Abstract
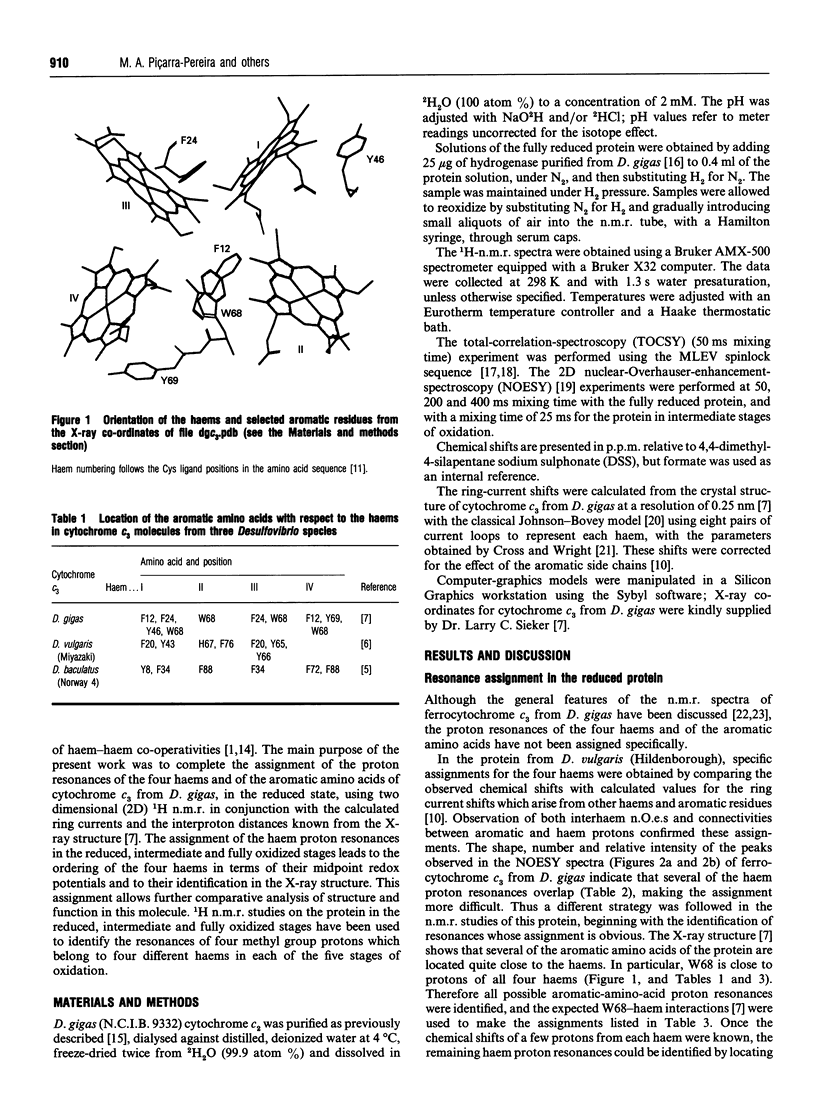
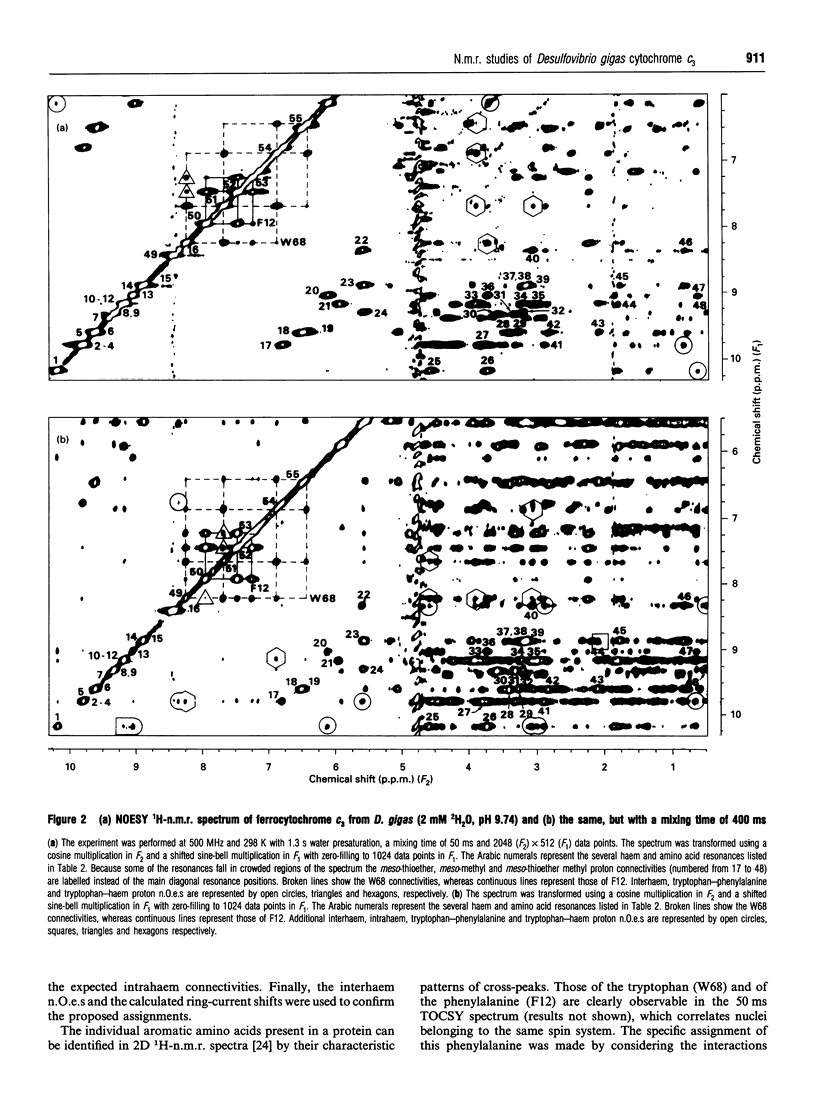
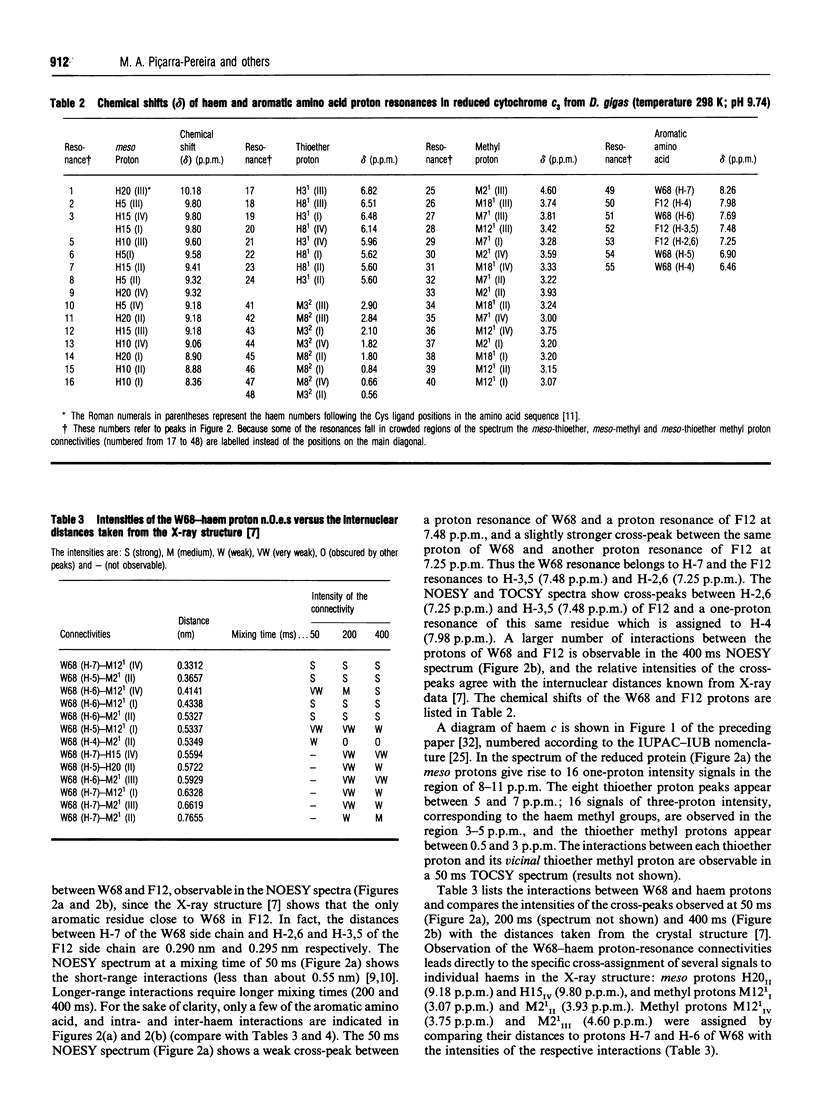
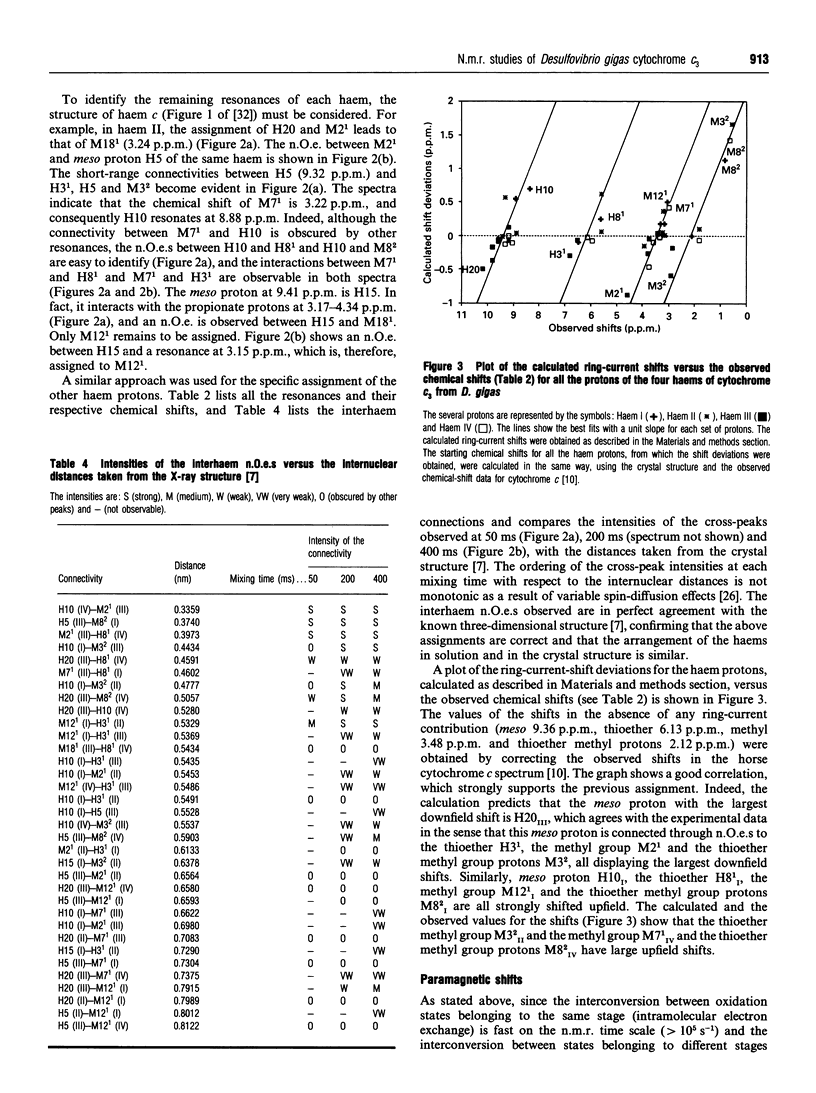
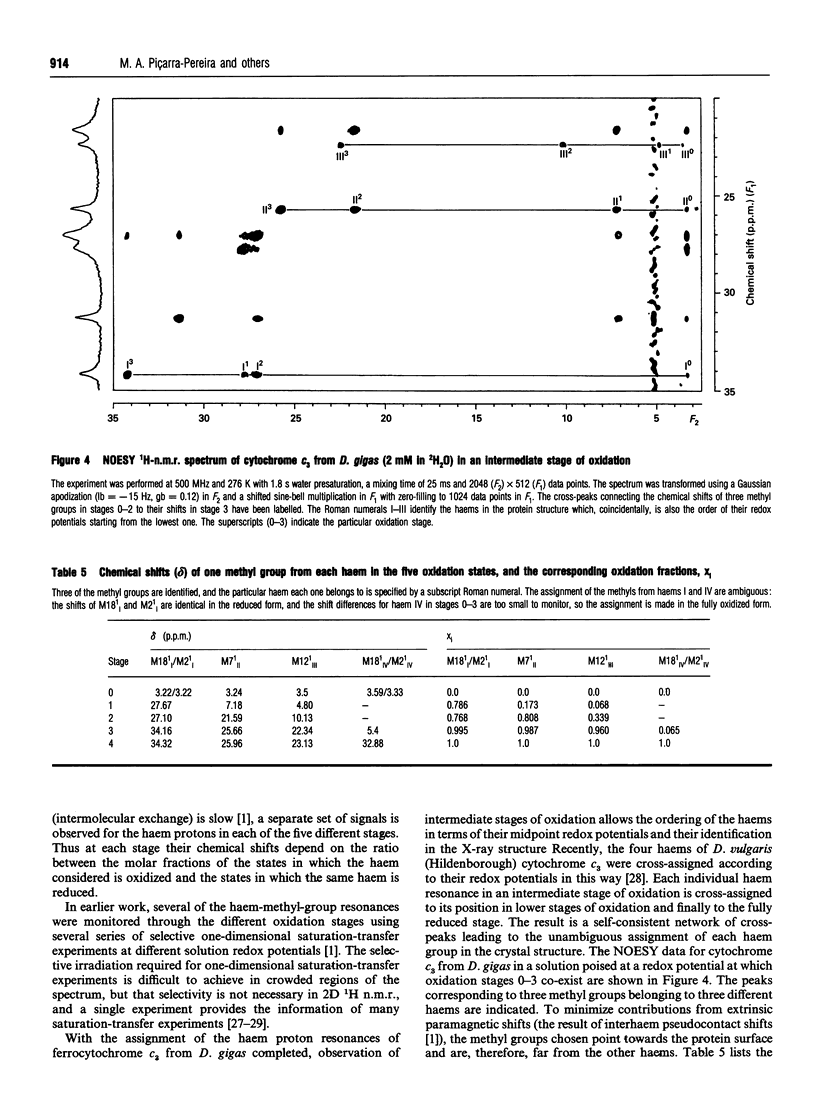
Several aromatic amino acid residues and haem resonances in the fully reduced form of Desulfovibrio gigas cytochrome c3 are assigned, using two-dimensional 1H n.m.r., on the basis of the interactions between the protons of the aromatic amino acids and the haem protons as well as the intrahaem distances known from the X-ray structure [Kissinger (1989) Ph.D. Thesis, Washington State University]. The interhaem interactions observed in the n.m.r. spectra are in full agreement with the D. gigas X-ray structure and also with the n.m.r. data from Desulfovibrio vulgaris (Hildenborough) [Turner, Salgueiro, LeGall and Xavier (1992) Eur. J. Biochem. 210, 931-936]. The good correlation between the calculated ring-current shifts and the observed chemical shifts strongly supports the present assignments. Observation of the two-dimensional nuclear-Overhauser-enhancement spectra of the protein in the reduced, intermediate and fully oxidized stages led to the ordering of the haems in terms of their midpoint redox potentials and their identification in the X-ray structure. The first haem to oxidize is haem I, followed by haems II, III and IV, numbered according to the Cys ligand positions in the amino acid sequences [Mathews (1985) Prog. Biophys. Mol. Biol. 54, 1-56]. Although the haem core architecture is the same for the different Desulfovibrio cytochromes c3, the order of redox potentials is different.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coletta M., Catarino T., LeGall J., Xavier A. V. A thermodynamic model for the cooperative functional properties of the tetraheme cytochrome c3 from Desulfovibrio gigas. Eur J Biochem. 1991 Dec 18;202(3):1101–1106. doi: 10.1111/j.1432-1033.1991.tb16476.x. [DOI] [PubMed] [Google Scholar]
- Coutinho I. B., Turner D. L., LeGall J., Xavier A. V. Characterization of the structure and redox behaviour of cytochrome c3 from Desulfovibrio baculatus by 1H-nuclear-magnetic-resonance spectroscopy. Biochem J. 1993 Sep 15;294(Pt 3):899–908. doi: 10.1042/bj2940899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho I. B., Turner D. L., Legall J., Xavier A. V. Revision of the haem-core architecture in the tetraheam cytochrome c3 from Desulfovibrio baculatus by two-dimensional 1H NMR. Eur J Biochem. 1992 Oct 1;209(1):329–333. doi: 10.1111/j.1432-1033.1992.tb17293.x. [DOI] [PubMed] [Google Scholar]
- Fan K. J., Akutsu H., Kyogoku Y., Niki K. Estimation of microscopic redox potentials of a tetraheme protein, cytochrome c3 of Desulfovibrio vulgaris, Miyazaki F, and partial assignments of heme groups. Biochemistry. 1990 Mar 6;29(9):2257–2263. doi: 10.1021/bi00461a008. [DOI] [PubMed] [Google Scholar]
- Guigliarelli B., Bertrand P., More C., Haser R., Gayda J. P. Single-crystal electron paramagnetic resonance study of cytochrome c3 from Desulfovibrio desulfuricans Norway Strain. Assignment of the heme midpoint redox potentials. J Mol Biol. 1990 Nov 5;216(1):161–166. doi: 10.1016/S0022-2836(05)80067-1. [DOI] [PubMed] [Google Scholar]
- Higuchi Y., Kusunoki M., Matsuura Y., Yasuoka N., Kakudo M. Refined structure of cytochrome c3 at 1.8 A resolution. J Mol Biol. 1984 Jan 5;172(1):109–139. doi: 10.1016/0022-2836(84)90417-0. [DOI] [PubMed] [Google Scholar]
- Higuchi Y., Kusunoki M., Yasuoka N., Kakudo M., Yagi T. On cytochrome c3 folding. J Biochem. 1981 Dec;90(6):1715–1723. doi: 10.1093/oxfordjournals.jbchem.a133648. [DOI] [PubMed] [Google Scholar]
- LEGALL J., MAZZA G., DRAGONI N. LE CYTOCHROME C3 DE DESULFOVIBRIO GIGAS. Biochim Biophys Acta. 1965 May 18;99:385–387. [PubMed] [Google Scholar]
- LeGall J., Ljungdahl P. O., Moura I., Peck H. D., Jr, Xavier A. V., Moura J. J., Teixera M., Huynh B. H., DerVartanian D. V. The presence of redox-sensitive nickel in the periplasmic hydrogenase from Desulfovibrio gigas. Biochem Biophys Res Commun. 1982 May 31;106(2):610–616. doi: 10.1016/0006-291x(82)91154-8. [DOI] [PubMed] [Google Scholar]
- Mathews F. S. The structure, function and evolution of cytochromes. Prog Biophys Mol Biol. 1985;45(1):1–56. doi: 10.1016/0079-6107(85)90004-5. [DOI] [PubMed] [Google Scholar]
- McDonald C. C., Phillips W. D., LeGall J. Proton magnetic resonance studies of Desulfovibrio cytochromes c3. Biochemistry. 1974 Apr 23;13(9):1952–1959. doi: 10.1021/bi00706a027. [DOI] [PubMed] [Google Scholar]
- Morimoto Y., Tani T., Okumura H., Higuchi Y., Yasuoka N. Effects of amino acid substitution on three-dimensional structure: an X-ray analysis of cytochrome c3 from Desulfovibrio vulgaris Hildenborough at 2 A resolution. J Biochem. 1991 Oct;110(4):532–540. doi: 10.1093/oxfordjournals.jbchem.a123615. [DOI] [PubMed] [Google Scholar]
- Moss G. P. Nomenclature of tetrapyrroles. Recommendations 1986 IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Eur J Biochem. 1988 Dec 15;178(2):277–328. doi: 10.1111/j.1432-1033.1988.tb14453.x. [DOI] [PubMed] [Google Scholar]
- Moura J. J., Xavier A. V., Cookson D. J., Moore G. R., Williams R. J. Redox states of cytochrome c3 in the absence and presence of ferredoxin. FEBS Lett. 1977 Sep 15;81(2):275–280. doi: 10.1016/0014-5793(77)80534-6. [DOI] [PubMed] [Google Scholar]
- Odom J. M., Peck H. D., Jr Hydrogenase, electron-transfer proteins, and energy coupling in the sulfate-reducing bacteria Desulfovibrio. Annu Rev Microbiol. 1984;38:551–592. doi: 10.1146/annurev.mi.38.100184.003003. [DOI] [PubMed] [Google Scholar]
- Pierrot M., Haser R., Frey M., Payan F., Astier J. P. Crystal structure and electron transfer properties of cytochrome c3. J Biol Chem. 1982 Dec 10;257(23):14341–14348. [PubMed] [Google Scholar]
- Salgueiro C. A., Turner D. L., Santos H., LeGall J., Xavier A. V. Assignment of the redox potentials to the four haems in Desulfovibrio vulgaris cytochrome c3 by 2D-NMR. FEBS Lett. 1992 Dec 14;314(2):155–158. doi: 10.1016/0014-5793(92)80963-h. [DOI] [PubMed] [Google Scholar]
- Santos H., Moura J. J., Moura I., LeGall J., Xavier A. V. NMR studies of electron transfer mechanisms in a protein with interacting redox centres: Desulfovibrio gigas cytochrome c3. Eur J Biochem. 1984 Jun 1;141(2):283–296. doi: 10.1111/j.1432-1033.1984.tb08190.x. [DOI] [PubMed] [Google Scholar]
- Turner D. L., Salgueiro C. A., LeGall J., Xavier A. V. Structural studies of Desulfovibrio vulgaris ferrocytochrome c3 by two-dimensional NMR. Eur J Biochem. 1992 Dec 15;210(3):931–936. doi: 10.1111/j.1432-1033.1992.tb17497.x. [DOI] [PubMed] [Google Scholar]


