Abstract
Total femur replacement is a well-recognized salvage procedure and an alternative to hip disarticulation in patients with massive femoral bone loss. Compared to conventional total femur replacement, intramedullary total femur (IMTF) requires less soft tissue dissection and preserves femoral bone stock and soft-tissue attachments. Despite these advantages, patients can still anticipate compromised functional outcomes and high complication rates following IMTF. Prior studies describe IMTF with the patient positioned laterally and utilizing posterior or anterolateral approaches to the hip. We describe our IMTF technique performed via the direct anterior approach in the supine position. In our experience, this is an effective method, with potential benefits including intraoperative limb length and rotational assessment, use of fluoroscopy, more convenient exposure of the knee, and potential lower rates of hip instability.
Keywords: Intramedullary total femur, Total femur replacement, Direct anterior approach, Revision, Total hip arthroplasty, Total knee arthroplasty
Introduction
There is a rising number of patients seeking revision total knee and hip arthroplasty surgeries for compromised femora because of complications of primary total knee and hip arthroplasty such as osteolysis, instability, prosthetic joint infection (PJI), aseptic loosening, and periprosthetic fracture [[1], [2], [3], [4], [5]]. The vast majority of revision surgeries can attempt to augment lost bone stock with isolated hip or knee component revision [5]. In certain complex situations, such as interprosthetic fracture or loosening and significant femoral bone loss from a failed long-stem prosthesis adjacent to an arthritic hip or knee may require a more aggressive approach, such as total femoral replacement (TFR) [[6], [7], [8], [9], [10]].
TFR was originally described in 1952 as an alternative to amputation for complex oncologic and nononcologic etiologies [11]. Compared to alternative treatment strategies, such as immobilization, open reduction and internal fixation, and isolated hip or knee revision, the main advantage of TFR is immediate fixation, which allows for early mobilization and weight bearing. However, conventional TFR, which includes removal of the femur and detachment of its soft tissue attachments, has multiple disadvantages including high rates of hip instability, patella maltracking, limb length discrepancy, PJI, gait disturbance, and perioperative morbidity [9,10,[12], [13], [14], [15]]. Alternatively, an intramedullary total femur (IMTF) construct, which mates together a hip and knee arthroplasty, allows for less soft tissue dissection and maintenance of femoral bone stock [7]. Despite these theoretical advantages, IMTF similarly demonstrates a high complication profile, which may be related, at least in part, to surgical approach, operative technique, and difficulties related to patient positioning [7,8,13,15].
IMTF has been previously described with the hip exposed through a posterolateral or anterolateral approach and the knee thereby exposed with the patient in a lateral decubitus or “sloppy lateral” position [7,8,13,16]. Alternatively, approaching the hip via a direct anterior approach (DAA) has several advantages including (1) supine positioning, which allows for intraoperative limb length assessment and facilities the standard positioning for approaching the knee, as well as convenient use of intraoperative fluoroscopy for live feedback on acetabular, intramedullary (IM) canal preparation, and component placement; and (2) direct anterior hip exposure that utilizes intramuscular planes while maintaining posterior capsular and muscular structures, which allows for a faster recovery and theoretically lowers the dislocation rate [[17], [18], [19], [20], [21], [22]]. Despite these advantages, there are no studies, to our knowledge, describing the utilization of the DAA for IMTF. In this article, we detail our surgical technique and provide a case series of 6 patients who underwent IMTF via a DAA as a revision procedure for failed total hip and/or knee arthroplasty.
Surgical technique
Patient positioning
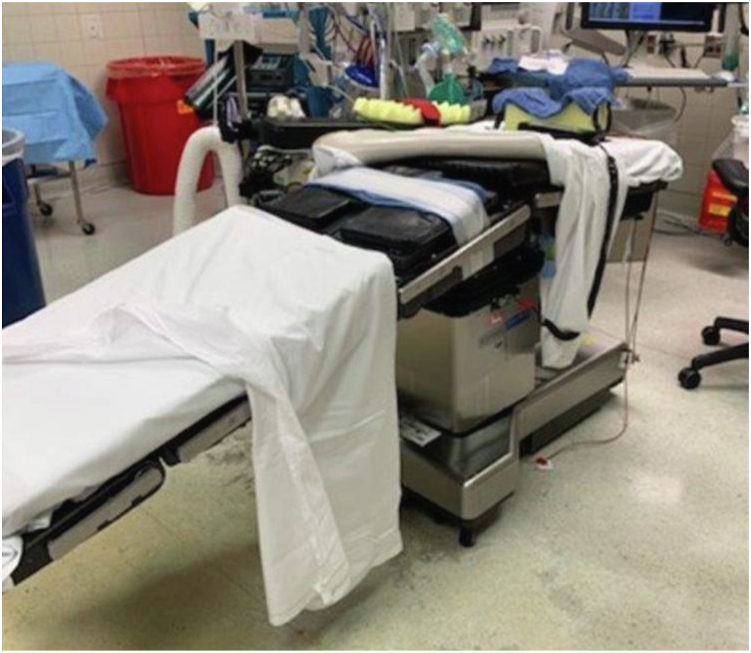
The patient is positioned supine on a standard operating table (AMSCO Surgical Table; Steris Healthcare, Mentor, OH). We find the use of a standard table instead of a radiolucent table advantageous as it allows for table leg flexion, which may be useful for femoral exposure through increased hip extension and adduction with the patient’s greater trochanters at the level of the table flex point. To enable more extensive fluoroscopic imaging without C-arm impingement, the table can be flipped such that the base is toward the head with the head extension at the foot, so there is more room distally under the table for the fluoroscope to maneuver (Fig. 1).
Figure 1.
