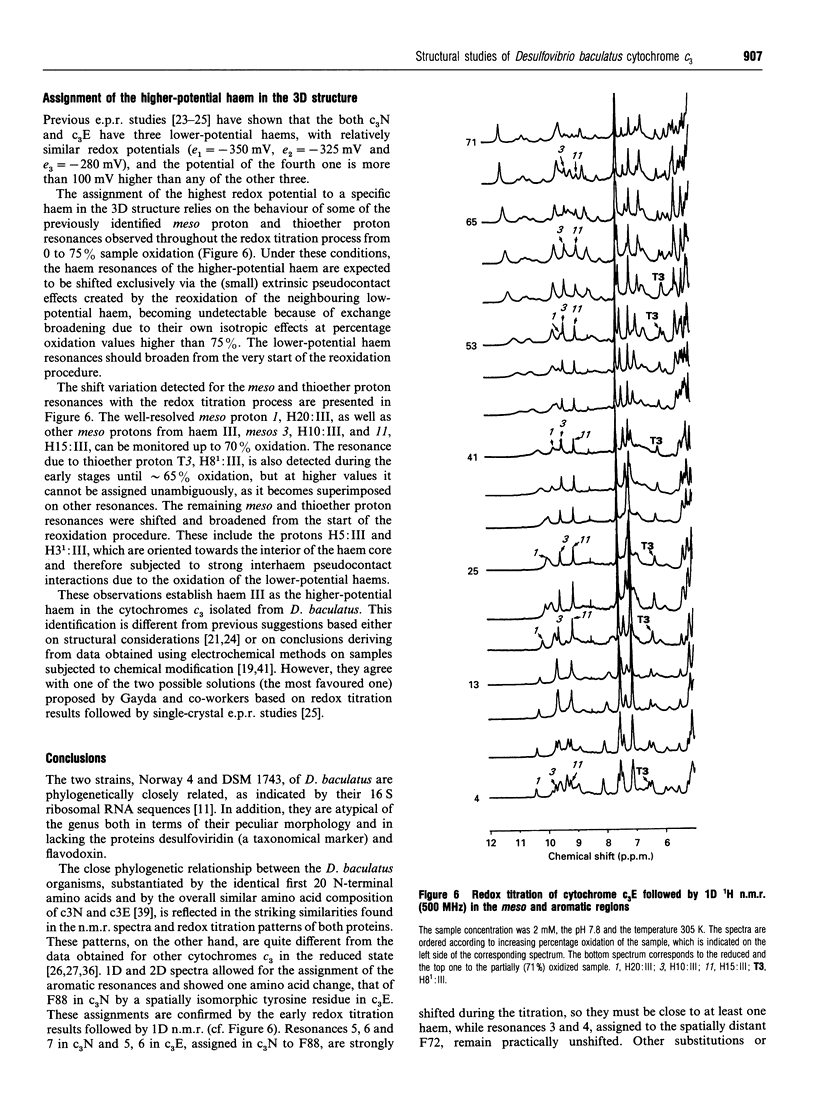
Complete assignment of the aromatic and haem proton resonances in the cytochromes c3 isolated from Desulfovibrio baculatus strains (Norway 4, DSM 1741) and (DSM 1743) was achieved using one- and two-dimensional 1H n.m.r. Nuclear Overhauser enhancements observed between haem and aromatic resonances and between resonances due to different haems, together with the ring-current contributions to the chemical shifts of haem resonances, support the argument that the haem core architecture is conserved in the various cytochromes c3, and that the X-ray structure of the D. baculatus cytochrome c3 is erroneous. The relative orientation of the haems for both cytochromes was determined directly from n.m.r. data. The n.m.r. structures have a resolution of approximately 0.25 nm and are found to be in close agreement with the X-ray structure from D. vulgaris cytochrome c3. The proton assignments were used to relate the highest potential to a specific haem in the three-dimensional structure by monitoring the chemical-shift variation of several haem resonances throughout redox titrations followed by 1H n.m.r. The haem with highest redox potential is not the same as that in other cytochromes c3.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry C. D., North A. C., Glasel J. A., Williams R. J., Xavier A. V. Quantitative determination of mononucleotide conformations in solution using lanthanide ion shift and broadenine NMR probes. Nature. 1971 Jul 23;232(5308):236–245. doi: 10.1038/232236a0. [DOI] [PubMed] [Google Scholar]
- Bruschi M., Hatchikian C. E., Golovleva L. A., Gall J. L. Purification and characterization of cytochrome c3, ferredoxin, and rubredoxin isolated from Desulfovibrio desulfuricans Norway. J Bacteriol. 1977 Jan;129(1):30–38. doi: 10.1128/jb.129.1.30-38.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruschi M., Loutfi M., Bianco P., Haladjian J. Correlations studies between structural and redox properties of cytochromes C3. Biochem Biophys Res Commun. 1984 Apr 30;120(2):384–389. doi: 10.1016/0006-291x(84)91265-8. [DOI] [PubMed] [Google Scholar]
- Coutinho I. B., Turner D. L., Legall J., Xavier A. V. Revision of the haem-core architecture in the tetraheam cytochrome c3 from Desulfovibrio baculatus by two-dimensional 1H NMR. Eur J Biochem. 1992 Oct 1;209(1):329–333. doi: 10.1111/j.1432-1033.1992.tb17293.x. [DOI] [PubMed] [Google Scholar]
- Devereux R., He S. H., Doyle C. L., Orkland S., Stahl D. A., LeGall J., Whitman W. B. Diversity and origin of Desulfovibrio species: phylogenetic definition of a family. J Bacteriol. 1990 Jul;172(7):3609–3619. doi: 10.1128/jb.172.7.3609-3619.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson C. M., Hoyle N. J., Geraldes C. F., Bruschi M., LeGall J., Wright P. E., Williams R. J. Outline structure of cytochrome c3 and consideration of its properties. Nature. 1974 May 31;249(456):425–429. doi: 10.1038/249425a0. [DOI] [PubMed] [Google Scholar]
