Abstract
The rise of operational noise as an environmental pollutant for farm animals is an emerging concern. The mechanisms through which music can alleviate oxidative stress, inflammation, and apoptosis induced by noise exposure remain underexplored. This study aims to investigate the alleviating effects and underlying mechanisms of long-term music exposure on noise-induced damage to the chicken spleen. Male Arbor Acres (AA) broilers were divided into four groups: control (C), acute noise stimulation (NS), noise stimulation with music mitigation (NSM), and music only (M). NS and NSM groups were exposed to noise (simulating sudden intensity noise, 115 to 120dB) for 10 minutes daily for a week, starting at 14-days-old. NSM and M groups then received 28 days of 6-hour daily music (Mozart K.448, 60–65 dB). The results showed that noise stimulation significantly activated the Keap-1/Nrf2 and NF-κB signaling pathways. Long-term music intervention has also been demonstrated to successfully mitigate oxidative stress and abnormal apoptosis induced by acute noise stimulation. Microscopic examination of the spleen revealed that acute noise stimulation resulted in an increase in splenic cells, a decrease in lymphocytes, and blurred boundaries between the red and white pulps in the NS group. However, these pathological changes were alleviated in the NSM group following music intervention. Compared with the control group, the NS group exhibited significantly elevated oxidative stress parameters. In contrast, music intervention in the NSM group notably improved antioxidant capacity and partially alleviated morphological abnormalities in the spleen. Additionally, noise stimulation activated the NF-κB pathway, upregulating the downstream genes of the inflammatory factors IL-1β, IL-6, and TNF-α. Noise-induced mitochondrial damage led to apoptosis, as observed by TUNEL staining, along with increased gene and protein expression of Bcl-2, Bax, Cyt-C, Casp-3, Casp-8, and Casp-9. These findings indicate that acute noise exposure can induce splenic damage via oxidative stress, inflammation, and apoptosis by modulating the Keap-1/Nrf2 and NF-κB pathways. Prolonged music stimulation effectively mitigates noise-induced damage, offering a vital experimental foundation for further research on noise pollution's impact on organisms and music's alleviating role.
Key words: animal welfare, apoptosis, inflammation, oxidative stress, chicken spleen
INTRODUCTION
Noise is acknowledged as a principal factor influencing health, especially in settings such as farms, where animals are routinely subjected to loud environments (Kupcikova et al., 2021). Dramatic fluctuations in noise levels represent a substantial source of stress and trepidation in poultry, undermining productivity and posing a profound risk to well-being (Campo et al., 2005) . In particular, high-intensity noise has been demonstrated to impair immune function and induce inflammatory reactions in poultry (Kight and Swaddle, 2011). Noise also serves as a significant physiological stressor that activates the central nervous system, influences mood responses, and can trigger oxidative stress (Daiber et al., 2019). According to a study conducted by Shukla and colleagues, noise has an adverse effect on spatial learning and memory functions by exacerbating oxidative stress and inflammatory responses (Shukla et al., 2020). Studies have shown that oxidative stress is crucial for the development of health issues related to noise exposure, including increased reactive oxygen species (ROS) and a compromised antioxidant defense system (Demirel et al., 2009; Mirmohammadi et al., 2020).
Changes in ROS levels stimulate the activation of various transcription factors that are sensitive to oxidative stress, including nuclear factor erythroid 2-related factor 2 (Nrf2), nuclear factor-κB (NF-κB), and peroxisome proliferator-activated receptor (PPAR) (Qi et al., 2012). ROS can prompt the activation of the NF-κB signaling pathway, leading to the initiation of the inflammatory response, and ROS overproduction can further intensify oxidative stress (Sesti et al., 2012). If antioxidant enzymes, including glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD), are ineffective at neutralizing the accumulation of pro-oxidants, lipid peroxidation can further aggravate cellular damage through the secretion of malondialdehyde (MDA) (Zhu et al., 2024). Under normal physiological conditions, Nrf2 is pivotal and is typically maintained in the cytoplasm in an inactive state by binding to Kelch-like ECH-associated protein 1 (Keap-1). Upon activation, Nrf2 orchestrates the expression of downstream antioxidant enzymes, including GSH and heme oxygenase-1 (HO-1), to combat oxidative stress and inflammation (Saha et al., 2020). Oxidative damage can initiate apoptosis and inflammatory reactions, which in turn can inhibit immune functions (Chen et al., 2018). Although we have foundational knowledge of the detrimental effects of noise on the immune system, the precise mechanisms by which noise induces apoptosis in chicken spleen cells and causes tissue inflammation are still mostly unknown and require urgent exploration.