A standard operating table with base turned toward the head to allow for fluoroscopic imaging of the entire pelvis. A Montreal post can be placed midline as a peroneal post to allow for traction without the patient drifting down the table.
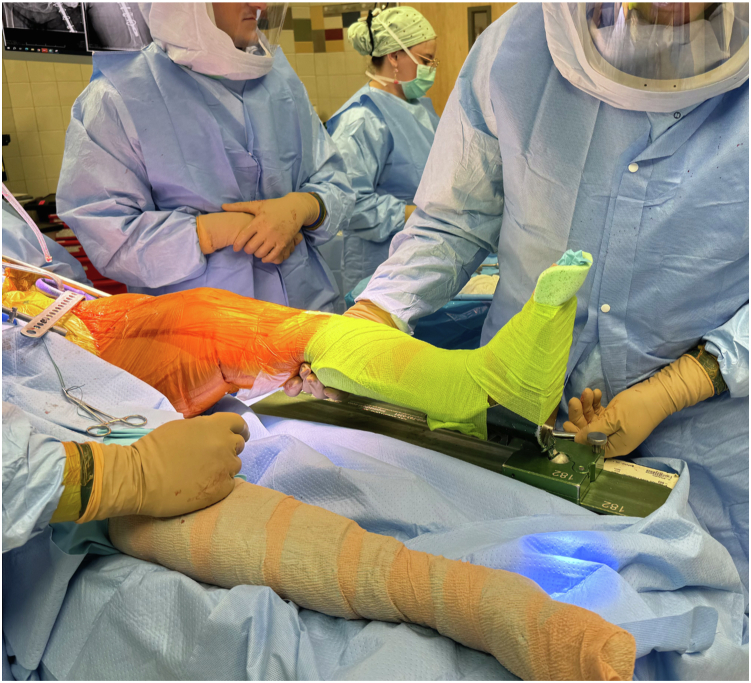
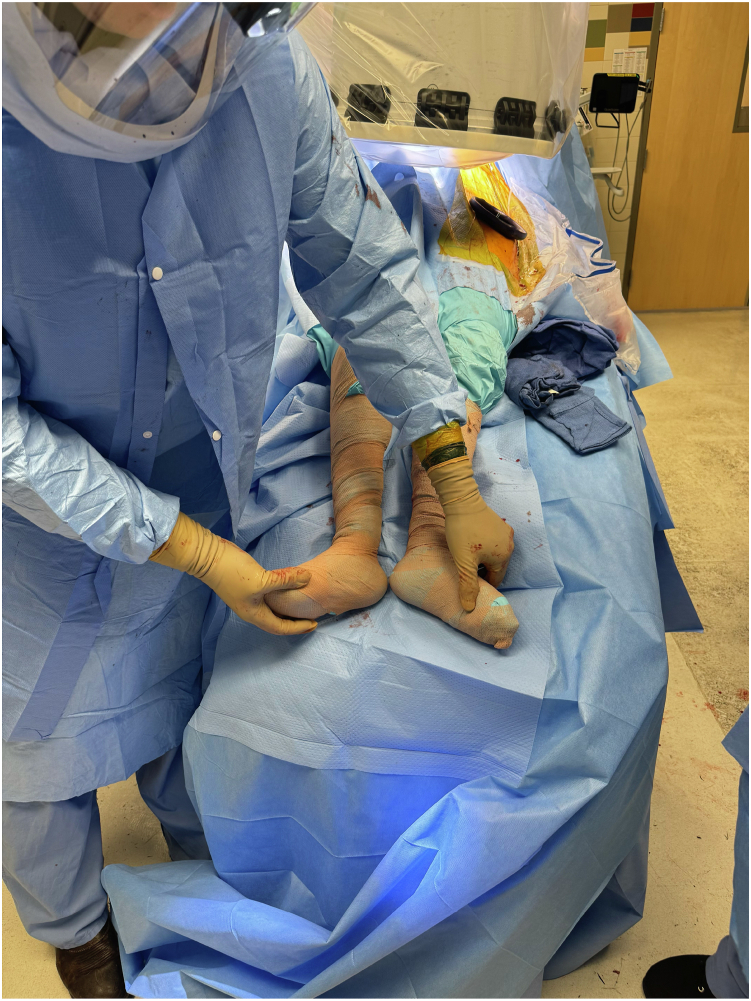
Both legs are prepped into the surgical field and draped free, allowing for easy clinical determination of leg lengths, which is especially useful in revision settings where there may be a loss of normal proximal femoral architecture to use for radiographic leg length determination. Furthermore, having the patient supine with the leg free allows for rotational assessment and simultaneous testing of hip stability and patellofemoral tracking. Our preference is to utilize a De Mayo Knee Positioner (Innovative Medical Products, Plainville, CT) to control the lower extremity during exposure and instrumentation of the knee, which may also be utilized to apply static traction to the leg as necessary for hip exposure, similar to a dedicated traction table (Fig. 2). For final leg length determination and component selection, the boot is removed to allow palpation of the heels and medial malleoli (Fig. 3).
Figure 2.
Use of a De Mayo Knee Positioner (Innovative Medical Products, Plainville, CT) to control the lower extremity during exposure and instrumentation of the knee; may be utilized to apply static traction to the leg as necessary for hip exposure, similar to a dedicated traction table.
Figure 3.
Clinical length and rotation determination via palpation of the heels and medial malleoli.
Surgical approach
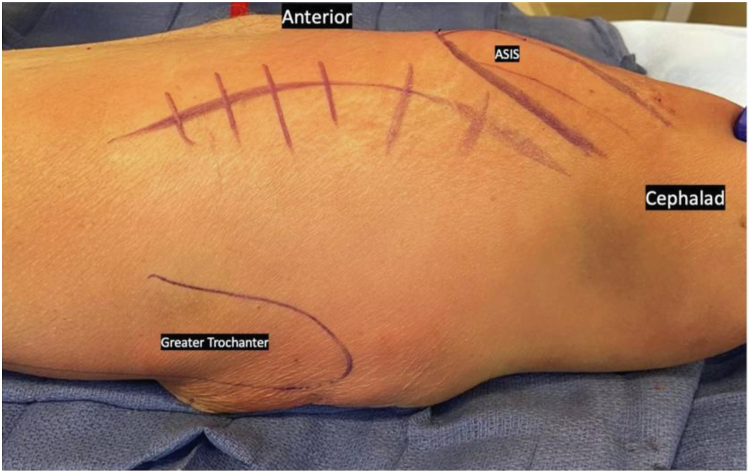
An extensile DAA to the hip utilizing the Hueter interval between tensor fascia lata (TFL) and sartorius is performed [23]. Proximally, the incision is curved along the outer border of the iliac crest allowing for access of the TFL origin just posterior to the anterior superior iliac spine (Fig. 4). Release of the leading edge of the TFL origin off the pelvic brim can be performed to improve visualization and femoral exposure for component explant and instrumentation. The TFL can later be directly repaired as long as an adequate cuff of soft tissue is maintained; we typically run a locking vicryl stitch to act as a rip-stop for later repair during TFL fascia closure. The capsular ligaments (pubofemoral, medial iliofemoral), if still present, can also be released sequentially from the femoral neck to further mobilize the femur as necessary. Distally, the incision can be extended along the lateral aspect of the femur through the iliotibial band if access to the femoral diaphysis is necessary; this was not needed in any of our cases [23]. For knee exposure, our preference is for a midvastus approach when possible, as this may theoretically expedite quadricep recovery and mitigate concerns of patellofemoral maltracking, although a standard medial parapatellar approach can be utilized per surgeon preference [24].
Figure 4.
The planned extensile DAA incision: a standard DAA approach with proximal extension, curving along the iliac crest.
IMTF specifics
Preoperatively, radiographs with standardized markers were obtained of the contralateral femur, from hip to knee, for determination of total femoral length. While the final determination of the exact construct length and size cannot be determined until the time of surgery, preoperative planning can help guide the operative plan. If there is concern about inadequate acetabular bone stock or acetabular component loosening or malpositioning, a preoperative CT scan of the ipsilateral hemipelvis is also recommended to aid in acetabular reconstruction planning.
In the setting of revision total hip arthroplasty (THA), our preference is to typically begin the procedure with an extensile DAA to the hip. We find this strategy useful in facilitating the limb traction and positioning typically necessary for femoral component explant via extensile DAA. Once the femoral component is explanted, attention can be given to the acetabulum. If a prior acetabular component is present and noted to be well fixed and positioned, consideration for retention may be given, especially if it can accommodate a modular dual-mobility or constrained liner; however, given the concern for hip instability postoperatively, a low threshold for acetabular component revision should be considered [7,13]. If a cup-cage construct is determined to be necessary, this can then be performed via the extensile DAA with the aid of intraoperative fluoroscopy, as previously described by our group [25].
After hip component explant and acetabular component placement are completed, attention is then typically brought to the knee. If prior total knee arthroplasty (TKA) components are present, these are typically explanted in a standard fashion. In the setting of a native knee, an appropriate distal femur resection and proximal tibial resection is performed in order to accommodate a distal femoral replacement (DFR) component. Most systems available to date require a 3-7 cm minimum resection to accommodate the shortest DFR component. To optimize workflow and efficiency, we found that completing tibial component preparation and final component placement at this time was effective. Consideration for metaphyseal cone placement can also be given to optimize tibial component fixation and survivorship, particularly in the setting of revision TKA with metaphyseal bone loss [26].
Once the tibial component is complete, the femoral canal is then prepared through retrograde reaming, starting with the smallest-diameter flexible reamer over a guide wire. In the setting of significant femoral bone loss, as is typical for IMTF patients, orthogonal views of the femur can be easily performed with fluoroscopy to confirm IM guide wire placement. We also recommend placement of a clamp at the proximal aspect of the guidewire through the hip incision to mitigate guidewire migration or plunging of the reamer proximally.
After the canal is adequately prepared, the trial DFR component with mated IM rod trial can be inserted based on preoperative length measurements. To optimize visualization and mating of the IM rod to femoral component proximally, we recommend erring toward the longer side of IM rod. If significant proximal femoral bone stock is still present, the metaphysis can then be reamed and prepared as necessary per specific implant requirements. Next, the trial proximal femoral body can be mated to the IM rod, and a trial reduction can be performed (Fig. 5). Component trialing is then performed and a length assessment is performed clinical by comparing to the contralateral extremity. Careful attention to component rotation is necessary to optimize hip stability as well as patellofemoral tracking; it should be noted that any additional anteversion at the hip can drive internal rotation at the knee, thereby increasing risk of patellar dislocation laterally. Herein lies the additional benefit of a DAA hip approach, as a neutral femoral version is better tolerated compared to posterior-based approaches to the hip. Once satisfied with limb length and component rotation, the distal femur is typically marked for guidance of final component placement.
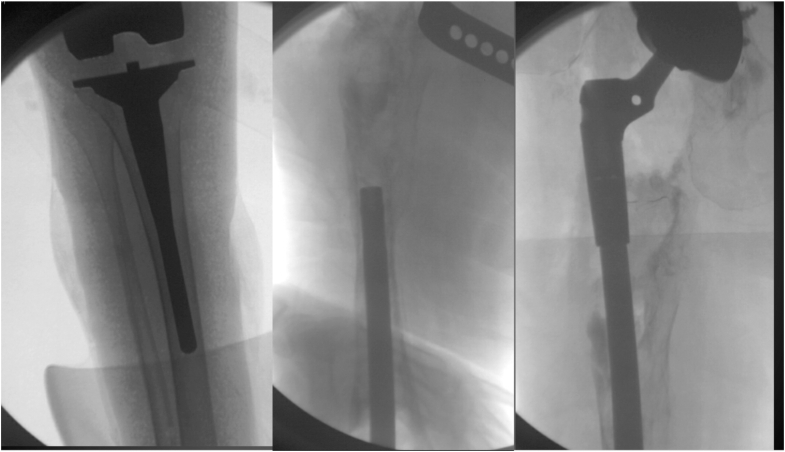
Figure 5.
Intraoperative fluoroscopy imaging depicting completion of the final tibial component followed by insertion of an IM rod and mating to the proximal body (Orthopedic Salvage System, Zimmer Biomet, Warsaw, IN).
Case examples
Two of the six cases were selected below to illustrate some of the key concepts.
Case 1
The patient is a 71-year-old female who originally underwent a primary posterior THA 17 years prior. Her postoperative course was complicated by a long history of revisions for recurrent instability, femoral and acetabular component loosening, periprosthetic femur fracture, and most recently, a stage 1 explant with placement of an antibiotic-coated intramedullary nail and hip disarticulation for recurrent PJI. She presented to our clinic approximately 6 months postoperatively after completing 6 weeks of culture-specific IV antibiotics with complaints of inability to ambulate and chronic pain resulting from her hip disarticulation. Her radiographs at that time demonstrated extensive proximal femoral bone loss with presence of an antibiotic-coated nail and multiple cerclage wires about a periprosthetic femoral fracture with apparent callous formation and consolidation (Fig. 6). Preoperative CT scan showed a Paprosky 3B acetabular defect with superomedial acetabular erosion and concern for pelvic discontinuity [27]. Inflammatory labs and hip aspiration were obtained within normal limits, suggesting clearance of prior infection. She had limited bone stock for revision arthroplasty and downstream symptomatic knee arthritis, and therefore, we indicated her for revision THA with IMTF and a cup-cage construct.
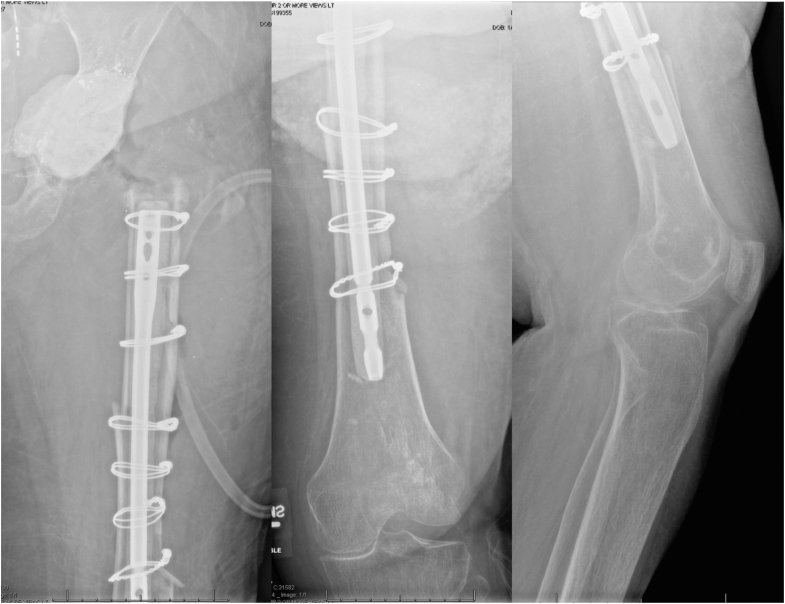
Figure 6.
Preoperative radiographs from Case 1. There was a presence of hip disarticulation with an antibiotic cement spacer in the acetabulum. Extensive proximal femoral bone loss was present with an antibiotic-coated nail and multiple cerclage wires about a prior periprosthetic femoral fracture that involved the entire femur down to the metadiaphysis with apparent callous formation and consolidation.
We performed an extensile DAA in our standard fashion, with partial reflection of TFL off of the anterior superior iliac spine to improve exposure. We noted that the anterior and medial wall of the acetabulum had been previously reamed away. We reverse reamed a fresh frozen cancellous allograft into the acetabular defect and implanted a press-fit Zimmer Biomet TM cup (Zimmer Biomet, Warsaw, IN) with a contralateral half cage. We then cemented in a Freedom Constrained Acetabular Liner (Zimmer Biomet, Warsaw, IN) into appropriate component version and inclination. Next, the knee was through a midvastus approach and resected 3 cm of distal femur as per the minimal resection for the DFR component (Orthopedic Salvage System, Zimmer Biomet, Warsaw, IN). A neutral cut of the proximal tibia was performed, followed by tibial component trialing and cementation of the final tibial component. We then removed the prior IM nail, reamed the femoral canal using flexible reamers over a guidewire, and trialed the distal femur segment with mated IM rod. After trialing with adequate restoration of limb length and rotation, final components were implanted (Fig. 7). At her most recent in-person follow-up visit 6 months postoperatively, she was doing well, ambulating with a front wheel walker without subsequent complications.
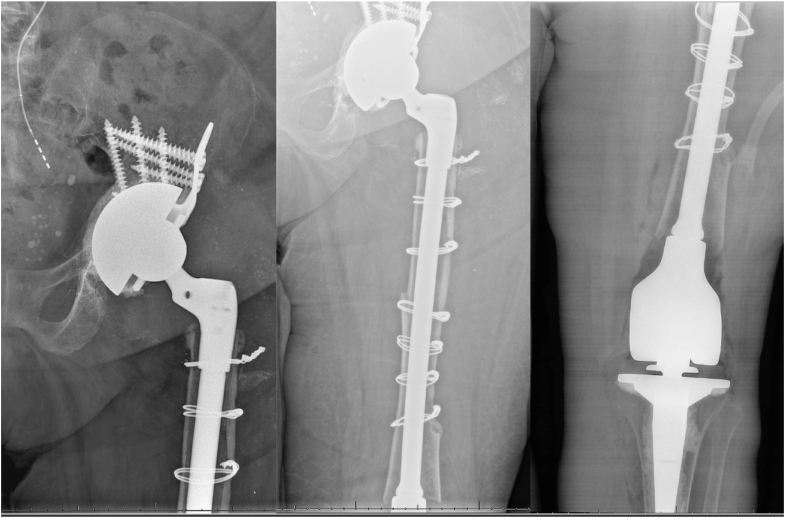
Figure 7.
Postoperative radiographs from Case 1. Cup cage construct using a contralateral half-cage and a cemented constrained liner and IMTF construct (Orthopedic Salvage System, Zimmer Biomet, Warsaw, IN).
Case 2
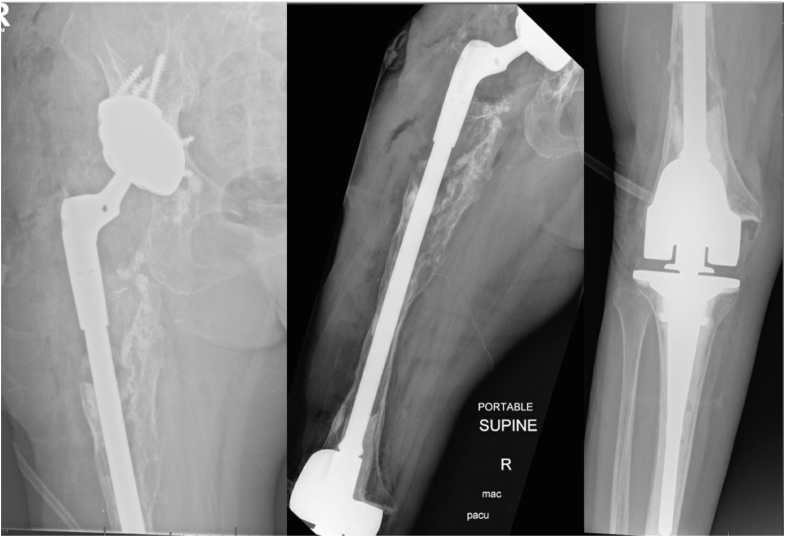
The patient is a 78-year-old female who originally had a posterior primary THA in 1977, which was subsequently revised multiple times for aseptic loosening, instability, and PJI including prior hip disarticulation. Most recently, the patient underwent both component stage 2 revision with proximal femoral replacement (PFR) and acetabular augmentation. Two years later, the patient presented to our clinic with significant right thigh pain and an inability to ambulate. Preoperative radiographs demonstrated subsidence of the PFR with progressive osteolysis and an incomplete cement mantle (Fig. 8). The acetabular component appeared to be well fixed and appropriately positioned in the presence of a cemented modular dual mobility liner. She had a 40-degree arch of motion in her knee with severe knee arthritis. Given the minimal proximal femoral bone stock with unsupportive diaphysis and downstream knee pathology, she was indicated for an IMTF with potential acetabular component retention.
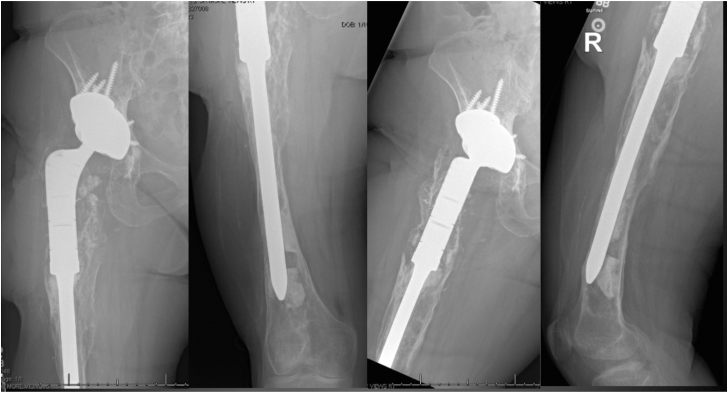
Figure 8.
Preoperative radiographs from Case 2. There was subsidence of the prior proximal femoral replacement with progressive osteolysis, an incomplete cement mantle, and distal third femoral bone loss involving the metadiaphyseal junction and adjacent knee arthritis. The acetabular component appeared to be well fixed and appropriately positioned in the presence of a cemented modular dual mobility liner.
An extensile DAA approach to the hip was first performed. The prior cemented PFR component was explanted, and the acetabular component was exposed and noted to be well fixed and positioned. A midvastus approach to the knee was performed, and 3 cm of distal femur was resected as per the minimal resection for the DFR component (Orthopedic Salvage System, Zimmer Biomet, Warsaw, IN). The tibial component was then trialed and the final components were cemented into place. The femoral canal was then reamed, the distal femur with mated IM rod trials were placed based on preoperative length measurements, and proximal femoral body was mated. Trial reduction using a dual mobility construct (Zimmer Biomet, Warsaw, IN) was then performed, and once satisfied with length and component rotation the final components were implanted (Fig. 9). At her most recent in-person follow-up visit 9 months postoperatively, she had improved knee range of motion from 0 to 90 degrees, minimal pain, and was ambulating with a front wheel walker without subsequent complications.
Figure 9.
Postoperative radiographs from Case 2. The prior acetabular component was maintained, and the IMTF construct was implanted, bypassing the femoral bone loss (Orthopedic Salvage System, Zimmer Biomet, Warsaw, IN).
Discussion
Massive femoral bone loss from failed THA and TKA can create significant challenges for successful reconstruction. Since its inception in the mid-20th century, TFR has become increasingly popular due to its capacity for limb preservation and immediate fixation in patients with severely compromised femora. IMTF is an attractive alternative to conventional TFR as it requires less soft tissue dissection and preserves femoral bone stock. Even with these theoretical advantages, the outcomes following IMTF continue to be sobering with a high complication profile and significant perioperative morbidity [7,13]. While these outcomes are largely correlated to the severely compromised state of patients who meet indications for TFR or IMTF, some complications such as hip instability, patella maltracking, and leg length discrepancy may be related, at least in part, to surgical approach and patient positioning. This article describes the first, to our knowledge, surgical technique and case series of 6 patients who underwent IMTF in the supine position via a DAA to the hip for failed THA and TKA.
In 2006, Peters et al. reported on 23 IMTFs using 2 separate approaches to create an IMTF construct: (1) IM attachment of a new revision TKA to an existing well-fixed THA using a customs IM sleeve; and (2) placement of a modular IMTF with new hip and knee arthroplasty, as performed in the current series [7]. Improvements in functional outcomes and significant reduction in pain scores were demonstrated in both cohorts; however, only 1 of the 16 patients in the modular IMTF group was able to ambulate without assistive devices postoperatively. Similar findings were demonstrated by Hoell et al. [13] and Fountain et al. [8] with 96% and 92% of patients, respectively, requiring assistive devices to ambulate postoperatively. In the present series, we demonstrated similar findings with only one patient ambulating without assistive devices at most recent follow-up; however, our series is limited in the total duration of follow-up (Table 1). While these functional results are certainly far from perfect, it is important to consider the significant morbidity, mortality, and psychological sequelae of a hip disarticulation—the likely alternative treatment option in many of these patients [28,29].
Table 1.
Case examples of indicated IMTF patients and their postoperative outcomes.
| Patient number | Age | Gender | BMI (kg/m2) | Indication | OR time (minutes) | Assistive devices | Follow-up (months) | Complication(s) |
|---|---|---|---|---|---|---|---|---|
| 1 | 71 | Female | 25.1 | Prior nonarticulating stage 1 hip spacer for arecurrent infection, instability, and periprosthetic femur fracture | 274 | FWW | 6 | |
| 2 | 56 | Female | 53.1 | Complex intra-articular distal femur fracture with an ipsilateral articulating spacer for prosthetic joint infection from prior THA | 222 | FWW | 6 | Superficial wound dehiscence |
| 3 | 78 | Female | 21.9 | Subsidence and loosening about a prior proximal femoral replacement with ipsilateral knee osteoarthritis | 211 | Cane | 9 | |
| 4 | 70 | Female | 41.7 | Prosthetic joint infection about a distal femur replacement and ipsilateral THA with subsequent stage 1 spacer | 310 | Cane | 40 | PJI s/p 2 stage and repeat IMTF |
| 5 | 69 | Male | 28.2 | Aseptic loosening of prior long distal femoral replacement for distal femur fracture with ipsilateral hip osteoarthritis | 316 | None | 58 | |
| 6 | 80 | Female | 27.6 | Subsidence and loosening about a prior proximal femoral replacement with ipsilateral knee osteoarthritis | 367 | FWW | 74 |
BMI, body mass index; FWW, front wheeled walker; OR, operating room.
Several studies have reported major complication rates as high as 25% to 50% following TFR for both oncologic and nononcologic etiologies [[7], [8], [9], [10],[12], [13], [14], [15]]. Patients must therefore be counseled preoperatively and warned of the possible subsequent need for hip disarticulation. In the series by Peters et al., there was 31% incidence of major complication in the modular IMTF group with the most common being hip instability and tibial component aseptic loosening at 13%, followed by PJI at 6% [7]. These series were prior to routine use of tibial cones with DFR revision arthroplasty, and so it is hoped that more modern case series will have reduced aseptic tibial loosening. In the present series, the tibial component was revised in all cases to accommodate an IMTF and a tibial cone was used in 4 of the 6 presented cases. Although some tibial baseplates can accommodate an IMTF, careful scrutiny and preoperative planning are essential to avoid unnecessary tibial component revision. More recently, Fountain et al. demonstrated a 50% incidence of major complication in their cohort with the most common being hip instability at 36%, followed by PJI at 14% [8]. It is important to note, however, that in both of these studies, the patient was positioned in the lateral decubitus position, and either a posterior or anterior-lateral approach to the hip was performed. While the etiology of hip instability after IMTF is largely multifactorial and related to massive proximal femoral bone loss with abductor muscular compromise, surgical techniques and approaches should also be considered.
In the present series, we report on 6 patients who underwent IMTF in the supine position with a DAA to the hip. While our cohort is limited by the number of patients and duration of follow-up, no patients have demonstrated hip instability thus far. Through preservation of the posterior capsule and short external rotators, prior studies have demonstrated a low rate of hip instability following DAA for both primary and revision THA [21,22,30,31]. Additionally, use of intraoperative fluoroscopy can improve acetabular component positioning, thereby increasing joint stability [20,32]. Further, we found that supine positioning had several unique advantages as it pertained to IMTF. First, it enabled a clinical limb length assessment intraoperatively, which is perhaps more reliable in the revision setting where radiographic landmarks are often lost. Second, it allowed for rotational assessment and simultaneous stability testing of the hip and knee joint during IMTF component trialing. Lastly, it facilitated the approach to the knee and use of a leg positioner for assistance in tibial preparation and cementing, which would otherwise be difficult if performed in the lateral decubitus position as previously reported. While the lack of follow-up in this cohort is a significant limitation and further studies are necessary to evaluate the long-term outcomes, we believe that with proper technique and revision skill set, IMTF can be safely performed through extensile DAA.
Summary
Concurrent with the increased incidence of primary THA and TKA is a rising number of patients requiring revision surgery for severely compromised femora. For patients with interprosthetic fracture or loosening and/or massive femoral bone loss from failed hip or knee arthroplasty adjacent to an arthritic hip or knee, IMTF is an attractive alternative to hip disarticulation or conventional TFR. Following IMTF, patients can anticipate improvements in pain and function, albeit at a compromised level and with high risks of complication and perioperative morbidity. Benefits of IMTF via a DAA include potential lower rates of hip instability and supine positioning, which facilitates intraoperative limb length and rotational assessment, use of fluoroscopy, and more convenient exposure of the knee. We believe that with proper preoperative planning and familiarity with extensile DAA, IMTF can be safely performed using our described technique.
Conflicts of interest
J. Gililland receives royalties from OrthoGrid; is a paid consultant for DJ Orthopedics, OrthoGrid, Smith & Nephew, Stryker, and Medacta; has stock options in OrthoGrid and CONextions; receives research support from Zimmer Biomet and Stryker; is in the editorial or publication board of the Journal of Arthroplasty; and is a board member of the American Association of Hip and Knee Surgeons and American Academy of Orthopaedic Surgeons. L. Anderson is a paid speaker and a paid consultant for Medacta; has stock options in OrthoGrid; and receives research support from Zimmer Biomet and Stryker. The other author declares no potential conflicts of interest.
For full disclosure statements refer to https://doi.org/10.1016/j.artd.2024.101474.
CRediT authorship contribution statement
Adam J. Taylor: Writing – original draft, Project administration, Data curation. Jeremy M. Gililland: Writing – review & editing, Supervision, Conceptualization. Lucas A. Anderson: Writing – review & editing, Supervision, Methodology, Investigation, Conceptualization.
Appendix A. Supplementary data
References
- 1.Maradit Kremers H., Larson D.R., Crowson C.S., Kremers W.K., Washington R.E., Steiner C.A., et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386–1397. doi: 10.2106/JBJS.N.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sloan M., Premkumar A., Sheth N.P. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100:1455–1460. doi: 10.2106/JBJS.17.01617. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz S., Mowat F., Ong K., Chan N., Lau E., Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87:1487–1497. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 4.Abdel M.P., Watts C.D., Houdek M.T., Lewallen D.G., Berry D.J. Epidemiology of periprosthetic fracture of the femur in 32 644 primary total hip arthroplasties: a 40-year experience. Bone Joint Lett J. 2016;98-b:461–467. doi: 10.1302/0301-620X.98B4.37201. [DOI] [PubMed] [Google Scholar]
- 5.Sheth N.P., Nelson C.L., Paprosky W.G. Femoral bone loss in revision total hip arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2013;21:601–612. doi: 10.5435/JAAOS-21-10-601. [DOI] [PubMed] [Google Scholar]
- 6.Melnic C.M., Lightsey H.M., 4th, Lozano Calderón S.A., Heng M. Megaprostheses in nononcologic hip and knee revision arthroplasty. J Am Acad Orthop Surg. 2021;29:e743–e759. doi: 10.5435/JAAOS-D-20-01052. [DOI] [PubMed] [Google Scholar]
- 7.Peters C.L., Hickman J.M., Erickson J., Lombardi A.V., Berend K.R., Mallory T.H. Intramedullary total femoral replacement for salvage of the compromised femur associated with hip and knee arthroplasty. J Arthroplasty. 2006;21:53–58. doi: 10.1016/j.arth.2004.12.061. [DOI] [PubMed] [Google Scholar]
- 8.Fountain J.R., Dalby-Ball J., Carroll F.A., Stockley I. The use of total femoral arthroplasty as a limb salvage procedure: the Sheffield experience. J Arthroplasty. 2007;22:663–669. doi: 10.1016/j.arth.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Putman S., Migaud H., Saragaglia D., Jenny J.Y., Dujardin F., Hue A.G., et al. Total femur replacement in non-oncologic indications: functional and radiological outcomes from a French survey with a mean 6 years' follow-up. Orthop Traumatol Surg Res. 2019;105:591–598. doi: 10.1016/j.otsr.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Sambri A., Parisi S.C., Zunarelli R., Di Prinzio L., Morante L., Lonardo G., et al. Megaprosthesis in non-oncologic settings-A systematic review of the literature. J Clin Med. 2023;12:4151. doi: 10.3390/jcm12124151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchman J. Total femur and knee joint replacement with a vitallium endoprosthesis. Bull Hosp Joint Dis. 1965;26:21–34. [PubMed] [Google Scholar]
- 12.Ramanathan D., Siqueira M.B., Klika A.K., Higuera C.A., Barsoum W.K., Joyce M.J. Current concepts in total femoral replacement. World J Orthop. 2015;6:919–926. doi: 10.5312/wjo.v6.i11.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoell S., Butschek S., Gosheger G., Dedy N., Dieckmann R., Henrichs M., et al. Intramedullary and total femur replacement in revision arthroplasty as a last limb-saving option: is there any benefit from the less invasive intramedullary replacement? J Bone Joint Surg Br. 2011;93:1545–1549. doi: 10.1302/0301-620X.93B11.27309. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez M.R., Inchaustegui M.L., Layme J., Ruiz-Arellanos K., Larios F., Pretell-Mazzini J. Postoperative outcomes of total femur replacement in oncologic and nononcologic patients: a systematic review of the literature. J Arthroplasty. 2023;39:1624–1631.e2. doi: 10.1016/j.arth.2023.11.035. [DOI] [PubMed] [Google Scholar]
- 15.Berend K.R., Lombardi A.V., Jr., Mallory T.H., Adams J.B., Dodds K.L. Total femoral arthroplasty for salvage of end-stage prosthetic disease. Clin Orthop Relat Res. 2004:162–170. doi: 10.1097/01.blo.0000142351.88039.e8. [DOI] [PubMed] [Google Scholar]
- 16.Walker P.S., Yoon W.W., Cannon S.R., Bentley G., Muirhead-Allwood S.K. Design and application of combined hip-knee intramedullary joint replacements. J Arthroplasty. 1999;14:945–951. doi: 10.1016/s0883-5403(99)90008-6. [DOI] [PubMed] [Google Scholar]
- 17.Hailer N.P., Weiss R.J., Stark A., Kärrholm J. The risk of revision due to dislocation after total hip arthroplasty depends on surgical approach, femoral head size, sex, and primary diagnosis. An analysis of 78,098 operations in the Swedish Hip Arthroplasty Register. Acta Orthop. 2012;83:442–448. doi: 10.3109/17453674.2012.733919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennon R., Keggi J., Zatorski L.E., Keggi K.J. Anterior approach for total hip arthroplasty: beyond the minimally invasive technique. J Bone Joint Surg Am. 2004;86-A Suppl 2:91–97. doi: 10.2106/00004623-200412002-00013. [DOI] [PubMed] [Google Scholar]
- 19.Sariali E., Leonard P., Mamoudy P. Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty. 2008;23:266–272. doi: 10.1016/j.arth.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Beamer B.S., Morgan J.H., Barr C., Weaver M.J., Vrahas M.S. Does fluoroscopy improve acetabular component placement in total hip arthroplasty? Clin Orthop Relat Res. 2014;472:3953–3962. doi: 10.1007/s11999-014-3944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charney M., Paxton E.W., Stradiotto R., Lee J.J., Hinman A.D., Sheth D.S., et al. A comparison of risk of dislocation and cause-specific revision between direct anterior and posterior approach following elective cementless total hip arthroplasty. J Arthroplasty. 2020;35:1651–1657. doi: 10.1016/j.arth.2020.01.033. [DOI] [PubMed] [Google Scholar]
- 22.Haynes J.A., Hopper R.H., Jr., Ho H., McDonald J.F., 3rd, Parks N.L., Hamilton W.G. Direct anterior approach for primary total hip arthroplasty lowers the risk of dislocation compared to the posterior approach: a single institution experience. J Arthroplasty. 2022;37:495–500. doi: 10.1016/j.arth.2021.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Mast N.H., Laude F. Revision total hip arthroplasty performed through the Hueter interval. J Bone Joint Surg Am. 2011;93 Suppl 2:143–148. doi: 10.2106/JBJS.J.01736. [DOI] [PubMed] [Google Scholar]
- 24.Katragadda B.C., Kumar S., Suresh A., Vk K. Midvastus versus medial parapatellar approach in simultaneous bilateral total knee arthroplasty. J Arthroplasty. 2023;38:2301–2306. doi: 10.1016/j.arth.2023.05.043. [DOI] [PubMed] [Google Scholar]
- 25.Lancaster A., Boes E., Gililland J., Anderson L. Direct anterior cup-half cage for revision and complex primary total hip arthroplasty: surgical technique. Arthroplast Today. 2022;16:140–149. doi: 10.1016/j.artd.2022.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heidenreich M.J., Lanting B.A., McCalden R.W., Naudie D.D., Howard J.L., MacDonald S.J., et al. Survivorship of metaphyseal cones and sleeves in revision total knee arthroplasty. J Arthroplasty. 2022;37:S263–S269. doi: 10.1016/j.arth.2022.02.074. [DOI] [PubMed] [Google Scholar]
- 27.Paprosky W.G., Bradford M.S., Younger T.I. Classification of bone defects in failed prostheses. Chir Organi Mov. 1994;79:285–291. [PubMed] [Google Scholar]
- 28.Schindler M., Baertl S., Walter N., Lang S., Szymski D., Alt V., et al. Retrospective analysis of mortality and quality of life after hip disarticulation or hemipelvectomy: a report on 15 patients. Arch Orthop Trauma Surg. 2023;143:4943–4949. doi: 10.1007/s00402-023-04783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unruh T., Fisher D.F., Jr., Unruh T.A., Gottschalk F., Fry R.E., Clagett G.P., et al. Hip disarticulation. An 11-year experience. Arch Surg. 1990;125:791–793. doi: 10.1001/archsurg.1990.01410180117019. [DOI] [PubMed] [Google Scholar]
- 30.Pincus D., Jenkinson R., Paterson M., Leroux T., Ravi B. Association between surgical approach and major surgical complications in patients undergoing total hip arthroplasty. JAMA. 2020;323:1070–1076. doi: 10.1001/jama.2020.0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh G., Khurana A., Gupta S. Evaluation of direct anterior approach for revision total hip arthroplasty: a systematic review. Hip Pelvis. 2021;33:109–119. doi: 10.5371/hp.2021.33.3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamilton W.G., Parks N.L., Huynh C. Comparison of cup alignment, jump distance, and complications in consecutive series of anterior approach and posterior approach total hip arthroplasty. J Arthroplasty. 2015;30:1959–1962. doi: 10.1016/j.arth.2015.05.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.