- Dolla A., Guerlesquin F., Bruschi M., Haser R. Ferredoxin electron transfer site on cytochrome c3. Structural hypothesis of an intramolecular electron transfer pathway within a tetra-heme cytochrome. J Mol Recognit. 1991 Feb;4(1):27–33. doi: 10.1002/jmr.300040105. [DOI] [PubMed] [Google Scholar]
- Dolla A., Leroy G., Guerlesquin F., Bruschi M. Identification of the site of interaction between cytochrome c3 and ferredoxin using peptide mapping of the cross-linked complex. Biochim Biophys Acta. 1991 Jun 17;1058(2):171–177. doi: 10.1016/s0005-2728(05)80234-8. [DOI] [PubMed] [Google Scholar]
- Fan K. J., Akutsu H., Kyogoku Y., Niki K. Estimation of microscopic redox potentials of a tetraheme protein, cytochrome c3 of Desulfovibrio vulgaris, Miyazaki F, and partial assignments of heme groups. Biochemistry. 1990 Mar 6;29(9):2257–2263. doi: 10.1021/bi00461a008. [DOI] [PubMed] [Google Scholar]
- Gayda J. P., Benosman H., Bertrand P., More C., Asso M. EPR determination of interaction redox potentials in a multiheme cytochrome: cytochrome c3 from Desulfovibrio desulfuricans Norway. Eur J Biochem. 1988 Oct 15;177(1):199–206. doi: 10.1111/j.1432-1033.1988.tb14362.x. [DOI] [PubMed] [Google Scholar]
- Guigliarelli B., Bertrand P., More C., Haser R., Gayda J. P. Single-crystal electron paramagnetic resonance study of cytochrome c3 from Desulfovibrio desulfuricans Norway Strain. Assignment of the heme midpoint redox potentials. J Mol Biol. 1990 Nov 5;216(1):161–166. doi: 10.1016/S0022-2836(05)80067-1. [DOI] [PubMed] [Google Scholar]
- Haser R., Pierrot M., Frey M., Payan F., Astier J. P., Bruschi M., Le Gall J. Structure and sequence of the multihaem cytochrome c3. Nature. 1979 Dec 20;282(5741):806–810. doi: 10.1038/282806a0. [DOI] [PubMed] [Google Scholar]
- Higuchi Y., Kusunoki M., Matsuura Y., Yasuoka N., Kakudo M. Refined structure of cytochrome c3 at 1.8 A resolution. J Mol Biol. 1984 Jan 5;172(1):109–139. doi: 10.1016/0022-2836(84)90417-0. [DOI] [PubMed] [Google Scholar]
- Higuchi Y., Kusunoki M., Yasuoka N., Kakudo M., Yagi T. On cytochrome c3 folding. J Biochem. 1981 Dec;90(6):1715–1723. doi: 10.1093/oxfordjournals.jbchem.a133648. [DOI] [PubMed] [Google Scholar]
- Keller R. M., Wüthrich K. Assignment of the heme c resonances in the 360 MHz H NMR spectra of cytochrome c. Biochim Biophys Acta. 1978 Mar 28;533(1):195–208. doi: 10.1016/0005-2795(78)90564-0. [DOI] [PubMed] [Google Scholar]
- LeGall J., Ljungdahl P. O., Moura I., Peck H. D., Jr, Xavier A. V., Moura J. J., Teixera M., Huynh B. H., DerVartanian D. V. The presence of redox-sensitive nickel in the periplasmic hydrogenase from Desulfovibrio gigas. Biochem Biophys Res Commun. 1982 May 31;106(2):610–616. doi: 10.1016/0006-291x(82)91154-8. [DOI] [PubMed] [Google Scholar]
- Moura I., Teixeira M., Huynh B. H., LeGall J., Moura J. J. Assignment of individual heme EPR signals of Desulfovibrio baculatus (strain 9974) tetraheme cytochrome c3. A redox equilibria study. Eur J Biochem. 1988 Sep 15;176(2):365–369. doi: 10.1111/j.1432-1033.1988.tb14290.x. [DOI] [PubMed] [Google Scholar]
- Moura J. J., Santos H., Moura I., LeGall J., Moore G. R., Williams R. J., Xavier A. V. NMR redox studies of Desulfovibrio vulgaris Cytochrome c3. Electron transfer mechanisms. Eur J Biochem. 1982 Sep;127(1):151–155. doi: 10.1111/j.1432-1033.1982.tb06849.x. [DOI] [PubMed] [Google Scholar]
- Park J. S., Kano K., Niki K., Akutsu H. Full assignment of heme redox potentials of cytochrome c3 of D. vulgaris Miyazaki F by 1H-NMR. FEBS Lett. 1991 Jul 8;285(1):149–151. doi: 10.1016/0014-5793(91)80746-p. [DOI] [PubMed] [Google Scholar]
- Pierrot M., Haser R., Frey M., Payan F., Astier J. P. Crystal structure and electron transfer properties of cytochrome c3. J Biol Chem. 1982 Dec 10;257(23):14341–14348. [PubMed] [Google Scholar]
- Piçarra-Pereira M. A., Turner D. L., LeGall J., Xavier A. V. Structural studies on Desulfovibrio gigas cytochrome c3 by two-dimensional 1H-nuclear-magnetic-resonance spectroscopy. Biochem J. 1993 Sep 15;294(Pt 3):909–915. doi: 10.1042/bj2940909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgueiro C. A., Turner D. L., Santos H., LeGall J., Xavier A. V. Assignment of the redox potentials to the four haems in Desulfovibrio vulgaris cytochrome c3 by 2D-NMR. FEBS Lett. 1992 Dec 14;314(2):155–158. doi: 10.1016/0014-5793(92)80963-h. [DOI] [PubMed] [Google Scholar]
- Santos H., Moura J. J., Moura I., LeGall J., Xavier A. V. NMR studies of electron transfer mechanisms in a protein with interacting redox centres: Desulfovibrio gigas cytochrome c3. Eur J Biochem. 1984 Jun 1;141(2):283–296. doi: 10.1111/j.1432-1033.1984.tb08190.x. [DOI] [PubMed] [Google Scholar]
- Senn H., Keller R. M., Wüthrich K. Different chirality of the axial methionine in homologous cytochromes c determined by 1H NMR and CD sectroscopy. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1362–1369. doi: 10.1016/0006-291x(80)90436-2. [DOI] [PubMed] [Google Scholar]
- Turner D. L., Salgueiro C. A., LeGall J., Xavier A. V. Structural studies of Desulfovibrio vulgaris ferrocytochrome c3 by two-dimensional NMR. Eur J Biochem. 1992 Dec 15;210(3):931–936. doi: 10.1111/j.1432-1033.1992.tb17497.x. [DOI] [PubMed] [Google Scholar]