The concept of an enriched environment (EE) was initially introduced into laboratories as an experimental tool to investigate animal learning behavior and was subsequently proven to have significant interventional effects on the treatment of animal diseases (Laviola et al., 2008). Studies have demonstrated that EE can influence the expression of cytokines, various immune components, and glial cells, suggesting a potential mechanism for modulating brain function (Garofalo et al., 2015). In addition to its notable impact on the nervous system, a few studies have reported the regulatory effects of EEs on immune responses, such as enhancing the effector function of white blood cells, reducing the levels of inflammatory cytokines in circulation, and increasing the number of natural killer cells (Arranz et al., 2010; Rattazzi et al., 2016; Brod et al., 2017). Music has been proven to modulate immune responses by increasing the activity of natural killer cells, lymphocytes, and interferon-γ (Wachi et al., 2009). Additionally, music is regarded as a simple enrichment tool for improving animal welfare, masking potentially disruptive backgrounds, and thereby mitigating anxiety, stress, and aggressive behaviors (Alworth and Buerkle, 2013; Li et al., 2021; Riemer et al., 2021). In contrast, music has been found to be a cost-effective and effective therapy that has demonstrated notable benefits in improving both physical and mental health as well as in overcoming psychological obstacles. This form of therapy has become increasingly popular in recent years (Trappe, 2010; Thaut, 2015). Research has also shown that listening to music can trigger the release of endogenous dopamine in the striatum during emotional peaks Reybrouck et al., 2021, Zaatar et al., 2024. Studies have shown that the classical music Mozart K448 can ameliorate stress-induced impairments in learning and memory, providing robust evidence for the use of classical music as a means to alleviate stress-induced immune damage in animals (Papadakakis et al., 2019; Saghari et al., 2021). Consequently, music, as an EE, exhibits potential benefits in regulating immune and nervous system functions in animals.
As one of the pivotal immune organs in poultry, the spleen plays a crucial role in maintaining body health. It is rich in lymphocytes and macrophages, which perform vital functions in immune responses and defense mechanisms (Bronte and Pittet, 2013). Despite the widespread recognition of the immunological significance of chicken spleens, there are few reports on the effects of noise on splenic function. Noise, a ubiquitous environmental stressor, may have deleterious effects on the immune system of poultry by triggering apoptosis and inflammation in splenic cells. Therefore, it is scientifically valuable and practically important to delve deeper into the impact of noise on chicken spleen and its underlying mechanisms. The present study aimed to explore the positive role of Mozart's K448 in ameliorating apoptosis and inflammation in chicken spleen under noise exposure. The results of this study offer novel ideas and methods for reducing the adverse effects of noise and optimizing poultry farming environments to enhance animal welfare.
MATERIALS AND METHODS
The Institutional Animal Care and Use Committee of Northeast Agricultural University in Heilongjiang Province, China, approved the experimental procedures (No. NEAUEC20210234) conducted in this study (Heilongjiang Province, China).
Experimental Animal Grouping and Sample Collection
The study was carried out in well-equipped animal facilities of Northeast Agricultural University. The details of the animal handling and experimental design are shown in Figure 1. A total of 400 one-day-old broiler chicks were purchased from a commercial hatchery. During the first week, five chicks died, potentially due to transportation-related stress. To guarantee even distribution, three additional chicks were randomly removed, resulting in a total of 392 experimental birds. At seven days of age, the chicks were randomly assigned to four environmentally controlled rooms, each room (3 × 3.8 m2) containing 98 chicks (Chang et al., 2024; Wang et al., 2024). The 4 treatment groups were as follows: control (C), acute noise stress (NS), acute noise stress with music mitigation (NSM), and music (M). Each treatment had seven replicates of 14 untreated chicks. The control group was left untreated experiment. Until 14 d of age, all chicks were reared under background white noise (<50 dB), measured using a sound decibel meter (TASI, Jiangsu, China). From d 14 to d 20, the NS and NSM groups were exposed to 115-120 dB of acute noise stress for 10 min/day (8:30-8:40 AM), simulating sudden intensity noise, which was broadcast from an external source and measured to be around 115-120 dB. Following noise stress exposure, the M and NSM groups received 28 consecutive days (d 14–41) of continuous Mozart K448 music exposure. The music was played from 9:00 am to 3:00 pm daily at 60 to 65 dB, and a high-precision digital sound level meter was used to maintain the target volume. The music player was placed in the center of the rearing room, and a high-precision digital sound level meter (VICTOR824) was used to measure the sound intensity to ensure that it remained within the specified 60 to 65 dB range (Wang et al., 2024). The pens were maintained under stable conditions with controlled temperature, ventilation, and lighting using a central controller. The overall temperature and lighting conditions were controlled using a central controller. One- to three-day-old room temperatures were set at 34 to 35°C, 4- to 7-day-old room temperatures were set at 32 to 33°C, and afterwards, the temperature was gradually decreased by 2 to 3°C every three days until the temperature was reduced to 20 to 21°C and then remained unchanged (Wei et al., 2024). The relative humidity was maintained at 50%. The light fixtures in each room were perpendicular to the floor, and there were two fixtures in each room to ensure that the chicks could have sufficient light and that the light was evenly distributed throughout the entire coop, preventing external light sources from seeping into the coop. All lights in each room were linked to a dimmer and a timer. To ensure good early feeding and watering activity to optimize early growth health and welfare, chicks were provided 23 h of light and 1 h of darkness from 1 to 7 d of age. After 7 d, 5 h of darkness and 19 h of light were provided. A 30 to 35 lux light intensity was supplied from 1 to 7 d of age. Provides 10-20 lux strength after 7 d (Bi et al., 2024; Su et al., 2024) (Table 1). In darkness, the light intensity was 0.2 to 0.4 lux. Light intensity was measured throughout the experiment (TASI, Jiangsu, China). At 42 d of age, chickens were treated by cervical dislocation for the spleens used for HE staining and Tunel, at least 3 intact spleens were taken from each group and fixed in 4% paraformaldehyde for 48 h for subsequent testing while ensuring their integrity and freshness. The spleen was used for TEM, with three replicates per group, each containing 6 to 10 pieces of 1 mm × 1 mm × 1 mm spleen tissue in 2.5% glutaraldehyde soaked in each replicate. The remaining spleen samples were labeled and numbered in enzyme-free EP tubes for subsequent tracking and identification. The remaining samples were stored in a freezer at -80°C, except for the paraformaldehyde-fixed samples.
Figure 1.
The time flow chart for chick treatment and sample collection.
Table 1.
Changes in experimental temperature corresponding to changes in chick age.
| Chicken age (days) | whole house brood temperature (°C) | Length of exposure (hours) | Light intensity (lux) |
|---|---|---|---|
| 3 | 35 | 23 | 30–35 |
| 6 | 33 | 23 | 30–35 |
| 9 | 31 | 19 | 10–20 |
| 12 | 29 | 19 | 10–20 |
| 15 | 27 | 19 | 10–20 |
| 18 | 25 | 19 | 10–20 |
| 21 | 23 | 19 | 10–20 |
| 24 | 20-21 | 19 | 10–20 |
| 27 | 20-21 | 19 | 10–20 |
Histopathological Examination and Ultrastructural Analysis
Histological staining: To prepare spleen tissue for histological staining, the slices were subjected to sequential immersion in xylene and ethanol solutions. This was followed by 95% ethanol solution for 5 min, 85% ethanol solution for 5 min, and finally, tap water to wash the slices. The cells were then stained with a staining solution for 1 to 2 min, washed again with tap water, and differentiated. Next, they were blued in a bluing solution and rinsed under running water. Finally, the slices were stained with eosin solution for 2 to 3 min, dehydrated in absolute ethanol, and mounted with neutral resin. The slides were observed under a microscope, and images were captured for analysis. Transmission electron microscopy (TEM) The fresh spleen tissue was cut into 1 mm³ pieces, which were then fixed for 4 h at 4°C. The tissue was then washed three times with PBS and fixed with 1% osmium tetroxide for 2 h at 20°C, followed by 3 washes with PBS. The tissue was then dehydrated in an alcohol gradient (50-100%) for 15 min per step. Next, the tissue was infiltrated with a mixture for transition and incubated overnight in pure resin. The tissue was then polymerized at 60°C for 48 h, and ultrathin 60 to 80 nm thick sections were cut. The sections were stained with uranyl acetate and lead citrate for 15 min each and then air-dried at room temperature. The sections were observed, photographed, and analyzed by transmission electron microscopy. Five fields of view were observed in each section, and the experimental equipment and reagents used are shown in Tables 2 and 3.
Table 2.
Laboratory apparatus used during histopathological examinations and ultrastructural experiments.
| Experimental equipment | Product number | Manufacturer | Manufacturer location |
|---|---|---|---|
| Dehydrator | JJ-12J | Junjie Electronics |
Wuhan, China |
| Embedding machine | JB-P5 | Junjie Electronics |
Wuhan, China |
| Refrigeration operating table | JB-L5 | Junjie Electronics |
Wuhan, China |
| Thermos Scientific | KD-P | Junjie Electronics |
Wuhan, China |
| Lab Oven | GFL-230 | Laibori Instrument |
Tianjin, China |
| Slides and coverslips | 10212432C | Shitai Experimental Equipment |
Jiangsu, China |
| Front-loaded optical microscope | Nikon Eclipse ci | Nikon | Japan |
| Imaging systems | NIKON DS-U3 | Nikon | Japan |
| Transmission electron microscope | JEOL LTD | Tokyo | Japan |
| Slicer | Leica UC27 | Leica Instruments | Germany |
| Diamond Slicer | ULTRA 45° | Daitome | Germany |
| Pathology Slicer | RM2016 | Leica Instruments | Germany |
Table 3.
Reagents for histopathological examination and ultrastructural analysis.
| Name of reagent | Product number | Manufacturer | Manufacturer location |
|---|---|---|---|
| Anhydrous ethanol | 10009218 | Sinopharm Chemical Reagent |
Beijing, China |
| Xylene | 10023418 | Sinopharm Chemical Reagent |
Beijing, China |
| HE Dye Solution | B1003 | Baiqiandu Biotechnology | Wuhan, China |
| Differentiating fluid | B1004 | Baiqiandu Biotechnology | Wuhan, China |
| Rebluing liquid | B1005 | Baiqiandu Biotechnology | Wuhan, China |
| Neutral gum | 10004160 | Sinopharm Chemical Reagent |
Beijing, China |
| 2.5%glutaraldehyde (CH20)2 | P1126 | Solarbio Technology | Beijing, China |
| PBS solution(0.01M) | B0002 | Baiqiandu Biotechnology | Wuhan, China |
| Acetone | 67-64-1 | Sinopharm Chemical Reagent |
Beijing, China |
| 812 Embedding agent | 90529-77-4 | SPI | USA |
Detection of Antioxidant Activity
To evaluate the antioxidant activity of the spleen, the samples were removed and placed into grinding tubes, which were then homogenized and centrifuged. The resulting supernatant was collected and analyzed for the MDA (A003-1-2) content, as well as the activities of CAT (A007-2-1), SOD (A001-3-2), and GSH-Px (A005-1-2), using reagent kits provided by Nanjing Jiancheng Bioengineering Institute.
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction
For qRT-PCR analysis, RNA was extracted from the spleen using TRIzol reagent (TaKaRa, China). Subsequently, cDNA synthesis was performed following the instructions provided with the M5 Sprint qPCR RT Kit (China). The primers used for qRT‒PCR were synthesized by Sangon Biotech (Shanghai, China). The reaction mixture included fluorescent dye, cDNA template, primers, and water. The qRT‒PCR protocol consisted of an initial denaturation step at 95°C for 10 min, followed by 45 cycles of denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 min. Each reaction was performed in triplicate. mRNA expression levels were determined using the 2-ΔΔCt method, with normalization to GAPDH as a reference (Wei et al., 2023). The forward and reverse primer sequences are listed in Table 4.
Table 4.
Sequences of the primers for PCR.
| Gene | Primer sequences (5′-3′) |
|---|---|
| Keap-1 | F: ACTTCGCTGAGGTCTCCAAG |
| R: CAGTCGTACTGCACCCAGTT | |
| Nrf2 | F: GGACGGTGACACAGGAACAAC |
| R: CTCCACAGCGGGAAATCAGAAAG | |
| HO-1 | F: AGCTTCGCACAAGGAGTGTT |
| R: CTCCGAGTTCTCCCCGAAAG | |
| NF-κB | F: GCAGGCAGAGGTGGTAGAAGAC |
| R: TCTTGTCATCTCCTTCAGCAGCAG | |
| IFN-γ | F: GCTCCCGATGAACGACTTGA |
| R: CATTCCCTTCCCATCTGGTCTGAG | |
| TNF-α | F: GTCTGCTCCTAGTGGCTTTCCTG |
| R: CATTCCCTTCCCATCTGGTCTGAG | |
| IL-1β | F: GCCGAGGAGCAGGGACTTTG |
| R: GAAGGACTGTGAGCGGGTGTAG | |
| IL-1 | F: CTCCTCCAGCCAGAAAGTGA |
| R: GAGCTTGTAGCCCTTGATGC | |
| INOS | F: CCTGTACTGAAGGTGGCTATTGG |
| R: AGGCCTGTGAGAGTGTGCAA | |
| COX-2 | F: TCCTACCCGCTATTGTCCTAGTCC |
| R: TCAGTATCATTGGTGTCCGATGGC | |
| Bcl-2 | F: GAGTTCGGCGGCGTGATGTG |
| R: TTCAGGTACTCGGTCATCCAGGTG | |
| Bax | F: ACTCTGCTGCTGCTCTCCTCTC |
| R: ATCCACGCAGTGCCAGATGTAATC | |
| P-53 | F: GGAGATGGAACCATTGCTGGAACC |
| R: GCTCCTGCCAGTTGCTGTGATC | |
| Cyt-C | F: CCTAATCGCCGTGGCCTTCTTAAC |
| R: GGAGGAGGTAGATGGTCGGATTGG | |
| Caspase-3 | F: TACCGGACTGTCATCTCGTTCAGG |
| R: ACTGCTTCGCTTGCTGTGATCTTC | |
| Caspase-8 | F: GGAAGCAGTGCCAGAACTCAGAAG |
| R: TTGTTGTGGTCCATGCACCGATAG | |
| Caspase-9 | R: CCGAAGGAGCAAGCACGACAG |
| F: CATCTAGCATGTCAGCCAGGTCAC | |
| GAPDH | R: AGTGAAGGCTGCTGCTGATGG |
| F: TCAAAGGTGGAGGAATGGCTGTC |
Detection of Apoptosis by the Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling (TUNEL) Assay
The process for deparaffinizing spleen paraffin sections involved heating them at 65°C for 2 h in an oven. Subsequently, the sections were dehydrated in xylene, absolute ethanol, and a gradient of ethanol (95-70%) in a stepwise manner, with each step lasting 5 to 20 min. The sections were then washed with PBS three times for 5 min each. Next, the sections were incubated with proteinase K at 37°C for 30 min, after which they were washed three times with PBS for 5 min each. Next, 50 μl of TUNEL reaction solution was added, and the sections were incubated at 37°C in the dark for 2 h. The slides were then washed with PBS three times for 5 min each and incubated with DAPI staining solution at room temperature in the dark for 10 min. The slides were then washed with PBS 3 times for 5 min each and sealed with antifluorescence quenching mounting medium. The apoptosis rate was calculated as the percentage of positive cells (green nuclei) relative to the total number of cells (blue nuclei) using Image-Pro Plus 6.0 software. Three chickens from each group were used, with each chicken having three slices and five fields of view observed from each slice. The reagents and apparatus used in the experiments are listed in Tables 5 and 6, respectively.
Table 5.
Experimental apparatus used during Tunel's experiments.
| Name of reagent | Product number | Manufacturer | Manufacturer location |
|---|---|---|---|
| A device for extracting water | JT-12K | Junjie Electronics | Wuhan, China |
| Embedder | JB-P5 | Junjie Electronics | Wuhan, China |
| Freezer station | JB-L5 | Junjie Electronics | Wuhan, China |
| Tissue spreader | JK-5 | Junjie Electronics | Wuhan, China |
| Slicing machine | RM2016 | Leica Instruments | |
| Ovens | DGX-9003B | Formosa Experimental Instrument | Shanghai, China |
| Microwaves | P70D20P-TF | Glance Microwave Oven & Appliance | Guangdong,China |
| Water bath | HH-W-600 | Jintan Jiangnan Instrument Factory | Jintan, China |
| Decolourisation shaker | WD-9405A | Liuyi Instrument Factory | Beijing, China |
| Vortex mixer | TYXH-II | Tianyue Electronics | Nanjing, China |
| Pipette gun | KE0003087/KA0056573 | Dragon | Beijing, China |
| General Optical Microscope | CX-21 | OLYMPUS | Japan |
| Inverted microscope | IX51 | OLYMPUS | Japan |
Table 6.
The experimental apparatus used during the Tunel experiments.
| Name of reagent | Model number | Manufacturer | Manufacturer location |
|---|---|---|---|
| 4% paraformaldehyde | B0010 | Baiqiandu Biotechnology | Wuhan, China |
| Anhydrous ethanol | 10009218 | Sinopharm Chemical Reagent | Beijing, China |
| Xylene | 10023418 | Sinopharm Chemical Reagent | Beijing, China |
| PBS solution(0.01M) | B0002 | Baiqiandu Biotechnology | Wuhan, China |
| 10×EDTA repair solution | B0015 | Baiqiandu Biotechnology | Wuhan, China |
| Hematoxylin Dye | B1001 | Baiqiandu Biotechnology | Wuhan, China |
| H2O2 | B12555 | Baiqiandu Biotechnology | Wuhan, China |
| DAB color development kit | 2005289 | DAKO | Denmark |
| Slides and coverslips | 10212432C | Shitai Experimental Equipment | Jiangsu, China |
| Neutral gums | 10004160 | Sinopharm Chemical Reagent | Beijing, China |
| Tunel kit | C1088 | Roche | Switzerland |
Enzyme-Linked Immunosorbent Assay
Spleen tissues, each weighing 0.1 grams, were retrieved from Eppendorf (EP) tubes and homogenized in PBS (Servicebio, China) (pH 7.4, 1x). Subsequently, the homogenized samples were centrifuged at 12,000 × g for 20 min at 4°C. The resulting supernatant was collected and analyzed using an ELISA kit (Huijia Biotechnology Co., Ltd., China). During the ELISA procedure, the prepared samples were applied to pretreated ELISA plates, where they were specifically bound to antibodies to form complexes. After washing to remove unbound materials, enzyme-labeled antibodies were added, which were recognized and bound to create enzyme-antibody complexes. A substrate solution was then introduced, and the enzyme facilitated the development of color from the substrate. The concentration of the target substance in the sample was determined by measuring the absorbance. The final outcome was quantified as pg/mg of protein.
Western Blot
First, the protein samples were extracted by taking 0.1 g of spleen tissue and mixing the tissue with IP lysis buffer (Biosharp, China) and PMSF (Biosharp, China) in a 99:1 ratio to release proteins. The protein concentration was determined using a colorimetric method. Next, 5× loading buffer (Beyotime, China) was added, and any surplus was added to separate EP tubes for storage in a refrigerator. The protein samples were subjected to SDS‒PAGE, transferred from the gel to a PVDF membrane (Cytiva, Marlborough, MA), and blocked with a rapid blocking solution for 10 min. The membrane was incubated with primary antibody overnight (Table 7), followed by three washes with Tris-buffered saline containing Tween (TBST) for 15 min each. The secondary antibody was added and incubated for 2 h. The membrane was then washed three times for 15 min each with TBST and prepared for visualization. For visualization, the entire membrane was covered with a developer solution (ECL, Biosharp Life Sciences, China). Protein expression levels were analyzed using ImageJ software. The density of each band was normalized relative to that of its respective internal reference (GAPDH) and (histone H3) (Bi et al., 2024).
Table 7.
The antibodies utilized in this investigation.
| Antibody | Dilution ratio | Source |
|---|---|---|
| Keap-1, histone H3, Nuclear Nrf2, NF-κB P65, IKB, IKK, IFN-γ |
1:300 | Wanlei, China |
| Bcl-2, Cyt-C, Caspase-3, Caspase-9, Bax | 1:1000 | ABclonal, China |
| GAPDH | 1:2000 | Servicebio, China |
Immunofluorescence Double Staining
The spleen tissues collected were fixed, dehydrated, embedded, and sectioned at a typical thickness of 5-10 μm. Antigen retrieval was then performed. Blocking was performed using a buffer containing serum or bovine serum albumin (BSA) to prevent nonspecific binding. Subsequently, primary antibodies against TNF-α (1:800) and IL-6 (1:800) (Proteintech, China) were added and incubated at 4°C overnight. After thorough washing with PBS to remove unbound primary antibodies, the membranes were incubated with secondary antibodies at room temperature for 1 h. DAPI was used for nuclear staining and microscopic examination. The software Image-Pro Plus 6.0 was used to convert the grayscale images of green and red fluorescent monochrome photos to grayscale. The optical density (IOD) and pixel area (AREA) of each positive image were analyzed, and the average density (IOD/AREA) was calculated.
Bioinformatics Analysis
Correlations between apoptosis, inflammation, and Keap-1/Nrf2 pathway-related gene expression were analyzed using Pearson's correlation coefficients (https://www.omicstudio.cn). Protein‒protein interactions of differentially expressed proteins were constructed using the online database STRING 12.0 and Cytoscape software to construct differentially expressed protein‒protein interactions (PPIs). Pathway enrichment analysis of the DEGs was performed using a hiplot (https://hiplot.com.cn).
Statistical Analysis
qRT‒PCR data were evaluated using the 2−ΔΔCt method, where the values of the control group were designated as 1. Immunofluorescence data were quantified using ImageJ to compute the optical density values, and the positive expression rate was determined by comparison with nuclear staining. Before conducting the statistical analysis, the normality of the qRT‒PCR and immunofluorescence data was assessed using the GraphPad Prism 9.5.1 test. Western blot, qRT‒PCR, immunofluorescence, and TUNEL data were analyzed using one-way ANOVA in GraphPad Prism 9.5.1, with multiple comparisons conducted . The results are expressed as the mean ± standard error, and statistical significance was set at P < 0.05.
RESULTS
Histological Structural Changes in the Spleen
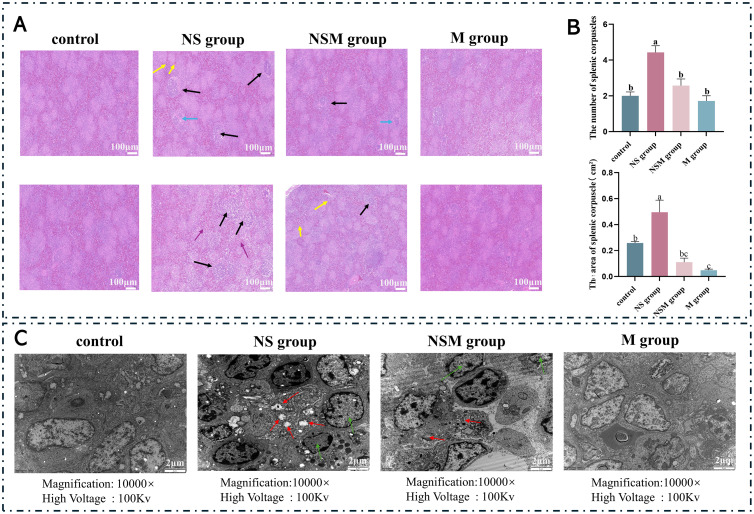
Photomicrographs of the histopathological examination of the spleen are shown in Figure 2. Histological observations revealed significant morphological differences between the groups: Groups C and M had normal spleen morphology with well-arranged splenocytes and no signs of congestion in the red pulp area. In contrast, the NS group showed significant pathologic and morphological changes after acute noise exposure, including irregular splenic nodules (black arrows) containing loosely arranged lymphocytes, macrophages, and a few plasma cells. Necrotic debris (yellow arrowheads) with a vacuolated appearance was observed, accompanied by inflammatory cell infiltration and mild disruption of splenic structures. In addition, scattered lymphocyte arrangements and prominent reticular epithelial cells (blue and purple arrows) were observed, as well as phagocytosis of cellular debris. After the musical intervention, the NSM group showed improvement over the NS group, with a decrease in necrotic debris and scattered lymphocytes. However, the prominent features of reticular epithelial cells remained, suggesting that some of the noise-induced changes had not been fully recovered. These findings highlight the detrimental effects of acute noise exposure on spleen morphology and also confirm that music intervention can mitigate these morphological changes to some extent. To gain further insights into the impact of acute noise exposure on the spleens of chicks, a quantitative analysis of the number and area of splenic corpuscles was performed (Figure 2B). A significant increase in both the number and area of splenic corpuscles was observed in the NS group (P < 0.05). A decrease in both the number and area of splenic corpuscles was observed in the NSM group after music mitigation (P < 0.05). Compared to those in the control group, no significant differences were observed in the cell number or area of splenocytes in the NSM group that received music intervention (P > 0.05). No significant differences in the number of splenic follicles were detected between the M group and the control group (P > 0.05), but the area of splenic follicles was significantly smaller in the M group than in the control group (P < 0.05). To further assess the impact of acute noise exposure on chicken spleens, ultrastructural observations were conducted, as illustrated in Figure 2C. Compared to the control group, after acute noise stimulation, some characteristics of cell apoptosis were observed in the spleen tissue of the NSM group, including mitochondrial cristae destruction (red arrows) and uneven cytoplasmic distribution (green arrows). In addition, we also observed the disappearance of mitochondrial cristae, which may affect the energy production function of mitochondria. In the NS group, the distribution of mitochondria changed slightly, with mitochondria lacking cristae clustering together. In contrast, after long-term music intervention, mitochondrial damage still existed in the NSM group, but the phenomena of cristae destruction and uneven cytoplasmic distribution were not as prominent in the NSM group compared to the NS group. These findings suggest that inflammation in splenic tissue can be triggered by acute noise exposure and that the inflammatory effects induced by noise stimulation may be alleviated by prolonged exposure to music.
Figure 2.
The attenuating influence of music on splenic damage caused by acute noise exposure in chicks. (A) Hematoxylin and eosin staining of spleen tissue sections: statistical analysis of splenic vesicle quantity and surface area. (B) Statistical analysis of splenic vesicle quantity and surface area. (C) Ultrastructural changes in the spleen of 42-day-old broiler chickens as observed under a transmission electron microscope (magnification of 10,000×); black arrows indicate splenic vesicles, yellow arrows indicate necrotic or apoptotic debris, blue arrows indicate scattered lymphocytes, purple arrows indicate prominent reticuloendothelial cells or macrophages, and green arrows indicate uneven cytoplasmic distribution. Red arrows indicate mitochondrial vacuolization and cristae disruption. Differences marked with lowercase letters indicate a P value of less than 0.05, and the same letter indicates a nonsignificant difference (P > 0.05) (mean ± SD).
Effect of Acute Noise Stimulation on the Antioxidant Activity of Chick Spleen Tissue
Inflammation and apoptosis can be induced by oxidative stress, prompting an investigation of the underlying mechanisms in animal models. The primary focus of this study was on the Keap-1/Nrf2 pathway. Activation of Nrf2 and HO-1, resulting in antioxidative effects, was observed following acute noise exposure (Figure 3). Our results demonstrated that following music mitigation, a significant decrease in the mRNA expression of Nrf2 and Keap-1 in the spleen of the NSM group was observed compared to that in the NS group (P < 0.05) (Figure 3A). In contrast, the mRNA expression of HO-1 in the NSM group was greater than that in the NS group (P < 0.05). Compared to those in the control group, the mRNA expression levels of Keap-1, Nrf2, and HO-1 in the NSM group were still significantly different (P < 0.05). Additionally, compared to those in the control group, the mRNA expression levels of Keap-1 in the M group were significantly lower (P < 0.05), while the mRNA expression levels of Nrf2 and HO-1 were not significantly different (P > 0.05). Although no significant difference was detected in the Keap-1 protein expression level compared to that in the other groups after music mitigation (P > 0.05), the protein expression pattern of Nrf2 reflected its mRNA expression trend (P < 0.05) (Figure 3B). To further validate the induction of oxidative stress by acute noise exposure, oxidative stress-related parameters were assessed (Figure 3C) to determine whether oxidative stress in the spleen triggered by acute noise could be alleviated by music. The MDA content in the spleen of the NS group was greater than that in the control group (P < 0.05). However, in the NSM group, the MDA content decreased relative to that in the NS group. Furthermore, the activities of SOD, CAT, and GSH-Px in the spleens of the NS group were reduced (P < 0.05). Remarkably, significant alterations in the activities of SOD, CAT, and GSH-Px were observed in the NSM group following music mitigation (P < 0.05). While the oxidative stress caused by acute noise exposure seemed to be alleviated by music, statistically significant differences remained between the NSM group and the control group (P < 0.05) in certain cases. Apart from CAT activity, which demonstrated no statistically significant differences between the control group and the M group (P > 0.05), the activities of SOD and GSH-Px, as well as the content of MDA, exhibited pronounced differences between the two groups (P < 0.05). These findings suggest that oxidative stress is induced in the spleen of chickens by acute noise exposure and that this oxidative stress may be mitigated by music.
Figure 3.
Music inhibits oxidative stress-mediated activation of the Keap-1/Nrf2 signaling pathway in chicken spleen. A: mRNA levels of Keap-1, Nrf2, and HO-1. B: Protein levels of Keap-1 and Nrf2. Nuclear Nrf2 is denoted by histone H3, and Keap-1 is denoted by GAPDH. C: SOD activity, CAT activity, GSH-Px activity, and MDA content. Statistical significance was analyzed using one-way ANOVA followed by the Tukey test. Different lowercase letters indicate P<0.05, and the same letter indicates that the difference is not significant (P > 0.05) (mean ± SD).
Long-Term Musical Stimulation Suppresses the Activation of the NF-κB Signaling Pathway Induced by Acute Noise Exposure
NF-κB plays a crucial role in the regulation of downstream inflammatory cytokines and mediators in the inflammatory signaling pathway. Therefore, changes in the NF-κB pathway were examined using quantitative real-time PCR and western blot analysis (Figure 4). Regarding gene mRNA expression, a significant downward trend in NF-κB, IFN-γ, TNF-α, IL-1, and IL-1β was observed in the NSM group compared to the NS group following long-term musical mitigation (P < 0.05) (Figure 4A). Moreover, compared to those in the control group, the levels of TNF-α, IL-1β, iNOS, and COX-2 in the M group tended to decrease (P < 0.05). To further confirm the efficacy of long-term music stimulation in alleviating inflammation induced by acute noise exposure, the expression levels of proteins linked to the NF-κB signaling pathway were evaluated using western blotting (Figure 4C) and immunofluorescence (Figure 4D) techniques. Our findings indicate that following long-term musical mitigation, the expression levels of IL-1, IL-1β, IKK, NF-κB P65, TNF-α, and IL-6 were significantly lower in the NSM group than in the NS group (P < 0.05). Importantly, notable differences in the expression of IL-1, IL-1β, IKK, NF-κB P65, TNF-α, and IL-6 persisted between the NSM group and both the C and M groups (P < 0.05). In addition to the significant increase in the expression of IκB, the expression levels of TNF-α, IL-6, NF-κB P-65, IKK, IFN-γ, IL-1, and IL-1β were significantly decreased (P < 0.05). Through these experimental analyses, activation of the NF-κB pathway was observed, and evidence indicated that the NF-κB signaling pathway was suppressed by long-term musical stimulation.
Figure 4.
Music has a potential anti-inflammatory effect and can effectively attenuate splenic inflammation by inhibiting the NF-κB signaling pathway triggered by noise. (A) mRNA expression levels of NF-κB, IFN-γ, TNF-α, IL-1β, IL-1, INOS, and COX-2. (B) Concentrations of IL-1 and IL-1β. C: Protein expression levels of NF-κB, IKB, IKK, and IFN-γ. D: Expression levels of TNF-α (red) and IL-6 (green) in the spleen after music-induced alleviation of acute noise were detected by immunofluorescence. The data were detected by protein blot analysis and are expressed as the mean ± SD. Statistical significance was analyzed using one-way ANOVA followed by the Tukey test. Different lowercase letters indicate P < 0.05, and the same letter indicates that the difference is not significant (P > 0.05) (mean ± SD).
Analysis of Apoptotic Molecule Expression in Spleen Tissue and TUNEL Assay
Figure 5 shows the mRNA and protein expression levels of the apoptotic genes in the spleen. The expression of genes related to apoptosis was assessed on the 28th day after acute noise exposure (Figure 5A). Notably, on the 28th day after the stimulus, significant upregulation of the expression of the apoptosis-related genes Bax, P-53, Cyt-C, Casp-3, Casp-8, and Casp-9 was detected in the spleens of the NS group (P < 0.05), whereas the expression of Bcl-2 exhibited a significant downward trend (P < 0.05). Following prolonged musical mitigation, a significant reduction in the expression of the apoptotic genes Bax, P-53, Cyt-C, Casp-3, Casp-8, and Casp-9 in the spleen of the NSM group was observed compared to that in the NS group (P < 0.05), whereas Bcl-2 was notably upregulated (P < 0.05). Compared to those in the control group, a marked increase in the expression of apoptotic genes, such as Bax, P-53, Cyt-C, Casp-3, Casp-9, and Casp-8, was observed in the NSM group (P < 0.05), while the expression of the antiapoptotic gene Bcl-2 was significantly decreased (P < 0.05). Furthermore, the protein expression data presented in Figure 5B indicate that the expression of apoptotic genes was triggered by acute noise exposure, except for Bcl-2, whose expression was significantly decreased. However, subsequent music mitigation led to a significant downregulation of apoptotic gene expression in the NSM group compared to that in the NS group, along with a notable upregulation of Bcl-2 expression (P < 0.05). To further confirm the occurrence of apoptosis, a TUNEL assay was conducted on the 28th day of the experiment. The results of this assay (Figure 5C) revealed a significant increase in the number of TUNEL-positive (green) nuclei in the spleens of chickens in the NS group compared to those in the control group (P < 0.05). Nevertheless, following musical mitigation, there was a substantial decrease in the number of TUNEL-positive nuclei in the spleen of the NSM group compared to that in the NS group (P < 0.05). In summary, these findings suggest that apoptosis in the spleen is triggered by acute noise exposure and can be alleviated by prolonged musical stimulation. This alleviation was evidenced by the downregulation of apoptotic genes, upregulation of Bcl-2, and a reduction in the number of TUNEL-positive nuclei, indicating the protective effect of music against noise-induced apoptosis in the spleen.
Figure 5.
Music alleviates excessive apoptosis induced by acute noise stimulation. (A) mRNA expression levels of Bcl-2, Bax, P-53, Cyt-C, Casp-3, Casp-8, and Casp-9 in the spleen. (B) Protein expression levels of Bcl-2, Cyt-C, Casp-3, Casp-9, and Bax in the spleen. C: TUNEL assay results for the spleen (assessed in section 42D). Apoptotic cells are shown in green, and apoptotic cells are shown in blue. (D) Protein‒protein interaction network diagram. (E) PPI network. (F) Correlation analysis of apoptosis, inflammation, and Nrf2/HO-1 pathway-related genes. Statistical significance was analyzed using one-way ANOVA followed by the Tukey test. Different lowercase letters indicate P < 0.05, and the same letter indicates a nonsignificant difference (P > 0.05) (mean ± SD).
Bioinformatics Analysis of the Effects of Music-Relieved Acute Noise Stimulation on the Spleen
Figure 5D illustrates a protein‒protein interaction network of spleen tissue, revealing strong relationships among the Keap-1/Nrf2 pathway, Keap1, Nfe2l2, NF-κB signaling pathway, Nfkb1, Ikbkg, apoptosis-related genes Bcl2, Bax, Cyct, Casp3, Casp9, cytokines il6, Irf3, Tnf, and il-b. Additionally, we carried out PPI analysis to identify interactions and correlations among 37 highly differentially expressed genes (DEGs) (Figure 5E). The PPI network was visualized using Cytoscape software to assess the interactions and correlations among these 37 DEGs. Notably, NFKB1, PTGS2, and MAPK8 showed high connectivity with other genes in the network, whereas NOS2, IL6, KEAP1, PIK3CA, IL1B, and MDM2 were highly relevant in the network. To evaluate the crosstalk among the Keap-1/Nrf2, NF-κB, and apoptosis signaling pathways, a correlation analysis of gene expression from these pathways was conducted, as shown in Figure 5F. The Bcl-2 gene displayed a significant negative correlation with other genes, while genes from the three pathways showed significant positive correlations (P < 0.05). HO-1 showed a positive correlation but was not significant (P > 0.05), indicating that acute noise stimulation may induce spleen injury by intervening in the Keap-1/Nrf2, NF-κB, and apoptosis signaling pathways.
DISCUSSION
Previous research has clearly demonstrated the negative impact of noise on organismal health (Daiber et al., 2019). Moreover, noise exposure in poultry imposes significant stress, resulting in cognitive deficits and neural damage (Turner et al., 2005; Chaudhury et al., 2013; Rodenburg et al., 2017). Prolonged exposure to noise can cause damage to the auditory, nervous, cardiovascular, endocrine, and visual systems to different extents (Westman and Walters 1981; Hahad et al., 2022). Notably, sustained exposure to high decibel noise can trigger inflammation in immune tissues and lymphocyte apoptosis, thereby increasing the risk of immune deficiency (Gröschel et al., 2018). Chen et al. (2022) reported that exposure to noise rapidly increased inflammatory cytokines, while gene expression associated with outer hair cell death also elevated (Chen et al., 2022). