Abstract
The benefits of regular physical exercise on cancer prevention, as well as reducing fatigue, treatment side effects and recurrence, and improving quality of life and overall survival of cancer patients, are increasingly recognised. Initial studies showed that the concentration of extracellular vesicles (EVs) increases during physical activity and that EVs carry biologically active cargo. These EVs are released by blood cells, skeletal muscle and other organs involved in exercise, thus suggesting that EVs may mediate tissue crosstalk during exercise. This possibility triggered a great interest in the study of the roles of EVs in systemic adaptation to exercise and in their potential applications in the prevention and treatment of various diseases, including cancer. This review presents studies exploring the concentration and molecular cargo of EVs released during exercise. Furthermore, we discuss putative stimuli that may trigger EV release from various cell types, the biological functions and the impact of exercise‐induced EVs on cancer development and progression. Understanding the interplay between exercise, EVs, and cancer biology may offer insights into novel therapeutic strategies and preventive measures for cancer.
Keywords: cancer, EV protein cargo, EV RNA cargo, exercise‐induced extracellular vesicles, muscle‐derived EVs, physical exercise
1. INTRODUCTION
Regular physical activity is associated with a wide range of health benefits, including a reduced risk of cancer. Numerous epidemiological studies have provided evidence supporting the link between higher physical activity levels and a lower incidence of multiple cancer types (Kerr et al., 2017). One of the most extensive meta‐analyses published so far pooled data from 12 prospective cohorts including 1.44 million participants, 186,932 of whom were diagnosed with cancer during the follow‐up, and assessed the association of leisure‐time physical activity with the incidence of 26 common cancer types (Moore et al., 2016). High versus low levels (the 90th percentile compared with the 10th percentile) of physical activity were associated with lower risk of 13 cancer types with hazard ratios (HR) ranging from 0.58 for oesophageal adenocarcinoma to 0.90 for breast cancer. On the contrary, the incidence of malignant melanoma and prostate cancer was increased in individuals with higher levels of physical activity (HR of 1.27 and 1.05, respectively). The increased melanoma risk is likely due to higher exposure to solar UV radiation in physically active persons, whereas the reasons for increased prostate cancer risk are not entirely clear (Moore et al., 2016). Many cancer types, such as breast, colon, gastric, liver, endometrial, head and neck cancer and oesophageal adenocarcinoma (Diao et al., 2023; Matthews et al., 2020) showed a linear dose‐response association indicating that higher levels of physical activity are consistently associated with lower risks. Whereas the dose‐response curve appears to be non‐linear or even U‐shaped for some other types, such as lung cancer (Diao et al., 2023) and myeloma (Matthews et al., 2020), showing that higher activity levels than the recommended (7.5–15 MET hours per week) do not have additional benefit.
Although physicians rarely consider physical exercise as a part of cancer treatment, an increasing number of exercise intervention trials demonstrate that supervised exercise is safe and feasible even for patients with bone metastases (Engle et al., 2024; Singh et al., 2020). Moreover, higher post‐diagnosis physical activity reduces fatigue, treatment side effects, anxiety and depression thus leading to improved quality of life (QoL), as well as decreases recurrence rate and improves overall survival in cancer patients (Kraschnewski & Schmitz, 2017; Morishita et al., 2020; Schmidt et al., 2015).
Experimental animal studies provided further evidence of the beneficial effects of exercise both for the reduction of cancer risk and delaying the progression of cancer, while also offering some insights into the underlying mechanisms. Several large‐scale meta‐analyses pooling data from several thousands of rodents consistently reported that physical exercise leads to a reduction of cancer incidence, a decrease in tumour size, and a prolonged life span of animals (Eschke et al., 2019; Figueira et al., 2018; Li et al., 2022). Notably, the beneficial effects of exercise on tumour growth in preclinical models manifested across various training modalities, such as high‐intensity aerobic workouts (Jee et al., 2022), endurance training (Hagar et al., 2019), and resistance exercise (Padilha et al., 2019). Independent studies have also reported exercise effects on the tumour microenvironment, including vasculature normalisation (Jones et al., 2010), NK cell mobilisation and trafficking (Pedersen et al., 2016) and reduction of infiltrating immunosuppressive cells (Hagar et al., 2019; Jee et al., 2022; Wennerberg et al., 2020). Moreover, exercise was shown to be a favourable factor for reverting cancer‐induced cardiac remodelling (Padrão et al., 2018) and countering cancer‐related cognitive impairments on the structural and functional level in rodents (Zimmer et al., 2016).
At least partially, these effects are mediated by bioactive molecules secreted into the circulation during the exercise. These molecules can be secreted in a soluble form or packaged into carriers such as extracellular vesicles (EVs). The term “EVs” refers to nano‐ to micro‐sized particles released from cells, delimited by a lipid bilayer, and that cannot replicate on their own (Welsh et al., 2024). It comprises a wide variety of vesicles that differ in their mode of biogenesis, size, molecular content, membrane composition and specific functions. EVs are released by virtually all cell types in the body; hence the blood and other biofluids contain a mixture of various types of EVs originating from multiple cell types (Murillo et al., 2019; Yáñez‐Mó et al., 2015; Zonneveld et al., 2021). In addition, a variety of non‐vesicular extracellular particles (NVEPs) such as lipoprotein particles, ribonucleoprotein particles, exomeres and supermeres exist in the circulation and are often co‐isolated with EVs (Zhang et al., 2023). EVs as well as NVEPs interact with the recipient cells and mediate intercellular communication. They transfer proteins, lipids, metabolites, mRNAs, various non‐coding RNAs and even DNA fragments between cells, thus changing the functions and phenotypes of recipient cells (Yáñez‐Mó et al., 2015).
Several recent studies have shown that the concentrations of circulating EVs rapidly increase during physical exercise (Frühbeis et al., 2015; Warnier et al., 2022; Whitham et al., 2018), thus raising a great interest in their roles in systemic adaptation to exercise and disease prevention. In the past few years, a significant amount of data has accumulated regarding the molecular cargo and biological functions of EVs released during physical exercise (exercise‐induced EVs). This review aims to summarise these data and consolidate the current understanding of the relationship between the molecular cargo of exercise‐induced EVs and their impact on cancer development and progression.
2. RELEASE OF EVS DURING PHYSICAL EXERCISE
2.1. Changes in circulating EV concentration during exercise
Studies reporting circulating EV concentrations before and after physical exercise in humans and experimental animals are summarised in Table 1. Most of the studies analysing the acute responses to high‐intensity exercise, such as cycling or running to exhaustion, reported an increase in EV levels in the blood (Annibalini et al., 2019; Brahmer et al., 2019; Frühbeis et al., 2015; Warnier et al., 2022; Whitham et al., 2018). However, the response seems to be transient as EV levels typically normalise shortly after cessation of exercise (Frühbeis et al., 2015; Whitham et al., 2018). In contrast, moderate‐intensity exercise or resistance training studies have presented mixed results. Some studies report a significant elevation (Bei et al., 2017; Dimassi et al., 2018; Ma et al., 2018; Mohammad et al., 2021; Nair et al., 2020; Oliveira et al., 2018), while others have found no change (Barcellos et al., 2020; Hou et al., 2019; Just et al., 2020; Lovett et al., 2018; Xiang et al., 2020) or even a decrease (Rigamonti et al., 2020) in EV levels after exercise. These findings suggest that intense physical activity could be a potent stimulator of EV release and the degree of EV increase might be proportional to the exercise intensity. This idea is supported by a study demonstrating that the levels of endothelial cell‐derived EVs increase in an exercise dose‐dependent manner in mice (Ma et al., 2018). In addition, it has been shown that exhaustive, but not moderate exercise, induces apoptosis in peripheral blood lymphocytes (Mooren et al., 2002). It would therefore be interesting to investigate if this leads to an increase in apoptotic bodies that could contribute to higher EV levels. The composition of these apoptosis‐related EVs can be expected to be different from other released EVs (Battistelli & Falcieri, 2020). Furthermore, variables such as the type of exercise and the timing of sample collection seem to influence EV counts across studies, suggesting that different types, intensities, and durations of exercise may affect the kinetics of EV release from various cell types or their clearance from circulation in various ways. Interestingly, sex‐specific differences in the EV size and marker profile have also been found in response to resistance exercise (Conkright et al., 2022).
TABLE 1.
Studies investigating changes in EV concentration during exercise.
| No. | Effect on EV concentration | Methods of EV isolation | Methods of EV quantification | Type of exercise | Time of blood collection | Species | Gender | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Cycling: Sharp ↑, normalises within 90 min#Running: moderate, prolonged ↑ | Differential UC | WB, NTA | Acute, high intensity: cycling, running until exhaustion | Pre‐ex, immediately post‐ex, 90 min post‐ex | Human | Male | Frühbeis et al. (2015) |
| 2.1 | Human: ↑ of plasma EVs | No pre‐isolation of EVs | Nano‐FCM | Acute, moderate to high intensity: stress test | Rest, peak, 15 min post‐ex | Human | Male & Female | Bei et al. (2017) |
| 2.2 | Mouse: 1.85‐fold ↑ in baseline serum EV concentration | ExoQuick™ | TEM, AFM, NTA, WB | Regular, moderate to high‐intensity: swimming | Pre‐ex, 3 weeks of regular exercise | Mouse | Male | |
| 3 | Post‐exercise: ↑ of small EVs | UC | NTA, Cryo‐TEM | Acute, high‐intensity cycling | Baseline, +60 min post‐ex, +4 h post‐ex | Human | Male | Whitham et al. (2018) |
| 4 | No change in EV concentration | SEC | NTA, TEM | Acute, mixed intensity: plyometric jumping, downhill running | Pre‐ex, +2 h, +24 h post‐ex | Human | Male | Lovett et al. (2018) |
| 5 | Post‐ex: ↑ circulating EV level | UC | FC | Regular, high intensity: aerobic exercise | Pre‐ex, 2h post‐ex | Human | Female | Dimassi et al. (2018) |
| 6 | Exercise intensity influences the rise in circulating EPC‐derived EVs and miR‐126 levels | UC | NTA, WB, qPCR | Regular, low and moderate intensity: treadmill running | Pre‐ex and 24 h post‐ex | Mouse | ND | Ma et al. (2018) |
| 7.1 | No change in EV concentration | ExoQuick™ | TEM, WB, NTA | Regular, moderate intensity: swimming | Pre‐ex and 24 h post‐ex | Rat | Male | Hou et al. (2019) |
| 7.2 | No change in EV concentration | Regular, high‐intensity: team‐based rowing | Human | Male | ||||
| 8 | Post‐ex: circulating EVs ↑ 2‐fold | UC | NTA | Acute, high intensity: flywheel resistance training | Pre‐ex, +2 h post‐ex | Human | Male | Annibalini et al. (2019) |
| 9 | NTA: No change in total EV concentration#WB: ↑ of EV‐associated CD9, CD63 and CD81#MACSPlex: ↑ of leukocyte, endothelial cell and platelet‐derived EVs | SEC, immuno‐isolation | NTA, WB, MACSPlex, EV Array | Incremental cycling test until exhaustion | Pre‐ex, during and immediately post‐ex | Human | Male | Brahmer et al. (2019) |
| 10 | No change in EV concentration | Polymer‐based precipitation and SEC | NTA, TEM, WB | Acute, moderate intensity with blood flow restriction | Pre‐ex, +1 h post‐ex | Human | Male | Just et al. (2020) |
| 11 | No change in EV concentration | ExoQuick™ | BCA (protein levels), TEM, WB | Acute, moderate intensity: treadmill running | Not described | Mice | Male | Xiang et al. (2020) |
| 12 | Post‐ex: EVs ↓ significantly | UC | NTA, FC | Acute, moderate intensity: exercise at moderate constant workload | Pre‐ex, immediately post‐ex, +3 h, next day | Human | Male & Female | Rigamonti et al. (2020) |
| 13 | No change in EV concentration | ExoQuick™ | NTA, ELISA, AChE | Regular, varied types/intensities: aerobic, acrobatic, resistance, combined | Pre‐ex; +1 h post‐ex | Rat | Male | Barcellos et al. (2020) |
| 14 | Post‐ex: small EVs ↑ significantly | UC | WB, NTA, TEM | Acute, moderate intensity: treadmill walking | Pre‐ex, post‐snack, post‐ex | Human | Female | Mohammad et al. (2021) |
| 15 | Post‐ex: serum EVs ↑ significantly | ExoQuick™ | IEM, RPST, WB | Acute, varied intensity: treadmill exercise | Pre‐ex, post‐ex | Rat | ND | Oliveira et al. (2018) |
| 16 | During exercise: EVs ↑ 313% at 30min, ↓ 53% at 60min (NTA)#Post‐ex: ↑ of TSG101, CD81, HSP60 | SEC, UC | NTA, WB | Acute, high‐intensity: cycling | Pre, during, post‐ex | Human | Male | Warnier et al. (2022) |
| 17 | Resistance exercise: ↑ CD63+ EVs in both sexes; men: ↑ small EVs; women: ↑ SGCA+ EVs | SEC | FC | Acute heavy resistance exercise | Pre‐ex, immediately post‐ex | Human | Male & Female | Conkright et al. (2022) |
| 18 | No change in total EV concentration. Increase in CD81+/SGCA+/CD45− EVs in response to AAE | SEC, UC | NTA, WB, FC | Different exercise protocols (mode, intensity, altitude) | Pre‐ex, immediately post‐ex, +1/2/6/24 h | Human | Male & Female | Maggio et al. (2023) |
| 19 | Post‐ex: ↑ of CD9+, CD63+, and CD81+ EVs | No pre‐isolation of EVs | Single EV particle analysis (Exoview) | High‐intensity intermittent training | Pre‐ex, immediately post‐ex | Human | Male & Female | McIlvenna et al. (2023) |
Abbreviations: AAE, acute aerobic exercise; AChE, acetylcholinesterase; AFM, atomic force microscopy; EPC, endothelial progenitor cell; FC, flow cytometry; IEM, immunoelectron microscopy; ND, Not described; NTA, nanoparticle tracking analysis; post‐ex, post‐exercise; pre‐ex, pre‐exercise; qPCR, quantitative PCR; RNA‐Seq, RNA sequencing analysis; RPST, resistive pulse sensing technology; SEC, size exclusion chromatography; TEM, transmission electron microscopy; UC, ultracentrifugation; WB, Western blot.
It is important to mention that the discrepancies among the studies may also arise from the variations in the methodological approaches for EV isolation and quantification. In the majority of studies, ultracentrifugation (UC), size‐exclusion chromatography (SEC) or precipitation‐based methods such as ExoQuick have been used to isolate EVs. These methods differ significantly in their efficiency, purity and co‐isolation of non‐EV components that subsequently may affect the measurements of EV concentrations (Coumans et al., 2017). For example, nanoparticle tracking analysis (NTA) results can be strongly affected by the presence of lipoprotein particles (Brahmer et al., 2019). Hence, the methods allowing the quantification of EVs directly in the biofluid without the pre‐isolation of EVs, such as nano‐FCM (Bei et al., 2017) or Exoview (McIlvenna et al., 2023) are likely to provide less biased and more accurate EV counts.
2.2. Cell types and stimuli for EV release
It seems plausible that multiple tissues involved in or responding to exercise release their EVs into the bloodstream during exercise. Given that skeletal muscle accounts for approximately 40% of total body weight and secretes a wide variety of biologically active signalling molecules, it could be expected that a substantial fraction of exercise‐induced EVs are derived from skeletal muscle. Skeletal muscle indeed produces EVs that are enriched in muscle‐specific proteins such as alpha‐sarcoglycan (SGCA) (Guescini et al., 2015), ATP2A1, β‐enolase, and desmin (Watanabe et al., 2022). However, the majority of muscle‐derived EVs accumulate in the muscle interstitium and only a minor fraction of them are released in the bloodstream, where they account for only 1%–5% of the total EVs (Guescini et al., 2015; Pinto et al., 2024; Watanabe et al., 2022). Although the levels of SGCA+ EVs were increased in post‐exercise blood samples (Maggio et al., 2023; Rigamonti et al., 2020), they are unlikely to be the major component of the pool of exercise‐induced EVs. The first and so far the only study providing a detailed phenotyping analysis of EVs released during vigorous exercise was published by Brahmer et al. in 2019. In this study, a multiplexed flow‐cytometry analysis demonstrated a significant increase of lymphocyte (CD4, CD8), monocyte (CD14), platelet (CD62P, CD41b and CD42a), endothelial cell (CD105, CD146) and antigen‐presenting cell (MHC‐II) markers, suggesting that these cell types may be the main contributors to the increase in circulating EV levels during and immediately after exercise (Brahmer et al., 2019). However, this study was limited to 37 EV surface markers and, currently, it is not known what fraction of the total EV pool these markers capture. Furthermore, it is not known to what extent these results can be generalised to other types, intensities and durations of exercise.
The molecular mechanisms responsible for the induction of EV release during exercise are poorly understood so far. One of the putative mechanisms could be lymphocyte mobilisation into the bloodstream—a well‐known phenomenon that happens during acute dynamic exercises such as cycling or running. It is driven by increased blood pressure and shear forces, and adrenaline stimulation, which cause detachment and recirculation of lymphocytes (mainly NK and T cells) from the vascular and tissue reservoirs such as the lung, liver, bone marrow, lymph nodes and spleen into the bloodstream resulting in up to 10‐fold increase in some cell subsets (reviewed in Fiuza‐Luces et al., 2024). Hence, lymphocytosis per se could be the cause of increased EV levels.
Furthermore, the release of EVs from various cell types could be stimulated by biomechanical forces (reviewed in Thompson & Papoutsakis, 2023). Exercise exposes cells to mechanical forces such as shear force, tension, and compression, impacting EV biogenesis and release. Several studies indicate that shear forces increase EV yield from various cell types like platelets, chondrocytes, osteocytes, and mesenchymal stem cells (MSCs). Similarly, cyclic tension or compression has been shown to boost EV release from cells like bronchial epithelial cells, fibroblasts, endothelial cells, ligament cells, chondrocytes, and skeletal muscle cells. However, the response of endothelial cells to shear forces may vary (Thompson & Papoutsakis, 2023). Hence, it seems reasonable to assume that different modes and intensities of exercise expose cells to distinct types and magnitudes of biomechanical forces, leading to varied EV release rates from various parental cells.
The EV release rate can also be stimulated by increased intracellular Ca2+ concentration which is a potent stimulus of plasma membrane EV biogenesis in a variety of cell types, including muscle cells (Taylor et al., 2020; Yamaguchi et al., 2023). In addition, an acidic environment generated by increased lactate production (Cairns, 2006; Federici et al., 2014) and reactive oxygen species (ROS) production by the mitochondrial electron transport chain in muscle cells may also contribute to increased EV release (Nørgård et al., 2023) (Figure 1).
FIGURE 1.
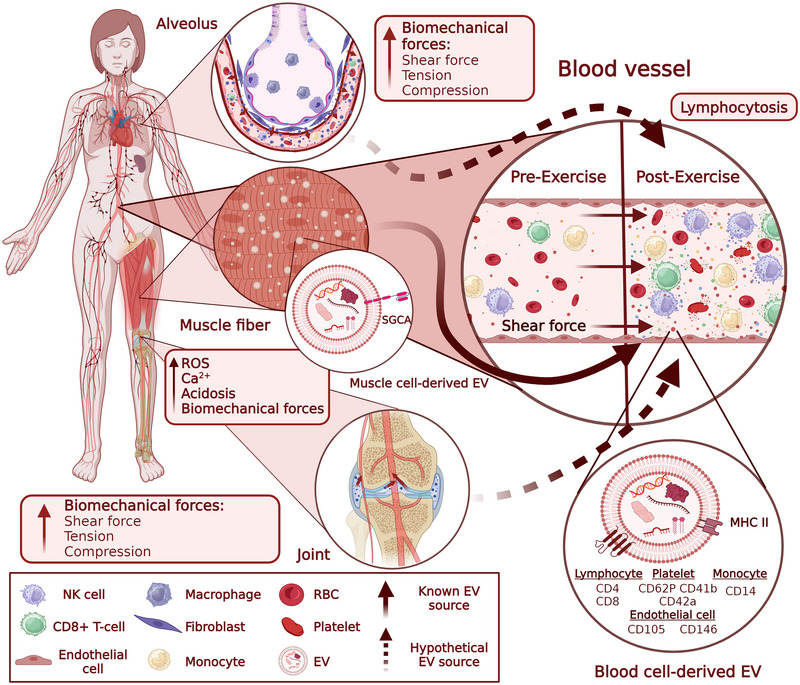
Parental cell types and potential stimuli that trigger EV release during physical exercise. Abbreviations: SGCA, alpha‐sarcoglycan; NK cell, natural killer cells; MHC, Major histocompatibility complex; RBC, red blood cells; ROS, reactive oxygen species.
2.3. Molecular cargo of exercise‐induced EVs
The molecular cargo of EVs consists of proteins, lipids, carbohydrates, metabolites and different types of nucleic acids. Several molecules are commonly found in EVs and are used as markers of these vesicles, such as the tetraspanins CD63, CD9 and CD81 (Welsh et al., 2024). However, the molecular composition of EVs largely reflects the composition of the cells from where they originate, and changes in response to different physiological and pathological conditions, such as cancer, neurological diseases or cardiovascular diseases (Hill, 2019; Kosaka et al., 2019; Saheera et al., 2021; Yáñez‐Mó et al., 2015). Interestingly, increasing evidence shows that physical exercise/activity affects the molecular composition of EVs.
The first studies aiming to identify the molecular cargo of exercise‐induced EVs date from approximately 10 years ago (Frühbeis et al., 2015; Guescini et al., 2015), and numerous studies have been published since then. This review does not intend to describe in detail the molecules that are affected by exercise, methodological limitations of EV isolation and quantification and/or to compare the different studies, because this has been done in several excellent reviews (Brahmer et al., 2020; Darragh et al., 2021). Here, we present the experimental approaches that have been used to study exercise‐induced EVs to identify gaps and/or future directions that would allow a better understanding of how the molecular composition of EVs is affected by exercise.
EV cargo in the context of exercise has been studied in both humans and animal models (mainly mice and rats). Tables 2 and 3 include information about human studies and show that most of the studies have been performed in healthy males. To the best of our knowledge, exercise‐induced changes in the EV cargo have not been investigated in cancer patients.
TABLE 2.
Studies investigating the nucleic acid cargo of exercise‐induced EVs.
| EV isolation | EV analysis | Type of exercise | Time of EV collection | EV source | Health status | Cohort | Gender | Molecular changes | EV term | References |
|---|---|---|---|---|---|---|---|---|---|---|
| DG | FC, WB | A 40‐min acute aerobic exercise session on a treadmill set at a 1% grade | At rest 1 h before and 1 h after the end of the exercise | Plasma | Healthy | 18 sedentary and physically fit (mean age 26) | Male | miR‐133b (muscle related) and miR‐181a‐5p were up‐regulated in EVs after exercise | EVs | Guescini et al. (2015) |
| SEC | NTA, EM | 2 consecutive bouts of muscle‐damaging exercise: combination of plyometric jumping and downhill running | At baseline, and at 2 h and 24 h after exercise. | Plasma | Healthy | 9 untrained (age range 18–30) | Male | miR‐31 (skeletal muscle‐related) decreased from baseline to 24 h post‐exercise | EVs | Lovett et al. (2018) |
| Sequential centrifugation, max. speed 20,000 × g | FC | High‐intensity interval aerobic exercise 3 times a week for 8 weeks | Before and after a 8‐week aerobic training period | Plasma | Healthy | 15 sedentary with normal weight (age 24.3 ± 1.5) or overweight (age 21.9 ± 0.9) | Female | After exercise the expression of:—miR‐150 and miR‐146a (monocyte/macrophage‐related) and of miR‐21 (endothelial‐related) were increased in both groups.—The expression of miR‐124a (monocyte/macrophage‐related) and miR‐320a (vessel‐linked related) was increased in controls.—miR‐223 (platelet‐related) was increased for subjects with obesity | Micro‐particles | Dimassi et al. (2018) |
| SEC | WB, NTA, EM | Single bout of high‐intensity interval cycling consisting of 10 × 60 s intervals of cycling at peak power output with 75 s rests between intervals | Before, immediately (within 5 min) and 4 h after exercise | Plasma | Healthy | 10 (age 24.6 ± 4.0) | Male | miR‐1‐3p, miR‐16‐5p, and miR‐222‐3p, miR‐23a‐3p, miR‐208a‐3p, and miR‐150‐5p miR‐486‐5p, miR‐126‐3p, and miR‐378a‐5p were increased after immediately and after 4 h exercise compared to before exercise | Exosomes | DʼSouza et al. (2018) |
| Sequential centrifugation max. 100,000 × g, ExoQuick plasma | NTA | Organised, team‐based rowing training for over 1 year (training for 1–2 h per day, 6 days per week) | 24 h after the last training session | Plasma | Healthy | 32 (age 19–22): 16 athletes and 16 untrained control | Male | miR‐342‐5p is increased in young athletes compared to untrained students | Exosomes | Hou et al. (2019) |
| Sequential centrifugation, max. 110,000 × g | NTA | Single flywheel based iso‐inertial training session. | Immediately before and 2 h after exercise. | Plasma | Healthy | 8 recreationally resistance‐trained (age 23.7 ± 2.8) | Male | miR‐206 and miR‐146a levels are increased after exercise | EVs | (Annibalini et al., 2019) |
| ExoQuick | NTA | A 40 min acute exercise bout on a cycle ergometer | At baseline, immediately after exercise and 3 h after exercise. | Plasma | Healthy | 5 endurance‐trained and 5 age‐matched sedentary (age > 65) | Male | miR‐486‐5p, miR‐215‐5p, miR‐941 are increased and miR‐151b is decreased at baseline. miR‐383‐5p, miR‐339‐5p, and miR‐874‐3p are upregulated and miR‐206, miR‐486‐5p, miR‐148a‐3p, and let‐7b‐5p are downregulated immediately after acute exercise in the trained group. miR‐34b‐3p, miR‐129‐2‐3p, miR‐138‐1‐3p, miR‐671‐3p and miR‐885‐5p are upregulated and miR‐486‐5p, miR‐629‐5p, and miR‐16‐2‐3p downregulated 3 h after exercised in the trained group. miR‐4433b‐3p was downregulated and miR‐505‐3p, miR‐29b‐3p, miR‐203a‐3p, miR‐384, miR‐451a, miR‐223‐3p, miR‐218‐5p, and miR‐495‐3p were upregulated immediately after exercise in the sedentary group. miR‐4433b‐3p, mir‐378c, miR‐151b, miR‐151a| and miR‐151b were downregulated 3h after exercise in the sedentary group | Exosomes | Nair et al. (2020) |
| Sequential centrifugation, max. speed 20,000 × g | not specified | Acute, moderate intensity exercise consisting on cycling for 60 min | Before (at rest), immediately after and 3 h after exercise | Plasma | Healthy | 12 males (age 22.9 ± 2.6) and 8 females (age 23.0 ± 3.4) | Male, Female | Acute exercise did not significantly alter the expression of miR‐1, miR‐16, miR‐23b and miR‐133a/b | EVs | Silver et al. (2020) |
| ExoRNeasy serum/plasma | EM, NTA, WB | 3 endurance tests, at maximal aerobic capacity, at anaerobic threshold (AnaT) and at aerobic threshold with a bicycle ergometer. A minimum of one recovery day between each test, except for AnaT test | Sweat was collected during the training. Blood was collected immediately before and 10 min after training | Sweat, serum | Healthy | 3 males and 5 females regularly participating in endurance training (age 26.3 ± 5.9) | Male, Female | In sweat, miR‐21 level increased after aerobic threshold and miR‐26 after all the endurance exercise tests compared to sauna control. In serum, miR‐21 and miR‐222 were increased after anaerobic exercise compared to before exercise | EVs | Karvinen et al. (2020) |
| Total exosome isolation reagent | NTA, fluorescence NTA, EM | Young sedentary: moderate‐intensity personal trainer‐supervised resistance and aerobic training of 60 min duration 3 times per week for 6 months. Senior trained: engaged at least twice a week for at least 25 years in endurance and resistance training | At baseline and after 0.5 year of training period and at baseline and over 25 years training | Plasma | Healthy | 14 sedentary young (age 23 ± 2) and 11 trained senior, regular exercise for at least 25 years (age 62 ± 6) | not specified | Let‐7a‐5p, let‐7g‐5p, miR‐130a‐3p, miR‐142‐3p, miR‐150‐5p, miR‐15a‐5p, miR‐15b‐5p, miR‐199a‐3p, miR‐199b‐3p, miR‐223‐3p, miR‐23a‐3p, miR‐451a‐3p, miR‐126‐3p, miR‐199a‐5p, miR‐21‐5p, miR‐25‐3p and miR‐374a‐5p were altered before and after 0.5 year of regular exercise. Let‐7a‐5p; let‐7g‐5p; miR‐130a‐3p; miR‐142‐3p; miR‐150‐5p; miR‐15a‐5p; miR‐15b‐5p; miR‐199a‐3p; miR‐199b‐3p; miR‐223‐3p; miR‐23a‐3p, and miR‐451a‐3p were down‐regulated both in the 0.5 year and the 25 + years trained groups as compared to the sedentary group. miR‐411‐5p was down‐regulated and miR‐144‐3p was up‐regulated in the 25 + years trained group compared to the 0.5‐year trained group | Exosomes | Garai et al. (2021) |
| SEC or immuno‐affinity with CD9, CD63, and CD81 | NTA, EM, WB | Incremental exercise test on a treadmill followed 1week later by an incremental exercise test on a bicycle ergometer or only an incremental exercise test on a treadmill | At rest, immediately after and 30 min after exercise | Plasma | Healthy | 9 male and 1 female (age 26.8 ± 4.5) | Male, Female | EVs released during exercise do not contain luminal DNA | EVs | Neuberger et al. (2021) |
| ExoEasy membrane affinity column | NTA | Single exercise bout consisting of 45 min of frame running or cycling | Before and 30 min after exercise | Plasma | Healthy and cerebral palsy | 9 cerebral palsy (age 27). 20 typically developed (age 30) | Male, Female | Exercise did not affect EV cargo in any of the groups analysed by small RNA sequencing | EVs | Vechetti et al. (2022) |
| SEC or membrane affinity columns exoRNeasy midi kit | NTA, WB | 12 weeks of supervised exercise consisting of 2 whole‐body strength training sessions of 60 min and 2 interval training sessions on an ergometer bicycle of 60 min duration per week | Before, immediately, and 2 h after the first exercise bout, and after 12 weeks training (in the resting state) | Plasma | Healthy and dys‐glycemia and overweight | miRNA profiling: 6 dys‐glycemic and 7 normo‐glycemic (age 40–65). Selected miRNAs were measured in a larger subset (n = 19) | Male | miRNA profiling/affinity: miR‐30a‐5p, miR‐140‐3p, let‐7b‐5p, miR‐99a‐5p, miR‐126‐5p, miR‐10b‐5p, miR‐424‐5p, miR‐338‐3p, miR‐29c‐3p, miR‐222‐3p, miR‐126‐3p, miR‐145‐5p, and miR‐192‐5p are enriched by exercise before an immediately after exercise. RT‐PCR/affinity: miR‐10b‐5p, miR‐222‐3p, miR‐23a‐3p, miR30a‐5p, miR484, miR‐652‐3p, and miR‐92a‐3p, miR991‐5p are enriched immediately after exercise and return back to baseline after 2 h of recovery. These miRNAs are not similarly affected after the 12‐week exercise intervention. RT‐PCR‐SEC: miR‐10b‐5p, miR‐222‐3p, miR‐30a‐5p, miR‐339‐3p and miR‐99a‐5p are increased immediately after exercise | EVs | Doncheva et al. (2022) |
| miRCURY exosome isolation kit | NTA | 3.6 km‐long run on a 1.03 km vertical ascension with a 29.5% slope | 30 min before and within 30 min after the competition | Plasma | Healthy | 14 proff sky‐runners participating in a vertical run (age 38.8 ± 10.2) | Male | miR‐143‐3p, miR‐17‐5p, miR‐532‐3p, miR‐874‐3p and miR‐885‐5p were up‐regulated and miR‐1‐3p, miR‐29a‐3p and miR‐424‐5p) were down‐regulated after the competition | EVs | Faraldi et al. (2022) |
| Sequential centrifugation, max. 100,000 × g | EM, WB | 1 week of 45 min of aerobic training in a cycle ergometer per day and a bout of resistance exercise (3 sets of 8–12 repetitions on the leg press at 80% of 1RM, with 2 min rest between sets) on days 2, 4 and 6 | Before and after exercise | Muscle biopsy | Healthy | 8 (3 women and 5 men) sedentary individuals with obesity (age range 18–35) | Male, Female | miR‐let‐7f‐2‐3p, miR‐101‐5p, miR‐1301‐3p, miR‐1307‐3p, miR‐146b‐5p, miR‐190a‐5p, miR‐199a‐5p, miR‐199b‐5p, miR‐208b‐5p, miR‐23a‐5p, miR‐296‐3p, miR‐3605‐3p, miR‐3609, miR‐3615, miR‐370‐3p, miR‐3960, miR‐409‐3p, miR‐4326, miR‐4485‐3p, miR‐4485‐5p, miR‐4488, miR‐4497, miR‐483‐3p, miR‐483‐5p, miR‐485‐5p, miR‐486‐5p, miR‐629‐5p, miR‐7‐5p were differentially expressed | small EVs | Sullivan et al. (2022) |
| Umibio extracellular vesicles extraction kit | EM, WB, NTA | 1500‐m freestyle swimming session at the speed of the best previously recorded swimming performance | Before and after the swimming session | Plasma | Healthy | 13 freestyle swimmers (age 19.2 ± 1.1) | Male | miR‐144‐3p, miR‐145‐3p, miR‐509‐5p, miR‐891b and miR‐890 were the miRNAs with the largest expression‐fold variation among the 70 miRNAs differentially expressed (45 up‐regulated and 25 down‐regulated) after training | EVs | Lai et al. (2023) |
| Sequential centrifugation, max. speed 100,000 × g | DLS, EM, AFM | Unspecified regular endurance training performed by triathlon male athletes | At rest | Urine | Healthy | 13 triathletes (age 47 ± 8.8) and 13 inactive (age 42 ± 3.4) | Male | miR‐92a‐3p, miR‐27a‐3p, miR‐23a‐3p, miR‐133a, miR‐206, miR‐126‐3p, miR378A‐5p and miR‐34a‐5p were differentially expressed in inactive versus endurance‐trained men | EVs | Pietrangelo et al. (2023) |
| exoRNeasy midi kit affinity membrane | Single acute exercise bout of: (i) 30 min of steady‐state cycle ergometry, followed by 30 total sets of whole‐body resistance exercise (TRAD) or ii) high‐intensity tactical training consisting of a 10‐round circuit of high‐intensity, explosive exercise followed by a 30 total sets of whole‐body resistance exercise (HIIT) | Before, immediately and 3 h and 24 h after exercise | Serum | Healthy | Total 40: i) TRAD 12 males and 9 females (age 22 ± 3), ii) HIIT 11 males, 8 females (age 22 ± 2) | Male, Female | 33,029, 29,766, 26,895 long transcripts are upregulated and 259, 198,78 downregulated immediately, 3 h and 24 h after TRAD respectively. 15,664, 20,629, 16,018 long transcripts are upregulated and 223,374, 177 downregulated immediately, 3 h and 24 h after HIIT respectively. 2 and 24 small transcripts were upregulated immediately after TRAD or HIIT respectively. 4 small transcripts were upregulated and 52 downregulated in TRAD, and 8 small transcripts were upregulated and 148 downregulated in HITT 3h after exercise. 7 small transcripts were upregulated and 50 downregulated in TRAD, and 126 downregulated in HIIT 24h after exercise. 1 circRNA was upregulated in TRAD versus 30 upregulated and 6 downregulated in HITT immediately after exercise. 2 circRNA were upregulated and 3 downregulated in HITT 3h after exercise. 3 circRNA were upregulated and 1 downregulated in HITT after 24 h | EVs | Lavin et al. (2023) | |
| SEC followed by centrifugation at 100,000 × g | NTA, WB, TEM, | A 6‐week sprint cycling interval training at sea level and at 2000, 3000, and 4000 m | At the pre‐test session, 2 weeks before the first training session and 72 h after the last session | Plasma | Healthy | 30 athletes (age 18–35) divided into a group performing the exercise at different altitudes | Male | miR‐21‐5p was upregulated and miR‐23a‐3p was downregulated under hypoxia training at 3000 m | Exosome‐like EVs | Warnier et al. (2023) |
Abbreviations: AFM, atomic force microscopy; DG, density gradient; DLS, dynamic light scattering; EM, electron microscopy; FC, flow cytometry; NTA, nanoparticle tracking analysis; SEC, size‐exclusion chromatography; WB, Western blot.
TABLE 3.
Studies investigating the protein, protein/nucleic acid or metabolite cargo of exercise‐induced EVs.
| EV isolation | EV analysis | Type of exercise | Time of EV collection | EV source | Health status | Cohort | Gender | Molecular changes | EV term | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Sequential centrifugation, max. speed 20,000 × g | NTA, EM | 1‐h bout of cycle ergometer exercise | Before, immediately after, and 4 h after exercise | Plasma | Healthy | 11 (mean age 27, SEM 1) | Male | 322 proteins were altered immediately after exercise. 3 proteins (Vascular non‐inflammatory molecule 2, S100‐A8, S100‐A9) were significantly different between rest and recovery samples | EVs | Whitham et al. (2018) |
| Acoustic trapping and sequential centrifugation max. speed 13,000 × g or 20,000 × g | FC, EM, NTA | 10 min workout with progressively increasing intensity on stationary bicycles | Before and 1 h after exercise | Plasma | Healthy | 20 (mean age 58) | not specified | Cardiovascular disease panels CVD II and III: 54 proteins were changed in response to physical exercise including chemokines and interleukins associated with the inflammatory response (e.g. CCL17, CCL24, CXCL1, CXCL16, IL1RA, IL18, and macrophage receptor with collagenous structure (MARCO)), as well as proteins associated with angiogenesis (e.g. ANG1 and TIE2) and coagulation (PAR1, SELP, SRC, and vWF) | EVs | Bryl‐Górecka et al. (2018) |
| SEC and immuno‐isolation | NTA, WB, EM, FC | Incremental cycling test until exhaustion | Before, during and immediately after exercise | Plasma | Healthy | 21 active (mean age 28.7 ± 4.2) | Male | EVs release after exercise contain the lymphocyte markers CD4 and CD8, the monocyte marker CD14, the platelet markers CD41, CD42 and CD62P, the endothelial markers CD105 and CD146 and the antigen presenting cell marker MHC‐II | EVs | Brahmer et al. (2019) |
| EV Array and polymer‐based precipitation | NTA, TEM, WB | Single bout of blood flow restricted resistance exercise consisting of 5 sets of knee extensions | Immediately before exercise and after 1 h | Plasma | Healthy | 6 recreationally active (age 21 ± 0.6) | Male |
The proteins CD41, NCAM, Alix and CD25 were upregulated and Flotillin‐1 was down‐regulated after exercise. The miRNAs miR‐182‐5p, miR‐1294, let‐7b‐5p, miR‐451a, miR‐16‐5p, and miR‐36‐3p were upregulated and miR‐19b‐3p, miR‐17‐5p, miR‐221‐3p, miR‐150‐5p, miR‐340‐5p and miR‐21‐5p were downregulated after exercise |
EVs | Just et al. (2020) |
| Sequential centrifugation, max. speed 100,000 × g | WB, NTA, TEM | 16 resistance sessions over 8 weeks (2 per week). 8 different resistance exercises, with three sets | Before and after training | Plasma | Healthy | 28 trained (age 73.6 ± 0.8) and 10 control (age 72.6 ± 0.4) | Male, Female | The percent change of CD63 was reduced in the trained group versus the control group after an 8‐week resistance training program | Exosomes | Estébanez et al. (2021) |
| SEC | NTA, TEM, WB | A progressive 12‐week supervised cycle ergometer training of 35 min. Exercise sessions were repeated 3 times weekly on non‐consecutive days | At baseline and 72 h after the last bout of the 12‐week period training | Serum | Healthy or type 2 diabetes | 5 type 2 diabetes, 5 insulin resistant nondiabetic, 5 insulin sensitive nondiabetic (age 55–59) | Male | 262 proteins were regulated (102 down‐regulated, 160 up‐regulated) by exercise, including ALIX, Rab proteins annexins, flotillin, moesin, tubulin, integrins. 13 of the 262 proteins were shared between the 3 groups including antithrombin III, kininogen I, histidine‐rich glycoprotein, and α1‐antitrypsin. 29 proteins, including the fibrinogens FGA, FGB, and FG, were up‐ or down‐regulated after exercise only in the 2 insulin‐resistant groups | Exosomessmall EVs | Apostolopoulou et al. (2021) |
| SEC | FC, NTA, WB | 4 min of high‐intensity interval training in a programmable bicycle ergometer consisting of 8 sets of bout (20 s) and rest (10 s) | Before, at the immediate conclusion, 30 min and 120 min after exercise | Serum | Healthy | Total cohort: 17 (median age 20). For proteomics 3 men were selected | Male | 20 proteins were changed when comparing before and after exercise: alpha‐2‐antiplasmin, fibronectin, peroxiredoxin‐2, von Willebrand factor, multimerin‐1, fibrinogen alpha chain, fibrinogen beta chain, fibrinogen gamma chain, tetraspanin 9, spectrin beta chain, cofilin‐1, carbonic anhydrase‐1, catalase, immunoglobulin heavy variable 1–69, cholinesterase, immunoglobulin heavy constant gamma 4, histidine‐rich glycoproteins, serotransferrin, complement factor H and haptoglobin | EVs | Kobayashi et al. (2021) |
| 10,000 × g centrifugation, followed by SEC and UC at 100,000 × g for 1.5 h | NTA, EM, Nanoscale flow cytometry, mass spectrometry |
Aerobic exercise: one bout of high‐intensity cycling. Resistance exercise: one moderate bout of single leg exercise |
Aerobic exercise: before, 10 min, 1 h and 3 h after exercise Resistance exercise: before, 10 min, 30 min and 60 min after exercise. |
Plasma | Healthy |
Aerobic exercise: 7 male (mean 37.0 ± 5.0), 7 female (mean 28.0 ± 4.4) Resistance exercise: 8 male (mean 32.6 ± 5.5), 8 female (mean 33.7 ± 10.2) |
Males, females |
10 min after aerobic exercise:321 proteins increased (Ingenuity Pathway Analysis: immune regulation, glycolysis, signaling via Rho GTPase, interleukin‐8, and integrin) and after resistance exercise 7 proteins up‐ and 1 protein downregulated. Common to both: MMP9, MPO3, HIST1H4A |
EVs | Vanderboom et al. (2021) |
| miRCURY exosome cell/urine/CSF Kit | Exoview | 20 m shuttle run test | Before, immediately after, and after 1 h rest. | Urine | Healthy | 13 (age 25.5 ± 2.0) | Male | CD9, CD63, and CD81 increased in post‐exercise. 1 h after exercise only CD9 was still increased. miR‐let‐7a‐5p, miR‐193b‐3p, miR‐23a‐3p, miR‐320a‐3p, miR‐423‐5p, miR‐4454, miR‐5100, miR‐7847‐3p and miR‐8485 were altered after exercise | EVs | Park and Moon (2022) |
| Sequential centrifugation, max. 100,000 × g | Spectradyne nCS1, WB | 20 min of moderate intensity cycling | Before and after exercise | Plasma | Healthy | 20 young (18–35) and 20 mature (50–70) fit and unfit | Male | NAMPT (nicotinamide phosphoribosyltransferase) is increased after exercise in young individuals with high aerobic fitness whereas mature fit and young unfit individuals exhibited a limited increase | EVs | Chong et al. (2022) |
| Sequential centrifugation, max. 110,000 × g; SEC | NTA, WB, FC | Four different exercise protocols: Acute Aerobic Exercise (AAE) and Aerobic Training (AT), Acute Maximal Aerobic Exercise (AMAE), Altitude Aerobic Training (AAT) | AAE and AT: before and after 0,1, 2, 6, and 24 h. AMAE: 1 h before, and after 0, 1 and 2 h. AAT: before and at the end of the training, at least at 48 h from exercise. | Plasma | Healthy | 13 not physically active (age 20.1 ± 0.6) and 6 male athletes (experience in the distance 5000–10.000 m of 5 ± 1 years) men (age 23.3 ± 6.8) | Male, Female | miR‐206, miR‐133b and miR‐146a were upregulated following AAE within the first hour after exercise in the sedentary group. miR‐146 is increased immediately post‐exercise in the AT group. miR‐206, miR‐133b, miR‐486‐5p, miR‐181a‐5p and miR‐16 are upregulated immediately after AMAE. AAT showed no statistically significant variations following the training regarding miRNA expression levels. CD42a, CD41b, CD29, CD40 and CD62P are increased at 1 h post‐exercise and CD56, CD105 and CD3 are detected only immediately after AAE. mtDNA (COX1) is increased 1 h after AAE | EVs | Maggio et al. (2023) |
| Immuno‐capture/ imaging system | Exoview | High intensity intermittent cycling: 4 × 30 s at 200% of individual max power | Before and immediately after exercise. | Plasma | Healthy | 7 recreationally active persons (age 21 ± 4) | Male, Female | The expression of CD9 and CD63 is increased after high intensity intermittent exercise | small EVs | McIlvenna et al. (2023) |
| Filtration followed by centrifugation at 100,000 × g | BCA protein assay, WB, NTA, EM | A cycling bout exercise starting with an intensity of 40 W and increasing resistance with 15 W for females and 20 W for males every 2 min until exhaustion | Sweat was collected during exercise and during a rest period of 30 min. | Sweat | Healthy | 10 (3 females age 33.7 ± 6.6; 7 males age 38.7 ± 3.6) | Male, Female | Glutamate and glutamine (glutamate metabolism pathway), alanine, arginine, glycine, proline, threonine, and serine (amino acid pathway) and lactate (glycolysis pathway) were significantly increased during the exercise test compared to during recovery. Lysine was only detected during the exercise test and not during recovery. Myristate (fatty acid pathway) was reduced during exercise compared to during recovery. | EVs | Ali et al. (2023) |
| ExoQuick, immuno‐captured with L1CAM | NTA, FC | Supervised moderate to high‐intensity aerobic exercise of 60‐min duration 3 times a week for 16 weeks | At baseline and after 16 weeks of training. | Plasma | Mild to moderate Alzheimer's disease | 48 (age 71.3 ± 6.5) in exercise group and 47 (age 71.2 ± 6.5) in control group | Male, Female | The neuroprotective proteins proBDNF, BDNF, and humanin are increased in the exercise but not in the control group | Neuron‐derived EVs | Delgado‐Peraza et al. (2023) |
| SEC | NTA, ImageStream, Bradford colorimetric assay, WB, EM |
Endurance training allowing men to have specific running or strength performance |
At rest (circa 40 h after the most recent exercise session) on two separate but identical lab visits | Plasma | Healthy | 13 endurance‐trained (age 30 ± 6), 13 strength‐trained (age 25 ± 5) and 12 recreationally active (age 26 ± 2) | Male | 96 metabolites are unchanged when comparing resting men with divergent histories of exercise training | small EVs | Darragh et al. (2023) |
| SEC | NTA, WB, EM, Surface plasmon resonance imaging | Single bout of endurance exercise consisting of 30 min exercise on a treadmill | At basal level and immediately after exercise | Plasma | Healthy | 21 (age 26.95 ± 3.07) with a medium/high fitness level | Male | 98 proteins were upregulated after exercise including MAP2K1, and carnosine dipeptidase 2, glutamate‐cysteine ligase modifier subunit and microsomal glutathione S‐transferase 2, which are essential for the glutathione biosynthetic process. 48 of the proteins have catalytic activity, and the most upregulated proteins are mitochondrial proteins involved in the maintenance of energetic balance. The activity of Glutathione Reductase and Catalase (antioxidant enzymes) was higher after exercise | EVs | Lisi et al. (2023) |
| ExoQuick | NTA, protein assay kit, WB | A single 30 min bout or 5 × 30 min on consecutive days of aerobic exercise in a treadmill with a specific speed and slope | Before, 3 and 24 h after a bout of exercise and after 24 h from the last session of a 5‐day consecutive training. | Plasma | Healthy | 19 (age 25 ± 3.2) divided into a trained (Schmidt et al., 2015) and an untrained (Li et al., 2022) group | Male | Protein carbonyl, catalase, SOD2, and HSF1 were higher at baseline in untrained compared to trained men, and SOD2 was also increased after 3 h. However, Tot‐HSP27 was higher in the trained group at each time point. Protein carbonyl, HSF1, Catalase, and SOD2 were lower after 5 days of regular aerobic compared with the same group before training and the untrained group | EVs | Lisi et al. (2023) |
| SEC followed by centrifugation at 120,000 × g | NTA, WB, EM, immuno‐capture | 12 week of home‐based progressive elastic band‐based resistance training 3 days a week | Prior to and following a 12‐week exercise program | Plasma | Healthy | 30 (age 74.9 ± 5.7) | not specified | TSG101 and the miRNAs miR‐23a, miR‐27a, miRNA‐199a, miR‐146a and miR‐92a increased after exercise | Exosome‐like vesicles | Xhuti et al. (2023) |
| SEC | NTA, EM | Stationary bicycle exercise with an increase in workload of 15 watts per minute until volitional exhaustion | At baseline immediately prior to exercise, and 15 min and 24 h after exercise. | Plasma | Healthy or myalgic encephalomyelitis/ chronic fatigue syndrome (ME/ CFS) | 18 (age 44.4 ± 7.9) patients and 17 sedentary (age 44.2 ± 13.4) | Female |
MYL9, PDIA6, PPIB, HSPA5, F8, HSP90B1, VDAC3, FN1, CANX, CLTC, F13A1 are less abundant and ANXA2, B2M, ORM1 are more abundant in the ME/CFS group 15 min after exercise. EMILIN is more abundant in ME/CFS patients 24 h after exercise. 63 proteins (all upregulated, including YWHAE, YWHAQ, YWHAB, YWHAZ, YWHAG, YWHAH, ITGB1, ITGB3 and ITGA2B) in the ME/CFS group and 187 proteins (178 upregulated including CCT2, CCT5, CCT6A, CCT8, TUBB, TUBB4B, TUBA4A, TUBA8, MYL6, MYL9, MYL12A, MYLK, TPM4, TMOD3) and 9 ‐AGT, C4B, CLU, COLEC11, FCN2, MBL2, ORM2, PON1 and VSIG6‐ downregulated) in the control group were found differentially expressed after 15 min exercise |
EVs | Giloteaux et al. (2024) |
| Sequential centrifugation at max. speed 100,000 × g | Particle analysis with Spectradyne nCS1 and WB | A single 20‐min boat of moderate‐to‐vigorous intensity cycling | Before and immediately after exercise | Plasma | Healthy | 14 young (age 27.1 ± 4.0) and 14 mature (age 60.9 ± 6.1), 14 unfit (age 44.4 ± 18.7), 14 fit (age 43.7 ± 17.8) | Male | ADAMTS13, ANXA1, ANXA6, BASP1, CTSG, F8, HIST1H4A, ITGB2, LCP1, LTF, LYZ, MPO, and S100A8 are increased after exercise in the total cohort. ANXA1, LCP1, LYZ, MPO, and S100A8 are upregulated after exercise in the young subset. ANXA1, BASP1, HIST1H4A, LTF, LYZ, MPO, S100A8, and S100A9 are upregulated after exercise in the mature subset. ANXA1, LYZ, S100A8, and S100A9 were upregulated in the unfit subset after exercise. ANXA1, ANXA6, EEF1A1, HIST1H4A, ITGB2, LCP1, LTF, LYZ, MPO, and S100A8 are upregulated in the fit subset | small EVs | Chong et al. (2024) |
Abbreviations: BCA, bicinchoninic acid; EM, electron microscopy; FC, flow cytometry; NTA, nanoparticle tracking analysis; SEC, size‐exclusion chromatography; WB, Western blot.
As shown in Tables 2 and 3, plasma is often the selected biofluid, but more recent studies have started to investigate other biofluids such as sweat or urine (Ali et al., 2023; Karvinen et al., 2020; Park & Moon, 2022; Pietrangelo et al., 2023). In addition, one study has investigated the composition of EVs released by cells isolated from muscle biopsies (Sullivan et al., 2022). The studies included in Tables 2 and 3 generally contain an acceptable description of the cohorts studied and the EV methodology used, which is important for analysing and reproducing the results. It should also be mentioned that these studies often include several methods to characterise EVs, mainly immunoblotting, NTA and electron microscopy (EM). This is an important control to ensure that EVs are, in fact, present in the samples under analysis. However, the variety of EV isolation methods used in these studies complicated the identification of general trends. This is because EV isolation methods may lead to the isolation of heterogeneous EV subpopulations, which are known to differ to some extent in their molecular composition (Jeppesen et al., 2019; Kowal et al., 2016; Lischnig et al., 2022). Moreover, biofluids contain EVs released from different cell types, and most of the studies presented in Tables 2 and 3 have investigated the composition of bulk EV samples. Therefore, it would be interesting to have more studies where the composition of EVs originating from specific cell types is independently investigated.
So far, many studies on exercise‐induced EVs have focused on analysing specific miRNAs, mainly on miRNAs associated explicitly with cells from where EVs released during exercise could originate, such as skeletal muscle cells. However, recent studies have also investigated how exercise changes EVs' proteome and metabolome. Moreover, it has become more common to use high‐throughput omics methodologies to analyse the effect of exercise on the composition of EVs (see for example (Giloteaux et al., 2024; Lavin et al., 2023; Whitham et al., 2018). Results of these studies consistently show that physical exercise induces molecular changes in EVs found in biofluids and that EVs released during exercise have different cellular origins. For example, exercise‐induced EVs have been reported to contain molecules associated with muscle cells (Annibalini et al., 2019; Guescini et al., 2015; Xhuti et al., 2023), blood and endothelial cells (Brahmer et al., 2019), neurons (Delgado‐Peraza et al., 2023) or adipose tissue (Doncheva et al., 2022). However, it was not possible to identify EV molecules universally affected by exercise. It is likely that such molecules may not exist and that the molecular changes induced by exercise depend to a large extent on the type, intensity and duration of the exercise and/or the investigated cohort (i.e. old vs. young individuals, men vs. women, healthy vs. individuals with different pathologies, athletes vs. sedentary or normally trained individuals) or biomaterial (plasma, serum, urine, sweat, saliva, tissue biopsies). The time after exercise when the EV composition is investigated also seems to play a role in terms of molecular content, with some effects being of a relatively short duration (Whitham et al., 2018). It is also important to mention that, as shown in Table 1, exercise may change the EV release rate. If this is the case, it is important to determine if the observed changes in molecular composition are due to a general increase/decrease in EV release.
Finally, even if it was not possible to identify specific EV molecules universally affected by exercise, it is possible to find some trends in the biological processes in which the molecules altered after exercise are involved. For example, recent proteomic studies suggest that the proteins differentially expressed after exercise compared to before exercise are involved in signal transduction, the immune response, metabolism and oxidative stress (Apostolopoulou et al., 2021; Chong et al., 2024; Giloteaux et al., 2024; Kobayashi et al., 2021; Lisi et al., 2023; Vanderboom et al., 2021; Whitham et al., 2018).
2.4. The effects of exercise‐induced EVs on cancer development and progression
While the exact mechanisms remain incompletely understood, several hypotheses have been proposed to explain how physical activity may protect against cancer development and progression, including suppression of tumour growth by bioactive molecules secreted during exercise, reprogramming metabolic pathways in cancer cells, boosting cancer immunosurveillance and reducing chronic inflammation, and normalisation of tumour vasculature and blood flow (Pedersen et al., 2015). Do EVs play a role in these processes? To the best of our knowledge, so far only one study has investigated the role of exercise‐induced EVs in the progression of cancer (Sadovska et al., 2022). This study collected plasma EVs from trained, healthy rats immediately after the wheel‐running exercise and repeatedly administrated into F344 rats with orthotopically injected syngeneic prostate cancer cells. Results demonstrated a reduction of the primary tumour volume by 35% and possibly attenuating lung metastases. Although the mechanisms underlying these effects were not investigated in this study, it seems plausible that EVs may contribute to many of the above‐listed processes. In the following section, we will try to summarise evidence on the putative roles of exercise‐induced EVs in various anti‐cancer mechanisms (Figure 2).
FIGURE 2.
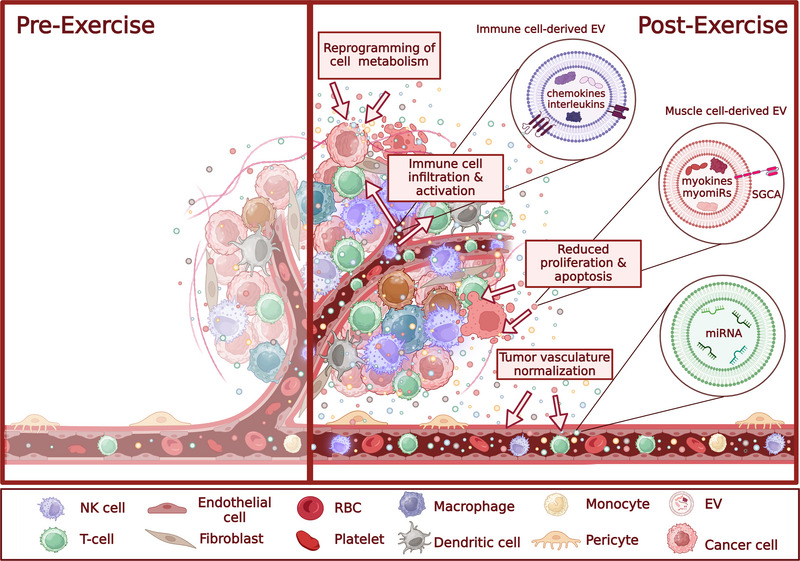
Contribution of exercise‐induced EVs to various anti‐cancer mechanisms. Abbreviations: SGCA, alpha‐sarcoglycan; NK cell, natural killer cell; RBC, red blood cell.
2.5. Suppression of tumour growth by myokines
Several studies have shown that exercise‐conditioned human serum reduces the growth of cancer cells, including breast, prostate and colon cancer (Baldelli et al., 2021; Dethlefsen et al., 2017; Orange et al., 2022). At least partially, this effect is mediated by myokines—several hundreds of cytokines or other peptides that are secreted by muscle fibres and exert either autocrine, paracrine, or endocrine effects on processes such as metabolism, angiogenesis, or inflammation. They mediate communication between muscles and other organs, including the brain, bone, liver, immune cells, etc. (Severinsen & Pedersen, 2020). Several myokines, such as oncostatin, Irisin, IL‐6 and SPARC, have been shown to reduce the proliferation and/or induce apoptosis in cancer cells, whereas IL‐6, IL‐10 and IL‐15 are involved in the remodelling of immune tumour microenvironment (reviewed in Huang et al., 2022).
Whitham et al. (2018) performed a quantitative proteomic analysis of EVs released before and after a 1‐h bout of cycling exercise in healthy males that resulted in the identification of 322 differentially expressed proteins. This list was significantly enriched with proteins involved in exosome biogenesis and glycolytic enzymes, suggesting that exercise induces an efflux of EVs into the circulation and that a fraction of EVs may originate from skeletal muscle. This study also identified 35 novel myokine candidates released from the contracting limb via femoral arteriovenous difference analysis. Fifteen of these proteins did not contain a signal peptide, suggesting that sorting of myokines into EVs could be an alternative way of tissue crosstalk to classic secretory pathways. Taken together, this study provided evidence that myokines are released and travel through the body enclosed in EVs (Whitham et al., 2018). However, which fraction of myokines is released and reaches the bloodstream in an EV‐enclosed form, and which—in a soluble form—remains to be established.
2.6. Reprogramming of the immune tumour microenvironment
Many studies have shown that dynamic exercise induces rapid mobilisation of effector lymphocytes, including mature NK cells, specific subsets of T cells and monocytes, in the bloodstream (reviewed in Fiuza‐Luces et al., 2024). Campbell et al. (2009) showed that the counts of CD8+ T cells with a high effector and tissue‐migrating potential and cytotoxic CD56(dim) NK cells are markedly increased (up to 10‐fold above baseline levels) in the bloodstream after a 20 min bout of high‐intensity cycling exercise (Campbell et al., 2009). Similarly, selective mobilisation of differentiated NK cell subsets with high cytotoxic activity against HLA‐expressing target cells (Bigley et al., 2014), γδ T cells (Anane et al., 2009) and CD14+CD16+ monocytes (Steppich et al., 2000) immediately after high‐intensity exercise have been reported. Lymphocyte mobilisation is driven by increased blood pressure, shear forces and adrenaline signalling via β2‐adrenergic receptors on the surface of lymphocytes. Lymphocyte counts return to baseline or even slightly decrease 1–2 h after exertion (Fiuza‐Luces et al., 2024). The cyclic mobilisation of lymphocytes into the bloodstream appears to be required for efficient immune cell trafficking and infiltration into tumours. For instance, preoperative high‐intensity interval training has been shown to increase the infiltration of NK cells into prostate cancer tissues and the NK cell counts were correlated with the number of training sessions (Djurhuus et al., 2023). Experiments with murine tumour models have shown that aerobic training results in a notable decrease in both tumour size and incidence. These effects were mediated by the mobilisation and redistribution of NK cells in an epinephrine and IL‐6‐dependent manner (Pedersen et al., 2016). IL‐6, a pleiotropic interleukin and the first myokine identified (Febbraio et al., 2004), elicits both anticancer and cancer‐promoting effects depending on the tissue context (Orange et al., 2023). IL‐6, produced by leukocytes and stromal cells in areas of chronic inflammation, has been demonstrated to promote tumour growth. Conversely, IL‐6 secreted by skeletal muscles during exercise exhibits anti‐tumour effects by mobilising cytotoxic immune cells and reducing DNA damage (Orange et al., 2023). Interestingly, although many cell types secrete IL‐6 in a soluble form, some cells, such as T cells have been reported to favour EV‐encapsulated IL‐6 release. Hence, in plasma, IL‐6 exists in EV‐encapsulated, EV surface‐attached and soluble forms (Fitzgerald et al., 2018).
Proteomic and transcriptomic profiling of EVs released during exercise revealed enrichment in molecules involved in processes such as immune cell proliferation, regulation of immune response, T cell effector functions, and trafficking of immune cells (Table 2). For example, analysis of plasma EVs collected before and after cycling exercise using Olink proteomics panels revealed the upregulation of various chemokines, interleukins and immune cell receptors (e.g. CCL17, CCL24, CXCL1, CXCL16, IL1RA, IL18 and MARCO) (Bryl‐Górecka et al., 2018). Multiple studies have shown that exercise triggers shifts in EV‐enclosed miRNA levels. Some of them might enhance the anti‐tumour immune responses if internalised by the respective immune cells. For example, miR‐126‐3p is an endothelial‐specific miRNA whose levels were increased in plasma EVs immediately after exercise (Doncheva et al., 2022; DʼSouza et al., 2018), as well as at baseline after 0.5 years of regular exercise (Garai et al., 2021). A recent study by Gondaliya et al (2024) showed that administration of milk‐derived EVs loaded with miR‐126‐3p in mice carrying hepatocellular cancer xenografts elicited pleiotropic effects including enhancing NK cell cytotoxic activity, impairing angiogenesis and modulating macrophage chemotaxis (Gondaliya et al., 2024). Similarly, EV miR‐29c‐3p levels increased after acute exercise (Doncheva et al., 2022), whereas overexpression of miR‐29c‐3p has been shown to enhance NK cell cytotoxicity by reducing Myc activity (Lee et al., 2021). Taken together, these findings indirectly suggest that the exercise‐induced cargo of EVs can exert immunomodulatory effects in the tumour microenvironment.
2.7. Reprogramming of energy metabolism in cancer cells
As the essence of neoplastic disease, losing regular control over cellular proliferation is accompanied by reprogramming metabolic pathways that provide cellular energy—adenosine triphosphate (ATP). The two central energy‐producing metabolic pathways (glycolysis and oxidative phosphorylation) are also, at the same time, sources for metabolic routes where the production of essential metabolites for nucleotide and lipid biosynthesis takes place (Hanahan & Weinberg, 2011; Pavlova et al., 2022; Vander Heiden et al., 2009). There is evidence showing that exercise can induce changes in intratumoral central carbon metabolism (especially mitochondrial metabolism) in mice human cancer xenograft model, independent of whether exercise was able to inhibit tumour growth (Lu et al., 2018). However, there is a lack of functional studies showing a direct effect of exercise‐induced EVs on the rate of glycolysis or/and oxidative phosphorylation (OXPHOS) in recipient cells.
We are just beginning to explore direct associations between muscle cell health, exercise, and cancer cell metabolism. Exercise‐conditioned serum and myokines have been shown to act as tumour suppressors in breast, colon and lung cancer and have been suggested to impact several hallmark features of cancer (Hojman et al., 2011; Ruiz‐Casado et al., 2017). EVs can contain modulators of cellular energy metabolism. The miRNAs contained in EVs released by drug‐resistant tumour cells or cells from the tumour microenvironment have an established role in the intercellular transfer of drug‐resistance traits, with accompanying metabolic reprogramming, to sensitive tumour cells (reviewed in Polónia et al., 2023; Vasconcelos et al., 2019; Xavier et al., 2022). Also, EVs from various sources can affect mitochondria (reviewed in Carles‐Fontana et al., 2022). However, there is a lack of studies showing the direct effect of factors contained in EVs (e.g. miRNA) on the energy production function in mitochondria of recipient cancer cells.
Among the exercise‐induced miRNA families, miR‐1, ‐133 and ‐206 are the most well‐studied (Mitchelson & Qin, 2015; Townley‐Tilson et al., 2010). Members of these are shown enriched in EVs released after exercise in several studies (Annibalini et al., 2019; DʼSouza et al., 2018; Guescini et al., 2015; Pietrangelo et al., 2023, also given in Tables 2 and 3). However, miR‐1, miR‐133a, miR‐133b and miR‐206 are mostly down‐regulated in various cancers and operate as tumour suppressors (Khasraghi et al., 2023; Nohata et al., 2012). A direct effect on cancer energy metabolism has only been shown in miR‐133b. The regulatory network of miR‐133b is involved in the altered energy metabolism of cancer cells. MiR‐133b controls pyruvate kinase expression, thereby repressing (aerobic)glycolysis (Li et al., 2017; Sugiyama et al., 2016; Wu et al., 2022), a property that can confuse the metabolism of cancer cells if, at the same time, OXPHOS activity increases with the production of oxygen free radicals. Cancer cells can become vulnerable to drugs that induce oxidative stress such as doxorubicin or radiation in such conditions.
Several studies show that communication within the tumour microenvironment regarding energy metabolism can also occur via EVs and the miRNAs they contain (Sepúlveda et al., 2023; Yan et al., 2018; Zhang et al., 2023). For example, the breast cancer cell‐secreted and EV‐encapsulated miR‐105 reprogrammed stromal cells to increase glucose and glutamine catabolism to fuel adjacent cancer cells when nutrients were sufficient. When nutrients are in shortage and metabolic by‐products accumulate, these reprogrammed fibroblasts detoxify metabolic wastes, including lactic acid and ammonium, by converting them into energy‐rich metabolites, again useful for the surrounding cancer cells (Yan et al., 2018). How and to what extent exercise‐induced EVs can interfere with cell‐to‐cell communication within the tumour is unknown.
To summarise, there is evidence to suggest that exercise affects the reprogramming of energy metabolism of cancer cells. However, there is still little detailed information about the mechanisms. EVs certainly have a role in this process. In addition, tumour energy metabolism relies on the cooperation of cancer cells with the cells in the tumour microenvironment; exercise effects on this also need further investigation. This knowledge could help support cancer treatment by amplifying or mimicking the positive effects of physical exercise.
2.8. Normalisation of tumour vasculature and blood flow
To sustain tumour growth, cancer cells need adequate amounts of oxygen and nutrients, thus explaining why angiogenesis is one of the hallmarks of tumour progression. Angiogenesis is required for normal physiological processes, such as wound repair and the menstrual cycle; however, newly formed vessels in tumours differ from those formed in healthy tissue (Hanahan & Weinberg, 2011). Tumour capillaries are often irregularly branched, unorganised, unusually large and hyperpermeable (Nagy et al., 2010). Under normal circumstances, proangiogenic factors are tightly regulated and are in balance with antiangiogenic factors, but tumours often exhibit what is known as the “angiogenic switch”, when this balance is disrupted, leading to a quickly developing but inefficient vascular network (Dudley & Griffioen, 2023; Papetti & Herman, 2002). This creates an even more hypoxic and acidic tumour microenvironment, which further promotes continuous angiogenesis. In addition, a malformed vascular network increases the metastatic potential and encumbers drug and immune cell delivery to the tumour site (Azzi et al., 2013). Normalising the existing vascular network of a tumour has been proposed as a potential treatment strategy (Yang et al., 2021). This can be done by targeting several signalling pathways, including vascular endothelial growth factor (VEGF), Ang‐Tie, Notch and oncogenic signalling (Yang et al., 2021). A non‐pharmaceutical approach to this therapy could be physical exercise, as it promotes angiogenesis and arteriogenesis in skeletal muscle and myocardium by both growth factor signalling and mechanical forces (Esteves et al., 2021). Indeed, a study examining intratumoral vascularisation of human breast cancer xenografts implanted in mice showed a significantly normalised vascular network after a 44‐day regiment of voluntary wheel running exercise (Jones et al., 2010).
Emerging evidence suggests that EVs may play a role in mediating this process. They have been found to carry a variety of bioactive molecules that mediate angiogenesis, like VEGF (Ko et al., 2019), angiopoietin‐2 (Xie et al., 2020), fibroblast growth factors (FGFs) (Proia et al., 2008) and various microRNAs (Vu et al., 2020). A study examining EV miRNA content in rats subjected to treadmill exercise protocols showed differential expression of several miRNAs implicated in angiogenesis (Hou et al., 2019). For example, one up‐regulated molecule was miR‐122, which has been shown to inhibit the VEGFC signalling pathway in bladder cancer (Wang et al., 2016). Another study compared miR‐126 expression in EVs released by endothelial progenitor cells between mice with differing exercise regiments and found that miR‐126 was more abundant in the exercise group (Ma et al., 2018). MiR‐126 has been found to exhibit both positive and negative effects on cancer angiogenesis (Jalil et al., 2023). This being said, there is a lack of research focusing specifically on exercise‐induced EV effects on angiogenesis in tumours.
2.9. Further research directions and challenges
A growing body of evidence shows that increased EV release is an integral part of the acute response to physical exercise and EVs play significant roles in mediating intercellular and inter‐organ communication during exercise. Experimental evidence on their implication in cancer prevention is starting to emerge, and recently, their use as exercise mimetics has been proposed (Pinto et al., 2024). However, several important questions and challenges remain.
It is crucial to identify the specific cells from which EVs are released during exercise. The current studies suggest that the majority of EVs are derived from endothelial and blood cells and only a minor fraction from skeletal muscle. More studies are needed to further elucidate the cellular origins of EVs released during various modes of exercise. Researchers can gain a more detailed understanding of the heterogeneity of exercise‐induced EV populations and their cellular sources by employing single EV analysis techniques. Furthermore, the dynamics of EV release and clearance in response to exercise are not well understood. Investigating the rates at which EVs are released into circulation and cleared from the body could help elucidate their roles in exercise physiology and potential therapeutic applications. However, the main challenges hampering such studies are difficulties in continuous blood sample collection during exercise without affecting the training process and the lack of robust EV quantification and phenotyping techniques that do not require prior EV isolation. Considering that there is no standard methodology for EV isolation and analysis, it becomes crucial to accurately report the experimental details used in each study to be able to interpret the results correctly and compare them among different studies.
Individual variability is a common problem for all research based on human samples. In the context of EVs and exercise it is especially important to take into account differences in individual fitness. This variability could be reduced by allowing the study participants to reach baseline plasma conditions between training periods/bouts. This would allow to better separate the effects of exercise from the effects due to the body's adaptation to exercise. Other parameters that should be considered are the timing of blood sample collection, the processing of blood samples before EV isolation, diurnal variation, dietary parameters and previous or concurrent medical conditions and/or treatments of patients.
It also has to be considered that the same stimuli that trigger the EV release from normal cells could also stimulate the release of EVs from cancer tissues. Massive amounts of data convincingly show that cancer‐derived EVs promote cancer progression by transferring aggressive phenotypic traits and drug‐resistant phenotypes to other cancer cells, modulating the anti‐tumour immune response, as well as contributing to remodelling of the tumour microenvironment and formation of pre‐metastatic niches (reviewed in Vasconcelos et al., 2019). While the net effect of preoperative physical activity appears to be positive, increased release of cancer‐derived EVs in the bloodstream may counteract the beneficial effects of exercise. However, the proportion of cancer‐derived EVs and other exercise‐induced EVs is likely to be variable among patients and may depend on a variety of factors, including tumour size, vascularisation and EV release rate, and this variation in turn may contribute to the heterogeneity of patients’ response to exercise. Hence, this issue certainly warrants further investigation and should be taken into account when considering exercise‐based therapeutic interventions in a preoperative setting.
Current data indicate that exercise changes the molecular composition of EVs; however, it is not known if the same changes occur in healthy individuals and cancer patients whose EV composition is altered by the presence of cancer. Additional studies integrating several omics analyses are needed to have a more complete overview of the exercise‐induced changes in the EV cargo in healthy people and cancer patients. It is also essential to further investigate molecular changes in specific EV populations, including both EVs originating from different cells and/or different mechanisms, and investigate molecular mechanisms that help the sorting of specific molecules in EVs during exercise.
Furthermore, there is a gap in understanding how EVs' cell‐of‐origin, molecular cargo, and biological effects are interconnected. Elucidating this link is essential for deciphering the functional significance of EVs in exercise physiology and cancer treatment.
AUTHOR CONTRIBUTIONS
Alicia Llorente: Conceptualization (equal); formal analysis (equal); funding acquisition (equal); investigation (lead); methodology (equal); project administration (equal); writing—original draft (equal); writing—review and editing (equal). Agnese Brokāne: Formal analysis (equal); investigation (equal); methodology (equal); visualization (equal); writing—review and editing (equal). Agata Mlynska: Formal analysis (equal); investigation (equal); methodology (equal); writing—review and editing (equal). Marju Puurand: Formal analysis (equal); investigation (equal); methodology (equal); writing—review and editing (equal). Krizia Sagini: Formal analysis (equal); investigation (equal); methodology (equal); writing—review and editing (equal). Signe Folkmane: Formal analysis (equal); investigation (equal); visualization (equal); writing—review and editing (equal); Marit Hjorth: Formal analysis (equal); investigation (equal); methodology (equal); writing—review and editing (equal). Beatriz Martin‐Gracia: Formal analysis (equal); investigation (equal); methodology (equal); writing—review and editing (equal). Silvana Romero: Formal analysis (equal); investigation (equal); writing—review and editing (equal). Diana Skorinkina: Formal analysis (equal); investigation (equal); writing—review and editing (equal). Mārtiņš Čampa: Formal analysis (equal); investigation (equal); writing—review and editing(equal); Rūdolfs Cešeiko: Formal analysis (equal); investigation (equal); writing—review and editing (equal). Nadezhda Romanchikova: Formal analysis (equal); investigation (equal); writing—review and editing (equal). Aija Kļaviņa: Formal analysis (equal); funding acquisition (equal); investigation (equal); project administration (equal); writing—review and editing (equal). Tuuli Käämbre: Funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal); writing—review and editing (equal); Aija Linē: Conceptualization (lead); formal analysis (equal); funding acquisition (lead); investigation (equal); methodology (equal); project administration (lead); supervision (equal); writing—original draft (lead).
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ACKNOWLEDGEMENT
This work was supported by EEA and Norway Grants under project No EEA‐RESEARCH‐164 (CancerBeat).
Llorente, A. , Brokāne, A. , Mlynska, A. , Puurand, M. , Sagini, K. , Folkmane, S. , Hjorth, M. , Martin‐Gracia, B. , Romero, S. , Skorinkina, D. , Čampa, M. , Cešeiko, R. , Romanchikova, N. , Kļaviņa, A. , Käämbre, T. , & Linē, A. (2024). From sweat to hope: The role of exercise‐induced extracellular vesicles in cancer prevention and treatment. Journal of Extracellular Vesicles, 13, e12500. 10.1002/jev2.12500
REFERENCES
- Ali, N. , Rahat, S. T. , Mäkelä, M. , Nasserinejad, M. , Jaako, T. , Kinnunen, M. , Schroderus, J. , Tulppo, M. , Nieminen, A. I. , & Vainio, S. (2023). Metabolic patterns of sweat‐extracellular vesicles during exercise and recovery states using clinical grade patches. Frontiers in Physiology, 14, 1295852. 10.3389/fphys.2023.1295852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anane, L. H. , Edwards, K. M. , Burns, V. E. , Drayson, M. T. , Riddell, N. E. , van Zanten, J. J. , Wallace, G. R. , Mills, P. J. , & Bosch, J. A. (2009). Mobilization of gammadelta T lymphocytes in response to psychological stress, exercise, and beta‐agonist infusion. Brain, Behavior, and Immunity, 23(6), 823–829. 10.1016/j.bbi.2009.03.003 [DOI] [PubMed] [Google Scholar]
- Annibalini, G. , Contarelli, S. , Lucertini, F. , Guescini, M. , Maggio, S. , Ceccaroli, P. , Gervasi, M. , Ferri Marini, C. , Fardetti, F. , Grassi, E. , Stocchi, V. , Barbieri, E. , & Benelli, P. (2019). Muscle and systemic molecular responses to a single flywheel based iso‐inertial training session in resistance‐trained men. Frontiers in physiology, 10, 554. 10.3389/fphys.2019.00554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolopoulou, M. , Mastrototaro, L. , Hartwig, S. , Pesta, D. , Straßburger, K. , de Filippo, E. , Jelenik, T. , Karusheva, Y. , Gancheva, S. , Markgraf, D. , Herder, C. , Nair, K. S. , Reichert, A. S. , Lehr, S. , Müssig, K. , Al‐Hasani, H. , Szendroedi, J. , & Roden, M. (2021). Metabolic responsiveness to training depends on insulin sensitivity and protein content of exosomes in insulin‐resistant males. Science Advances, 7(41), eabi9551. 10.1126/sciadv.abi9551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi, S. , Hebda, J. K. , & Gavard, J. (2013). Vascular permeability and drug delivery in cancers. Frontiers in Oncology, 3, 211. 10.3389/fonc.2013.00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahari Khasraghi, L. , Nouri, M. , Vazirzadeh, M. , Hashemipour, N. , Talebi, M. , Aghaei Zarch, F. , Majidpoor, J. , Kalhor, K. , Farnia, P. , Najafi, S. , & Aghaei Zarch, S. M. (2023). MicroRNA‐206 in human cancer: Mechanistic and clinical perspectives. Cellular Signalling, 101, 110525. 10.1016/j.cellsig.2022.110525 [DOI] [PubMed] [Google Scholar]
- Baldelli, G. , De Santi, M. , Gervasi, M. , Annibalini, G. , Sisti, D. , Højman, P. , Sestili, P. , Stocchi, V. , Barbieri, E. , & Brandi, G. (2021). The effects of human sera conditioned by high‐intensity exercise sessions and training on the tumorigenic potential of cancer cells. Clinical & Translational Oncology: Official Publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico, 23(1), 22–34. 10.1007/s12094-020-02388-6 [DOI] [PubMed] [Google Scholar]
- Barcellos, N. , Cechinel, L. R. , de Meireles, L. C. F. , Lovatel, G. A. , Bruch, G. E. , Carregal, V. M. , Massensini, A. R. , Dalla Costa, T. , Pereira, L. O. , & Siqueira, I. R. (2020). Effects of exercise modalities on BDNF and IL‐1β content in circulating total extracellular vesicles and particles obtained from aged rats. Experimental Gerontology, 142, 111124. 10.1016/j.exger.2020.111124 [DOI] [PubMed] [Google Scholar]
- Battistelli, M. , & Falcieri, E. (2020). Apoptotic bodies: Particular extracellular vesicles involved in intercellular communication. Biology, 9(1), 21. 10.3390/biology9010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei, Y. , Xu, T. , Lv, D. , Yu, P. , Xu, J. , Che, L. , Das, A. , Tigges, J. , Toxavidis, V. , Ghiran, I. , Shah, R. , Li, Y. , Zhang, Y. , Das, S. , & Xiao, J. (2017). Exercise‐induced circulating extracellular vesicles protect against cardiac ischemia‐reperfusion injury. Basic Research in Cardiology, 112(4), 38. 10.1007/s00395-017-0628-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigley, A. B. , Rezvani, K. , Chew, C. , Sekine, T. , Pistillo, M. , Crucian, B. , Bollard, C. M. , & Simpson, R. J. (2014). Acute exercise preferentially redeploys NK‐cells with a highly‐differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Brain, Behavior, and Immunity, 39, 160–171. 10.1016/j.bbi.2013.10.030 [DOI] [PubMed] [Google Scholar]
- Brahmer, A. , Neuberger, E. , Esch‐Heisser, L. , Haller, N. , Jorgensen, M. M. , Baek, R. , Möbius, W. , Simon, P. , & Krämer‐Albers, E. M. (2019). Platelets, endothelial cells and leukocytes contribute to the exercise‐triggered release of extracellular vesicles into the circulation. Journal of Extracellular Vesicles, 8(1), 1615820. 10.1080/20013078.2019.1615820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer, A. , Neuberger, E. W. I. , Simon, P. , & Krämer‐Albers, E. M. (2020). Considerations for the analysis of small extracellular vesicles in physical exercise. Frontiers in Physiology, 11, 576150. 10.3389/fphys.2020.576150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryl‐Górecka, P. , Sathanoori, R. , Al‐Mashat, M. , Olde, B. , Jögi, J. , Evander, M. , Laurell, T. , & Erlinge, D. (2018). Effect of exercise on the plasma vesicular proteome: A methodological study comparing acoustic trapping and centrifugation. Lab on a Chip, 18(20), 3101–3111. 10.1039/c8lc00686e [DOI] [PubMed] [Google Scholar]
- Cairns, S. P. (2006). Lactic acid and exercise performance: Culprit or friend? Sports Medicine (Auckland, N.Z.), 36(4), 279–291. 10.2165/00007256-200636040-00001 [DOI] [PubMed] [Google Scholar]
- Campbell, J. P. , Riddell, N. E. , Burns, V. E. , Turner, M. , van Zanten, J. J. , Drayson, M. T. , & Bosch, J. A. (2009). Acute exercise mobilises CD8+ T lymphocytes exhibiting an effector‐memory phenotype. Brain, Behavior, and Immunity, 23(6), 767–775. 10.1016/j.bbi.2009.02.011 [DOI] [PubMed] [Google Scholar]
- Carles‐Fontana, R. , Heaton, N. , Palma, E. , & Khorsandi, S. E. (2022). Extracellular vesicle‐mediated mitochondrial reprogramming in cancer. Cancers, 14(8), 1865. 10.3390/cancers14081865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, M. C. , Shah, A. D. , Schittenhelm, R. B. , Silva, A. , James, P. F. , Wu, S. S. X. , & Howitt, J. (2024). Acute exercise‐induced release of innate immune proteins via small extracellular vesicles changes with aerobic fitness and age. Acta Physiologica (Oxford, England), 240(3), e14095. 10.1111/apha.14095 [DOI] [PubMed] [Google Scholar]
- Chong, M. C. , Silva, A. , James, P. F. , Wu, S. S. X. , & Howitt, J. (2022). Exercise increases the release of NAMPT in extracellular vesicles and alters NAD+ activity in recipient cells. Aging Cell, 21(7), e13647. 10.1111/acel.13647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conkright, W. R. , Beckner, M. E. , Sterczala, A. J. , Mi, Q. , Lovalekar, M. , Sahu, A. , Krajewski, K. T. , Martin, B. J. , Flanagan, S. D. , Greeves, J. P. , OʼLeary, T. J. , Wardle, S. L. , Ambrosio, F. , & Nindl, B. C. (2022). Resistance exercise differentially alters extracellular vesicle size and subpopulation characteristics in healthy men and women: An observational cohort study. Physiological Genomics, 54(9), 350–359. 10.1152/physiolgenomics.00171.2021 [DOI] [PubMed] [Google Scholar]
- Coumans, F. A. W. , Brisson, A. R. , Buzas, E. I. , Dignat‐George, F. , Drees, E. E. E. , El‐Andaloussi, S. , Emanueli, C. , Gasecka, A. , Hendrix, A. , Hill, A. F. , Lacroix, R. , Lee, Y. , van Leeuwen, T. G. , Mackman, N. , Mäger, I. , Nolan, J. P. , van der Pol, E. , Pegtel, D. M. , Sahoo, S. , … Nieuwland, R. (2017). Methodological guidelines to study extracellular vesicles. Circulation Research, 120(10), 1632–1648. 10.1161/CIRCRESAHA.117.309417 [DOI] [PubMed] [Google Scholar]
- Darragh, I. A. J. , McNamee, N. , Daly, R. , Pacheco, S. M. , OʼDriscoll, L. , & Egan, B. (2023). The separation and identification of circulating small extracellular vesicles from endurance‐trained, strength‐trained and recreationally active men. The Journal of Physiology, 601(22), 5075–5091. 10.1113/JP285170 [DOI] [PubMed] [Google Scholar]
- Darragh, I. A. J. , OʼDriscoll, L. , & Egan, B. (2021). Exercise training and circulating small extracellular vesicles: Appraisal of methodological approaches and current knowledge. Frontiers in Physiology, 12, 738333. 10.3389/fphys.2021.738333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado‐Peraza, F. , Nogueras‐Ortiz, C. , Simonsen, A. H. , Knight, D. D. , Yao, P. J. , Goetzl, E. J. , Jensen, C. S. , Høgh, P. , Gottrup, H. , Vestergaard, K. , Hasselbalch, S. G. , & Kapogiannis, D. (2023). Neuron‐derived extracellular vesicles in blood reveal effects of exercise in Alzheimer's disease. Alzheimer's Research & Therapy, 15(1), 156. 10.1186/s13195-023-01303-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen, C. , Hansen, L. S. , Lillelund, C. , Andersen, C. , Gehl, J. , Christensen, J. F. , Pedersen, B. K. , & Hojman, P. (2017). Exercise‐induced catecholamines activate the hippo tumor suppressor pathway to reduce risks of breast cancer development. Cancer Research, 77(18), 4894–4904. 10.1158/0008-5472.CAN-16-3125 [DOI] [PubMed] [Google Scholar]
- Diao, X. , Ling, Y. , Zeng, Y. , Wu, Y. , Guo, C. , Jin, Y. , Chen, X. , Feng, S. , Guo, J. , Ding, C. , Diao, F. , Du, Z. , Li, S. , & Qiu, H. (2023). Physical activity and cancer risk: A dose‐response analysis for the global burden of disease study 2019. Cancer Communications (London, England), 43(11), 1229–1243. 10.1002/cac2.12488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimassi, S. , Karkeni, E. , Laurant, P. , Tabka, Z. , Landrier, J. F. , & Riva, C. (2018). Microparticle miRNAs as biomarkers of vascular function and inflammation response to aerobic exercise in obesity? Obesity (Silver Spring, Md.), 26(10), 1584–1593. 10.1002/oby.22298 [DOI] [PubMed] [Google Scholar]
- Djurhuus, S. S. , Simonsen, C. , Toft, B. G. , Thomsen, S. N. , Wielsøe, S. , Røder, M. A. , Hasselager, T. , Østergren, P. B. , Jakobsen, H. , Pedersen, B. K. , Hojman, P. , Brasso, K. , & Christensen, J. F. (2023). Exercise training to increase tumour natural killer‐cell infiltration in men with localised prostate cancer: A randomised controlled trial. BJU International, 131(1), 116–124. 10.1111/bju.15842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doncheva, A. I. , Romero, S. , Ramirez‐Garrastacho, M. , Lee, S. , Kolnes, K. J. , Tangen, D. S. , Olsen, T. , Drevon, C. A. , Llorente, A. , Dalen, K. T. , & Hjorth, M. (2022). Extracellular vesicles and microRNAs are altered in response to exercise, insulin sensitivity and overweight. Acta Physiologica (Oxford, England), 236(4), e13862. 10.1111/apha.13862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza, R. F. , Woodhead, J. S. T. , Zeng, N. , Blenkiron, C. , Merry, T. L. , Cameron‐Smith, D. , & Mitchell, C. J. (2018). Circulatory exosomal miRNA following intense exercise is unrelated to muscle and plasma miRNA abundances. American Journal of Physiology. Endocrinology and Metabolism, 315(4), E723–E733. 10.1152/ajpendo.00138.2018 [DOI] [PubMed] [Google Scholar]
- Dudley, A. C. , & Griffioen, A. W. (2023). Pathological angiogenesis: Mechanisms and therapeutic strategies. Angiogenesis, 26(3), 313–347. 10.1007/s10456-023-09876-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle, J. , Marshall, G. , Lefkowitz, T. , & Maltser, S. (2024). Fractured knowledge: Making sense of exercise in patients with bone metastases. American Journal of Physical Medicine & Rehabilitation, 103(3S Suppl 1), S58–S61. 10.1097/PHM.0000000000002423 [DOI] [PubMed] [Google Scholar]
- Eschke, R. K. , Lampit, A. , Schenk, A. , Javelle, F. , Steindorf, K. , Diel, P. , Bloch, W. , & Zimmer, P. (2019). Impact of physical exercise on growth and progression of cancer in rodents – A systematic review and meta‐analysis. Frontiers in Oncology, 9, 35. 10.3389/fonc.2019.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estébanez, B. , Visavadiya, N. P. , de Paz, J. A. , Whitehurst, M. , Cuevas, M. J. , González‐Gallego, J. , & Huang, C. J. (2021). Resistance training diminishes the expression of exosome CD63 protein without modification of plasma miR‐146a‐5p and cfDNA in the elderly. Nutrients, 13(2), 665. 10.3390/nu13020665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves, M. , Monteiro, M. P. , & Duarte, J. A. (2021). Role of regular physical exercise in tumor vasculature: Favorable modulator of tumor milieu. International Journal of Sports Medicine, 42(5), 389–406. 10.1055/a-1308-3476 [DOI] [PubMed] [Google Scholar]
- Faraldi, M. , Sansoni, V. , Perego, S. , Gomarasca, M. , Gerosa, L. , Ponzetti, M. , Rucci, N. , Banfi, G. , & Lombardi, G. (2022). Acute changes in free and extracellular vesicle‐associated circulating miRNAs and myokine profile in professional sky‐runners during the Gran Sasso dʼItalia vertical run. Frontiers in Molecular Biosciences, 9, 915080. 10.3389/fmolb.2022.915080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio, M. A. , Hiscock, N. , Sacchetti, M. , Fischer, C. P. , & Pedersen, B. K. (2004). Interleukin‐6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes, 53(7), 1643–1648. 10.2337/diabetes.53.7.1643 [DOI] [PubMed] [Google Scholar]
- Federici, C. , Petrucci, F. , Caimi, S. , Cesolini, A. , Logozzi, M. , Borghi, M. , D'Ilio, S. , Lugini, L. , Violante, N. , Azzarito, T. , Majorani, C. , Brambilla, D. , & Fais, S. (2014). Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS ONE, 9(2), e88193. 10.1371/journal.pone.0088193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueira, A. C. C. , Cortinhas, A. , Soares, J. P. , Leitão, J. C. , Ferreira, R. P. , & Duarte, J. A. (2018). Efficacy of exercise on breast cancer outcomes: A systematic review and meta‐analysis of preclinical data. International Journal of Sports Medicine, 39(5), 327–342. 10.1055/s-0044-101149 [DOI] [PubMed] [Google Scholar]
- Fitzgerald, W. , Freeman, M. L. , Lederman, M. M. , Vasilieva, E. , Romero, R. , & Margolis, L. (2018). A system of cytokines encapsulated in extracellular vesicles. Scientific Reports, 8(1), 8973. 10.1038/s41598-018-27190-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiuza‐Luces, C. , Valenzuela, P. L. , Gálvez, B. G. , Ramírez, M. , López‐Soto, A. , Simpson, R. J. , & Lucia, A. (2024). The effect of physical exercise on anticancer immunity. Nature Reviews. Immunology, 24(4), 282–293. 10.1038/s41577-023-00943-0 [DOI] [PubMed] [Google Scholar]
- Frühbeis, C. , Helmig, S. , Tug, S. , Simon, P. , & Krämer‐Albers, E. M. (2015). Physical exercise induces rapid release of small extracellular vesicles into the circulation. Journal of Extracellular Vesicles, 4, 28239. 10.3402/jev.v4.28239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garai, K. , Adam, Z. , Herczeg, R. , Banfai, K. , Gyebrovszki, A. , Gyenesei, A. , Pongracz, J. E. , Wilhelm, M. , & Kvell, K. (2021). Physical activity as a preventive lifestyle intervention acts through specific exosomal miRNA species‐evidence from human short‐ and long‐term pilot studies. Frontiers in Physiology, 12, 658218. 10.3389/fphys.2021.658218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giloteaux, L. , Glass, K. A. , Germain, A. , Franconi, C. J. , Zhang, S. , & Hanson, M. R. (2024). Dysregulation of extracellular vesicle protein cargo in female myalgic encephalomyelitis/chronic fatigue syndrome cases and sedentary controls in response to maximal exercise. Journal of Extracellular Vesicles, 13(1), e12403. 10.1002/jev2.12403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondaliya, P. , Driscoll, J. , Yan, I. K. , Ali Sayyed, A. , & Patel, T. (2024). Therapeutic restoration of miR‐126‐3p as a multi‐targeted strategy to modulate the liver tumor microenvironment. Hepatology Communications, 8(3), e0373. 10.1097/HC9.0000000000000373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guescini, M. , Canonico, B. , Lucertini, F. , Maggio, S. , Annibalini, G. , Barbieri, E. , Luchetti, F. , Papa, S. , & Stocchi, V. (2015). Muscle releases alpha‐sarcoglycan positive extracellular vesicles carrying miRNAs in the bloodstream. PLoS ONE, 10(5), e0125094. 10.1371/journal.pone.0125094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagar, A. , Wang, Z. , Koyama, S. , Serrano, J. A. , Melo, L. , Vargas, S. , Carpenter, R. , & Foley, J. (2019). Endurance training slows breast tumor growth in mice by suppressing Treg cells recruitment to tumors. BMC Cancer, 19(1), 536. 10.1186/s12885-019-5745-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan, D. , & Weinberg, R. A. (2011). Hallmarks of cancer: The next generation. Cell, 144(5), 646–674. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Hill, A. F. (2019). Extracellular vesicles and neurodegenerative diseases. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 39(47), 9269–9273. 10.1523/JNEUROSCI.0147-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojman, P. , Dethlefsen, C. , Brandt, C. , Hansen, J. , Pedersen, L. , & Pedersen, B. K. (2011). Exercise‐induced muscle‐derived cytokines inhibit mammary cancer cell growth. American Journal of Physiology. Endocrinology and Metabolism, 301(3), E504–E510. 10.1152/ajpendo.00520.2010 [DOI] [PubMed] [Google Scholar]
- Hou, Z. , Qin, X. , Hu, Y. , Zhang, X. , Li, G. , Wu, J. , Li, J. , Sha, J. , Chen, J. , Xia, J. , Wang, L. , & Gao, F. (2019). Longterm exercise‐derived exosomal miR‐342‐5p: A novel exerkine for cardioprotection. Circulation Research, 124(9), 1386–1400. 10.1161/CIRCRESAHA.118.314635 [DOI] [PubMed] [Google Scholar]
- Huang, Q. , Wu, M. , Wu, X. , Zhang, Y. , & Xia, Y. (2022). Muscle‐to‐tumor crosstalk: The effect of exercise‐induced myokine on cancer progression. Biochimica et Biophysica Acta. Reviews on Cancer, 1877(5), 188761. 10.1016/j.bbcan.2022.188761 [DOI] [PubMed] [Google Scholar]
- Jalil, A. T. , Abdulhadi, M. A. , Al‐Ameer, L. R. , Abbas, H. A. , Merza, M. S. , Zabibah, R. S. , & Fadhil, A. A. (2023). The emerging role of microRNA‐126 as a potential therapeutic target in cancer: A comprehensive review. Pathology, Research and Practice, 248, 154631. 10.1016/j.prp.2023.154631 [DOI] [PubMed] [Google Scholar]
- Jee, H. , Park, E. , Hur, K. , Kang, M. , & Kim, Y. (2022). High‐intensity aerobic exercise suppresses cancer growth by regulating skeletal muscle‐derived oncogenes and tumor suppressors. Frontiers in Molecular Biosciences, 9, 818470. 10.3389/fmolb.2022.818470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen, D. K. , Fenix, A. M. , Franklin, J. L. , Higginbotham, J. N. , Zhang, Q. , Zimmerman, L. J. , Liebler, D. C. , Ping, J. , Liu, Q. , Evans, R. , Fissell, W. H. , Patton, J. G. , Rome, L. H. , Burnette, D. T. , & Coffey, R. J. (2019). Reassessment of exosome composition. Cell, 177(2), 428–445.e18. 10.1016/j.cell.2019.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, L. W. , Viglianti, B. L. , Tashjian, J. A. , Kothadia, S. M. , Keir, S. T. , Freedland, S. J. , Potter, M. Q. , Moon, E. J. , Schroeder, T. , Herndon, J. E., 2nd , & Dewhirst, M. W. (2010). Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. Journal of Applied Physiology (Bethesda, Md.: 1985), 108(2), 343–348. 10.1152/japplphysiol.00424.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just, J. , Yan, Y. , Farup, J. , Sieljacks, P. , Sloth, M. , Venø, M. , Gu, T. , de Paoli, F. V. , Nyengaard, J. R. , Bæk, R. , Jørgensen, M. M. , Kjems, J. , Vissing, K. , & Drasbek, K. R. (2020). Blood flow‐restricted resistance exercise alters the surface profile, miRNA cargo and functional impact of circulating extracellular vesicles. Scientific Reports, 10(1), 5835. 10.1038/s41598-020-62456-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvinen, S. , Sievänen, T. , Karppinen, J. E. , Hautasaari, P. , Bart, G. , Samoylenko, A. , Vainio, S. J. , Ahtiainen, J. P. , Laakkonen, E. K. , & Kujala, U. M. (2020). MicroRNAs in extracellular vesicles in sweat change in response to endurance exercise. Frontiers in Physiology, 11, 676. 10.3389/fphys.2020.00676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr, J. , Anderson, C. , & Lippman, S. M. (2017). Physical activity, sedentary behaviour, diet, and cancer: An update and emerging new evidence. The Lancet. Oncology, 18(8), e457–e471. 10.1016/S1470-2045(17)30411-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, S. Y. , Lee, W. , Kenny, H. A. , Dang, L. H. , Ellis, L. M. , Jonasch, E. , Lengyel, E. , & Naora, H. (2019). Cancer‐derived small extracellular vesicles promote angiogenesis by heparin‐bound, bevacizumab‐insensitive VEGF, independent of vesicle uptake. Communications Biology, 2, 386. 10.1038/s42003-019-0609-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, Y. , Eguchi, A. , Tamai, Y. , Fukuda, S. , Tempaku, M. , Izuoka, K. , Iwasa, M. , Takei, Y. , & Togashi, K. (2021). Protein composition of circulating extracellular vesicles immediately changed by particular short time of high‐intensity interval training exercise. Frontiers in Physiology, 12, 693007. 10.3389/fphys.2021.693007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka, N. , Kogure, A. , Yamamoto, T. , Urabe, F. , Usuba, W. , Prieto‐Vila, M. , & Ochiya, T. (2019). Exploiting the message from cancer: The diagnostic value of extracellular vesicles for clinical applications. Experimental & Molecular Medicine, 51(3), 1–9. 10.1038/s12276-019-0219-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal, J. , Arras, G. , Colombo, M. , Jouve, M. , Morath, J. P. , Primdal‐Bengtson, B. , Dingli, F. , Loew, D. , Tkach, M. , & Théry, C. (2016). Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proceedings of the National Academy of Sciences of the United States of America, 113(8), E968–E977. 10.1073/pnas.1521230113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraschnewski, J. L. , & Schmitz, K. H. (2017). Exercise in the prevention and treatment of breast cancer: What clinicians need to tell their patients. Current sports medicine reports, 16(4), 263–267. 10.1249/JSR.0000000000000388 [DOI] [PubMed] [Google Scholar]
- Lai, Z. , Lin, W. , Yan, X. , Chen, X. , & Xu, G. (2023). Fatiguing freestyle swimming modifies miRNA profiles of circulating extracellular vesicles in athletes. European Journal of Applied Physiology, 123(9), 2041–2051. 10.1007/s00421-023-05167-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin, K. M. , Graham, Z. A. , McAdam, J. S. , OʼBryan, S. M. , Drummer, D. , Bell, M. B. , Kelley, C. J. , Lixandrão, M. E. , Peoples, B. , Tuggle, S. C. , Seay, R. S. , Van Keuren‐Jensen, K. , Huentelman, M. J. , Pirrotte, P. , Reiman, R. , Alsop, E. , Hutchins, E. , Antone, J. , Bonfitto, A. , … Bamman, M. M. (2023). Dynamic transcriptomic responses to divergent acute exercise stimuli in young adults. Physiological Genomics, 55(4), 194–212. 10.1152/physiolgenomics.00144.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C. C. , Ho, K. H. , Huang, T. W. , Shih, C. M. , Hsu, S. Y. , Liu, A. J. , & Chen, K. C. (2021). A regulatory loop among CD276, miR‐29c‐3p, and Myc exists in cancer cells against natural killer cell cytotoxicity. Life Sciences, 277, 119438. 10.1016/j.lfs.2021.119438 [DOI] [PubMed] [Google Scholar]
- Li, D. , Xia, L. , Chen, M. , Lin, C. , Wu, H. , Zhang, Y. , Pan, S. , & Li, X. (2017). miR‐133b, a particular member of myomiRs, coming into playing its unique pathological role in human cancer. Oncotarget, 8(30), 50193–50208. 10.18632/oncotarget.16745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Xiao, X. , Zhang, Y. , Tang, W. , Zhong, D. , Liu, T. , Zhu, Y. , Li, J. , & Jin, R. (2022). Effect of exercise on breast cancer: A systematic review and meta‐analysis of animal experiments. Frontiers in Molecular Biosciences, 9, 843810. 10.3389/fmolb.2022.843810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischnig, A. , Bergqvist, M. , Ochiya, T. , & Lässer, C. (2022). Quantitative proteomics identifies proteins enriched in large and small extracellular vesicles. Molecular & Cellular Proteomics: MCP, 21(9), 100273. 10.1016/j.mcpro.2022.100273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisi, V. , Moulton, C. , Fantini, C. , Grazioli, E. , Guidotti, F. , Sgrò, P. , Dimauro, I. , Capranica, L. , Parisi, A. , Di Luigi, L. , & Caporossi, D. (2023). Steady‐state redox status in circulating extracellular vesicles: A proof‐of‐principle study on the role of fitness level and short‐term aerobic training in healthy young males. Free Radical Biology & Medicine, 204, 266–275. 10.1016/j.freeradbiomed.2023.05.007 [DOI] [PubMed] [Google Scholar]
- Lisi, V. , Senesi, G. , Bertola, N. , Pecoraro, M. , Bolis, S. , Gualerzi, A. , Picciolini, S. , Raimondi, A. , Fantini, C. , Moretti, E. , Parisi, A. , Sgrò, P. , Di Luigi, L. , Geiger, R. , Ravera, S. , Vassalli, G. , Caporossi, D. , & Balbi, C. (2023). Plasma‐derived extracellular vesicles released after endurance exercise exert cardioprotective activity through the activation of antioxidant pathways. Redox Biology, 63, 102737. 10.1016/j.redox.2023.102737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett, J. A. C. , Durcan, P. J. , & Myburgh, K. H. (2018). Investigation of circulating extracellular vesicle microRNA following two consecutive bouts of muscle‐damaging exercise. Frontiers in Physiology, 9, 1149. 10.3389/fphys.2018.01149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, M. , Sanderson, S. M. , Zessin, A. , Ashcraft, K. A. , Jones, L. W. , Dewhirst, M. W. , Locasale, J. W. , & Hsu, D. S. (2018). Exercise inhibits tumor growth and central carbon metabolism in patient‐derived xenograft models of colorectal cancer. Cancer & Metabolism, 6, 14. 10.1186/s40170-018-0190-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, C. , Wang, J. , Liu, H. , Chen, Y. , Ma, X. , Chen, S. , Chen, Y. , Bihl, J. I. , & Yang, Y. I. (2018). Moderate exercise enhances endothelial progenitor cell exosomes release and function. Medicine and Science in Sports and Exercise, 50(10), 2024–2032. 10.1249/MSS.0000000000001672 [DOI] [PubMed] [Google Scholar]
- Maggio, S. , Canonico, B. , Ceccaroli, P. , Polidori, E. , Cioccoloni, A. , Giacomelli, L. , Ferri Marini, C. , Annibalini, G. , Gervasi, M. , Benelli, P. , Fabbri, F. , Del Coco, L. , Fanizzi, F. P. , Giudetti, A. M. , Lucertini, F. , & Guescini, M. (2023). Modulation of the circulating extracellular vesicles in response to different exercise regimens and study of their inflammatory effects. International Journal of Molecular Sciences, 24(3), 3039. 10.3390/ijms24033039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews, C. E. , Moore, S. C. , Arem, H. , Cook, M. B. , Trabert, B. , Håkansson, N. , Larsson, S. C. , Wolk, A. , Gapstur, S. M. , Lynch, B. M. , Milne, R. L. , Freedman, N. D. , Huang, W. Y. , Berrington de Gonzalez, A. , Kitahara, C. M. , Linet, M. S. , Shiroma, E. J. , Sandin, S. , Patel, A. V. , & Lee, I. M. (2020). Amount and intensity of leisure‐time physical activity and lower cancer risk. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 38(7), 686–697. 10.1200/JCO.19.02407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlvenna, L. C. , Parker, H. J. , Seabright, A. P. , Sale, B. , Anghileri, G. , Weaver, S. R. C. , Lucas, S. J. E. , & Whitham, M. (2023). Single vesicle analysis reveals the release of tetraspanin positive extracellular vesicles into circulation with high intensity intermittent exercise. The Journal of Physiology, 601(22), 5093–5106. 10.1113/JP284047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlvenna, L. C. , & Whitham, M. (2023). Exercise, healthy ageing, and the potential role of small extracellular vesicles. The Journal of Physiology, 601(22), 4937–4951. 10.1113/JP282468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchelson, K. R. , & Qin, W. Y. (2015). Roles of the canonical myomiRs miR‐1, ‐133 and ‐206 in cell development and disease. World Journal of Biological Chemistry, 6(3), 162–208. 10.4331/wjbc.v6.i3.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad, S. , Hutchinson, K. A. , da Silva, D. F. , Bhattacharjee, J. , McInnis, K. , Burger, D. , & Adamo, K. B. (2021). Circulating small extracellular vesicles increase after an acute bout of moderate‐intensity exercise in pregnant compared to non‐pregnant women. Scientific Reports, 11(1), 12615. 10.1038/s41598-021-92180-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, S. C. , Lee, I. M. , Weiderpass, E. , Campbell, P. T. , Sampson, J. N. , Kitahara, C. M. , Keadle, S. K. , Arem, H. , Berrington de Gonzalez, A. , Hartge, P. , Adami, H. O. , Blair, C. K. , Borch, K. B. , Boyd, E. , Check, D. P. , Fournier, A. , Freedman, N. D. , Gunter, M. , Johannson, M. , … Patel, A. V. (2016). Association of leisure‐time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Internal Medicine, 176(6), 816–825. 10.1001/jamainternmed.2016.1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooren, F. C. , Blöming, D. , Lechtermann, A. , Lerch, M. M. , & Völker, K. (2002). Lymphocyte apoptosis after exhaustive and moderate exercise. Journal of Applied Physiology (Bethesda, Md.: 1985), 93(1), 147–153. 10.1152/japplphysiol.01262.2001 [DOI] [PubMed] [Google Scholar]
- Morishita, S. , Hamaue, Y. , Fukushima, T. , Tanaka, T. , Fu, J. B. , & Nakano, J. (2020). Effect of exercise on mortality and recurrence in patients with cancer: A systematic review and meta‐analysis. Integrative Cancer Therapies, 19, 1534735420917462. 10.1177/1534735420917462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo, O. D. , Thistlethwaite, W. , Rozowsky, J. , Subramanian, S. L. , Lucero, R. , Shah, N. , Jackson, A. R. , Srinivasan, S. , Chung, A. , Laurent, C. D. , Kitchen, R. R. , Galeev, T. , Warrell, J. , Diao, J. A. , Welsh, J. A. , Hanspers, K. , Riutta, A. , Burgstaller‐Muehlbacher, S. , Shah, R. V. , … Milosavljevic, A. (2019). exRNA atlas analysis reveals distinct extracellular RNA cargo types and their carriers present across human biofluids. Cell, 177(2), 463–477.e15. 10.1016/j.cell.2019.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy, J. A. , Chang, S. H. , Shih, S. C. , Dvorak, A. M. , & Dvorak, H. F. (2010). Heterogeneity of the tumor vasculature. Seminars in Thrombosis and Hemostasis, 36(3), 321–331. 10.1055/s-0030-1253454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair, V. D. , Ge, Y. , Li, S. , Pincas, H. , Jain, N. , Seenarine, N. , Amper, M. A. S. , Goodpaster, B. H. , Walsh, M. J. , Coen, P. M. , & Sealfon, S. C. (2020). Sedentary and trained older men have distinct circulating exosomal microRNA profiles at baseline and in response to acute exercise. Frontiers in Physiology, 11, 605. 10.3389/fphys.2020.00605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederveen, J. P. , Warnier, G. , Di Carlo, A. , Nilsson, M. I. , & Tarnopolsky, M. A. (2021). Extracellular vesicles and exosomes: Insights from exercise science. Frontiers in Physiology, 11, 604274. 10.3389/fphys.2020.604274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger, E. W. I. , Hillen, B. , Mayr, K. , Simon, P. , Krämer‐Albers, E. M. , & Brahmer, A. (2021). Kinetics and topology of DNA associated with circulating extracellular vesicles released during exercise. Genes, 12(4), 522. 10.3390/genes12040522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohata, N. , Hanazawa, T. , Enokida, H. , & Seki, N. (2012). microRNA‐1/133a and microRNA‐206/133b clusters: Dysregulation and functional roles in human cancers. Oncotarget, 3(1), 9–21. 10.18632/oncotarget.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nørgård, M. Ø. , Lund, P. M. , Kalisi, N. , Andresen, T. L. , Larsen, J. B. , Vogel, S. , & Svenningsen, P. (2023). Mitochondrial reactive oxygen species modify extracellular vesicles secretion rate. FASEB BioAdvances, 5(9), 355–366. 10.1096/fba.2023-00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, G. P., Jr , Porto, W. F. , Palu, C. C. , Pereira, L. M. , Petriz, B. , Almeida, J. A. , Viana, J. , Filho, N. N. A. , Franco, O. L. , & Pereira, R. W. (2018). Effects of acute aerobic exercise on rats serum extracellular vesicles diameter, concentration and small RNAs content. Frontiers in Physiology, 9, 532. 10.3389/fphys.2018.00532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange, S. T. , Jordan, A. R. , Odell, A. , Kavanagh, O. , Hicks, K. M. , Eaglen, T. , Todryk, S. , & Saxton, J. M. (2022). Acute aerobic exercise‐conditioned serum reduces colon cancer cell proliferation in vitro through interleukin‐6‐induced regulation of DNA damage. International Journal of Cancer, 151(2), 265–274. 10.1002/ijc.33982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange, S. T. , Leslie, J. , Ross, M. , Mann, D. A. , & Wackerhage, H. (2023). The exercise IL‐6 enigma in cancer. Trends in Endocrinology and Metabolism: TEM, 34(11), 749–763. 10.1016/j.tem.2023.08.001 [DOI] [PubMed] [Google Scholar]
- Padilha, C. S. , Testa, M. T. , Marinello, P. C. , Cella, P. S. , Voltarelli, F. A. , Frajacomo, F. T. , Cechini, R. , Duarte, J. A. R. , Guarnier, F. A. , & Deminice, R. (2019). Resistance exercise counteracts tumor growth in two carcinoma rodent models. Medicine and Science in Sports and Exercise, 51(10), 2003–2011. 10.1249/MSS.0000000000002009 [DOI] [PubMed] [Google Scholar]
- Padrão, A. I. , Nogueira‐Ferreira, R. , Vitorino, R. , Carvalho, D. , Correia, C. , Neuparth, M. J. , Pires, M. J. , Faustino‐Rocha, A. I. , Santos, L. L. , Oliveira, P. A. , Duarte, J. A. , Moreira‐Gonçalves, D. , & Ferreira, R. (2018). Exercise training protects against cancer‐induced cardiac remodeling in an animal model of urothelial carcinoma. Archives of Biochemistry and Biophysics, 645, 12–18. 10.1016/j.abb.2018.03.013 [DOI] [PubMed] [Google Scholar]
- Papetti, M. , & Herman, I. M. (2002). Mechanisms of normal and tumor‐derived angiogenesis. American Journal of Physiology. Cell Physiology, 282(5), C947–C970. 10.1152/ajpcell.00389.2001 [DOI] [PubMed] [Google Scholar]
- Park, S. , & Moon, H. Y. (2022). Urinary extracellular vesicle as a potential biomarker of exercise‐induced fatigue in young adult males. European Journal of Applied Physiology, 122(10), 2175–2188. 10.1007/s00421-022-04995-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova, N. N. , Zhu, J. , & Thompson, C. B. (2022). The hallmarks of cancer metabolism: Still emerging. Cell Metabolism, 34(3), 355–377. 10.1016/j.cmet.2022.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, L. , Christensen, J. F. , & Hojman, P. (2015). Effects of exercise on tumor physiology and metabolism. Cancer Journal (Sudbury, Mass.), 21(2), 111–116. 10.1097/PPO.0000000000000096 [DOI] [PubMed] [Google Scholar]
- Pedersen, L. , Idorn, M. , Olofsson, G. H. , Lauenborg, B. , Nookaew, I. , Hansen, R. H. , Johannesen, H. H. , Becker, J. C. , Pedersen, K. S. , Dethlefsen, C. , Nielsen, J. , Gehl, J. , Pedersen, B. K. , Thor Straten, P. , & Hojman, P. (2016). Voluntary running suppresses tumor growth through epinephrine‐ and IL‐6‐dependent NK cell mobilization and redistribution. Cell Metabolism, 23(3), 554–562. 10.1016/j.cmet.2016.01.011 [DOI] [PubMed] [Google Scholar]
- Pietrangelo, T. , Santangelo, C. , Bondi, D. , Cocci, P. , Piccinelli, R. , Piacenza, F. , Rosato, E. , Azman, S. N. A. , Binetti, E. , Farina, M. , Locatelli, M. , Brunetti, V. , Le Donne, C. , Marramiero, L. , Di Filippo, E. S. , Verratti, V. , Fulle, S. , Scollo, V. , & Palermo, F. (2023). Endurance‐dependent urinary extracellular vesicle signature: Shape, metabolic miRNAs, and purine content distinguish triathletes from inactive people. Pflugers Archiv: European Journal of Physiology, 475(6), 691–709. 10.1007/s00424-023-02815-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto, A. C. , Tavares, P. , Neves, B. , Oliveira, P. F. , Vitorino, R. , Moreira‐Gonçalves, D. , & Ferreira, R. (2024). Exploiting the therapeutic potential of contracting skeletal muscle‐released extracellular vesicles in cancer: Current insights and future directions. Journal of Molecular Medicine (Berlin, Germany), 102(5), 617–628. 10.1007/s00109-024-02427-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polónia, B. , Xavier, C. P. R. , Kopecka, J. , Riganti, C. , & Vasconcelos, M. H. (2023). The role of Extracellular Vesicles in glycolytic and lipid metabolic reprogramming of cancer cells: Consequences for drug resistance. Cytokine & Growth Factor Reviews, 73, 150–162. 10.1016/j.cytogfr.2023.05.001 [DOI] [PubMed] [Google Scholar]
- Proia, P. , Schiera, G. , Mineo, M. , Ingrassia, A. M. , Santoro, G. , Savettieri, G. , & Di Liegro, I. (2008). Astrocytes shed extracellular vesicles that contain fibroblast growth factor‐2 and vascular endothelial growth factor. International Journal of Molecular Medicine, 21(1), 63–67. [PubMed] [Google Scholar]
- Rigamonti, A. E. , Bollati, V. , Pergoli, L. , Iodice, S. , De Col, A. , Tamini, S. , Cicolini, S. , Tringali, G. , De Micheli, R. , Cella, S. G. , & Sartorio, A. (2020). Effects of an acute bout of exercise on circulating extracellular vesicles: Tissue‐, sex‐, and BMI‐related differences. International Journal of Obesity (2005), 44(5), 1108–1118. 10.1038/s41366-019-0460-7 [DOI] [PubMed] [Google Scholar]
- Ruiz‐Casado, A. , Martín‐Ruiz, A. , Pérez, L. M. , Provencio, M. , Fiuza‐Luces, C. , & Lucia, A. (2017). Exercise and the hallmarks of cancer. Trends in Cancer, 3(6), 423–441. 10.1016/j.trecan.2017.04.007 [DOI] [PubMed] [Google Scholar]
- Sadovska, L. , Auders, J. , Keiša, L. , Romanchikova, N. , Silamiķele, L. , Kreišmane, M. , Zayakin, P. , Takahashi, S. , Kalniņa, Z. , & Linē, A. (2022). Exercise‐induced extracellular vesicles delay the progression of prostate cancer. Frontiers in Molecular Biosciences, 8, 784080. 10.3389/fmolb.2021.784080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheera, S. , Jani, V. P. , Witwer, K. W. , & Kutty, S. (2021). Extracellular vesicle interplay in cardiovascular pathophysiology. American Journal of Physiology. Heart and Circulatory Physiology, 320(5), H1749–H1761. 10.1152/ajpheart.00925.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, M. E. , Wiskemann, J. , Armbrust, P. , Schneeweiss, A. , Ulrich, C. M. , & Steindorf, K. (2015). Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: A randomized controlled trial. International Journal of Cancer, 137(2), 471–480. 10.1002/ijc.29383 [DOI] [PubMed] [Google Scholar]
- Sepúlveda, F. , Mayorga‐Lobos, C. , Guzmán, K. , Durán‐Jara, E. , & Lobos‐González, L. (2023). EV‐miRNA‐mediated intercellular communication in the breast tumor microenvironment. International Journal of Molecular Sciences, 24(17), 13085. 10.3390/ijms241713085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severinsen, M. C. K. , & Pedersen, B. K. (2020). Muscle‐organ crosstalk: The emerging roles of myokines. Endocrine Reviews, 41(4), 594–609. 10.1210/endrev/bnaa016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver, J. L. , Alexander, S. E. , Dillon, H. T. , Lamon, S. , & Wadley, G. D. (2020). Extracellular vesicular miRNA expression is not a proxy for skeletal muscle miRNA expression in males and females following acute, moderate intensity exercise. Physiological Reports, 8(16), e14520. 10.14814/phy2.14520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, B. , Hayes, S. C. , Spence, R. R. , Steele, M. L. , Millet, G. Y. , & Gergele, L. (2020). Exercise and colorectal cancer: A systematic review and meta‐analysis of exercise safety, feasibility and effectiveness. The International Journal of Behavioral Nutrition and Physical Activity, 17(1), 122. 10.1186/s12966-020-01021-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppich, B. , Dayyani, F. , Gruber, R. , Lorenz, R. , Mack, M. , & Ziegler‐Heitbrock, H. W. (2000). Selective mobilization of CD14(+)CD16(+) monocytes by exercise. American Journal of Physiology. Cell Physiology, 279(3), C578–C586. 10.1152/ajpcell.2000.279.3.C578 [DOI] [PubMed] [Google Scholar]
- Sugiyama, T. , Taniguchi, K. , Matsuhashi, N. , Tajirika, T. , Futamura, M. , Takai, T. , Akao, Y. , & Yoshida, K. (2016). MiR‐133b inhibits growth of human gastric cancer cells by silencing pyruvate kinase muscle‐splicer polypyrimidine tract‐binding protein 1. Cancer Science, 107(12), 1767–1775. 10.1111/cas.13091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, B. P. , Nie, Y. , Evans, S. , Kargl, C. K. , Hettinger, Z. R. , Garner, R. T. , Hubal, M. J. , Kuang, S. , Stout, J. , & Gavin, T. P. (2022). Obesity and exercise training alter inflammatory pathway skeletal muscle small extracellular vesicle microRNAs. Experimental Physiology, 107(5), 462–475. 10.1113/EP090062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, J. , Azimi, I. , Monteith, G. , & Bebawy, M. (2020). Ca2+ mediates extracellular vesicle biogenesis through alternate pathways in malignancy. Journal of Extracellular Vesicles, 9(1), 1734326. 10.1080/20013078.2020.1734326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, W. , & Papoutsakis, E. T. (2023). The role of biomechanical stress in extracellular vesicle formation, composition and activity. Biotechnology Advances, 66, 108158. 10.1016/j.biotechadv.2023.108158 [DOI] [PubMed] [Google Scholar]
- Townley‐Tilson, W. H. , Callis, T. E. , & Wang, D. (2010). MicroRNAs 1, 133, and 206: Critical factors of skeletal and cardiac muscle development, function, and disease. The International Journal of Biochemistry & Cell Biology, 42(8), 1252–1255. 10.1016/j.biocel.2009.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderboom, P. M. , Dasari, S. , Ruegsegger, G. N. , Pataky, M. W. , Lucien, F. , Heppelmann, C. J. , Lanza, I. R. , & Nair, K. S. (2021). A size‐exclusion‐based approach for purifying extracellular vesicles from human plasma. Cell Reports Methods, 1(3), 100055. 10.1016/j.crmeth.2021.100055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden, M. G. , Cantley, L. C. , & Thompson, C. B. (2009). Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science (New York, N.Y.), 324(5930), 1029–1033. 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos, M. H. , Caires, H. R. , Ābols, A. , Xavier, C. P. R. , & Linē, A. (2019). Extracellular vesicles as a novel source of biomarkers in liquid biopsies for monitoring cancer progression and drug resistance. Drug Resistance Updates: Reviews and Commentaries in Antimicrobial and Anticancer Chemotherapy, 47, 100647. 10.1016/j.drup.2019.100647 [DOI] [PubMed] [Google Scholar]
- Vechetti, I. J. , Norrbom, J. , Alkner, B. , Hjalmarsson, E. , Palmcrantz, A. , Pontén, E. , Pingel, J. , von Walden, F. , & Fernandez‐Gonzalo, R. (2022). Extracellular vesicle characteristics and microRNA content in cerebral palsy and typically developed individuals at rest and in response to aerobic exercise. Frontiers in Physiology, 13, 1072040. 10.3389/fphys.2022.1072040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu, L. T. , Gong, J. , Pham, T. T. , Kim, Y. , & Le, M. T. N. (2020). microRNA exchange via extracellular vesicles in cancer. Cell Proliferation, 53(11), e12877. 10.1111/cpr.12877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Xing, Q. F. , Liu, X. Q. , Guo, Z. J. , Li, C. Y. , & Sun, G. (2016). MiR‐122 targets VEGFC in bladder cancer to inhibit tumor growth and angiogenesis. American Journal of Translational Research, 8(7), 3056–3066. [PMC free article] [PubMed] [Google Scholar]
- Warnier, G. , De Groote, E. , Britto, F. A. , Delcorte, O. , Nederveen, J. P. , Nilsson, M. I. , Pierreux, C. E. , Tarnopolsky, M. A. , & Deldicque, L. (2022). Effects of an acute exercise bout in hypoxia on extracellular vesicle release in healthy and prediabetic subjects. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 322(2), R112–R122. 10.1152/ajpregu.00220.2021 [DOI] [PubMed] [Google Scholar]
- Warnier, G. , DE Groote, E. , Delcorte, O. , Nicolas Martinez, D. , Nederveen, J. P. , Nilsson, M. I. , Francaux, M. , Pierreux, C. E. , & Deldicque, L. (2023). Effects of a 6‐wk sprint interval training protocol at different altitudes on circulating extracellular vesicles. Medicine and Science in Sports and Exercise, 55(1), 46–54. 10.1249/MSS.0000000000003031 [DOI] [PubMed] [Google Scholar]
- Watanabe, S. , Sudo, Y. , Makino, T. , Kimura, S. , Tomita, K. , Noguchi, M. , Sakurai, H. , Shimizu, M. , Takahashi, Y. , Sato, R. , & Yamauchi, Y. (2022). Skeletal muscle releases extracellular vesicles with distinct protein and microRNA signatures that function in the muscle microenvironment. PNAS Nexus, 1(4), pgac173. 10.1093/pnasnexus/pgac173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh, J. A. , Goberdhan, D. C. I. , O'Driscoll, L. , Buzas, E. I. , Blenkiron, C. , Bussolati, B. , Cai, H. , Di Vizio, D. , Driedonks, T. A. P. , Erdbrügger, U. , Falcon‐Perez, J. M. , Fu, Q. L. , Hill, A. F. , Lenassi, M. , Lim, S. K. , Mahoney, M. G. , Mohanty, S. , Möller, A. , Nieuwland, R. , … Witwer, K. W. (2024). Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. Journal of Extracellular Vesicles, 13(2), e12404. 10.1002/jev2.12404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerberg, E. , Lhuillier, C. , Rybstein, M. D. , Dannenberg, K. , Rudqvist, N. P. , Koelwyn, G. J. , Jones, L. W. , & Demaria, S. (2020). Exercise reduces immune suppression and breast cancer progression in a preclinical model. Oncotarget, 11(4), 452–461. 10.18632/oncotarget.27464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham, M. , Parker, B. L. , Friedrichsen, M. , Hingst, J. R. , Hjorth, M. , Hughes, W. E. , Egan, C. L. , Cron, L. , Watt, K. I. , Kuchel, R. P. , Jayasooriah, N. , Estevez, E. , Petzold, T. , Suter, C. M. , Gregorevic, P. , Kiens, B. , Richter, E. A. , James, D. E. , Wojtaszewski, J. F. P. , & Febbraio, M. A. (2018). Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metabolism, 27(1), 237–251.e4. 10.1016/j.cmet.2017.12.001 [DOI] [PubMed] [Google Scholar]
- Wu, T. , Han, N. , Zhao, C. , Huang, X. , Su, P. , & Li, X. (2022). The long non‐sacoding RNA TMEM147‐AS1/miR‐133b/ZNF587 axis regulates the Warburg effect and promotes prostatic carcinoma invasion and proliferation. The Journal of Gene Medicine, 24(11), e3453. 10.1002/jgm.3453 [DOI] [PubMed] [Google Scholar]
- Xavier, C. P. R. , Belisario, D. C. , Rebelo, R. , Assaraf, Y. G. , Giovannetti, E. , Kopecka, J. , & Vasconcelos, M. H. (2022). The role of extracellular vesicles in the transfer of drug resistance competences to cancer cells. Drug Resistance Updates: Reviews and Commentaries in Antimicrobial and Anticancer Chemotherapy, 62, 100833. 10.1016/j.drup.2022.100833 [DOI] [PubMed] [Google Scholar]
- Xhuti, D. , Nilsson, M. I. , Manta, K. , Tarnopolsky, M. A. , & Nederveen, J. P. (2023). Circulating exosome‐like vesicle and skeletal muscle microRNAs are altered with age and resistance training. The Journal of Physiology, 601(22), 5051–5073. 10.1113/JP282663 [DOI] [PubMed] [Google Scholar]
- Xiang, H. , Chen, S. , Zhou, J. , Guo, J. , Zhou, Q. , & Zhou, Q. (2020). Characterization of blood‐derived exosomal proteins after exercise. The Journal of International Medical Research, 48(9), 300060520957541. 10.1177/0300060520957541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, J. Y. , Wei, J. X. , Lv, L. H. , Han, Q. F. , Yang, W. B. , Li, G. L. , Wang, P. X. , Wu, S. B. , Duan, J. X. , Zhuo, W. F. , Liu, P. Q. , & Min, J. (2020). Angiopoietin‐2 induces angiogenesis via exosomes in human hepatocellular carcinoma. Cell Communication and Signaling: CCS, 18(1), 46. 10.1186/s12964-020-00535-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, A. , Maeshige, N. , Noguchi, H. , Yan, J. , Ma, X. , Uemura, M. , Su, D. , Kondo, H. , Sarosiek, K. , & Fujino, H. (2023). Pulsed ultrasound promotes secretion of anti‐inflammatory extracellular vesicles from skeletal myotubes via elevation of intracellular calcium level. Elife, 12, RP89512. 10.7554/eLife.89512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, W. , Wu, X. , Zhou, W. , Fong, M. Y. , Cao, M. , Liu, J. , Liu, X. , Chen, C. H. , Fadare, O. , Pizzo, D. P. , Wu, J. , Liu, L. , Liu, X. , Chin, A. R. , Ren, X. , Chen, Y. , Locasale, J. W. , & Wang, S. E. (2018). Cancer‐cell‐secreted exosomal miR‐105 promotes tumour growth through the MYC‐dependent metabolic reprogramming of stromal cells. Nature Cell Biology, 20(5), 597–609. 10.1038/s41556-018-0083-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez‐Mó, M. , Siljander, P. R. , Andreu, Z. , Zavec, A. B. , Borràs, F. E. , Buzas, E. I. , Buzas, K. , Casal, E. , Cappello, F. , Carvalho, J. , Colás, E. , Cordeiro‐da Silva, A. , Fais, S. , Falcon‐Perez, J. M. , Ghobrial, I. M. , Giebel, B. , Gimona, M. , Graner, M. , … De Wever, O. (2015). Biological properties of extracellular vesicles and their physiological functions. Journal of Extracellular Vesicles, 4, 27066. 10.3402/jev.v4.27066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, T. , Xiao, H. , Liu, X. , Wang, Z. , Zhang, Q. , Wei, N. , & Guo, X. (2021). Vascular Normalization: A new window opened for cancer therapies. Frontiers in Oncology, 11, 719836. 10.3389/fonc.2021.719836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q. , Jeppesen, D. K. , Higginbotham, J. N. , Franklin, J. L. , & Coffey, R. J. (2023). Comprehensive isolation of extracellular vesicles and nanoparticles. Nature Protocols, 18(5), 1462–1487. 10.1038/s41596-023-00811-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T. , Yu, H. , Bai, Y. , Song, J. , Chen, J. , Li, Y. , & Cui, Y. (2023). Extracellular vesicle‐derived LINC00511 promotes glycolysis and mitochondrial oxidative phosphorylation of pancreatic cancer through macrophage polarization by microRNA‐193a‐3p‐dependent regulation of plasminogen activator urokinase. Immunopharmacology and Immunotoxicology, 45(3), 355–369. 10.1080/08923973.2022.2145968 [DOI] [PubMed] [Google Scholar]
- Zimmer, P. , Baumann, F. T. , Oberste, M. , Wright, P. , Garthe, A. , Schenk, A. , Elter, T. , Galvao, D. A. , Bloch, W. , Hübner, S. T. , & Wolf, F. (2016). Effects of exercise interventions and physical activity behavior on cancer related cognitive impairments: A systematic review. BioMed Research International, 2016, 1820954. 10.1155/2016/1820954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonneveld, M. I. , van Herwijnen, M. J. C. , Fernandez‐Gutierrez, M. M. , Giovanazzi, A. , de Groot , A. M. , Kleinjan, M. , van Capel, T. M. M. , Sijts, A. J. A. M. , Taams, L. S. , Garssen, J. , de Jong, E. C. , Kleerebezem, M. , Nolte‐’t Hoen, E. N. M. , Redegeld, F. A. , & Wauben, M. H. M. (2021). Human milk extracellular vesicles target nodes in interconnected signalling pathways that enhance oral epithelial barrier function and dampen immune responses. Journal of Extracellular Vesicles, 10(5), e12071. 10.1002/jev2.12071 [DOI] [PMC free article] [PubMed] [Google Scholar]


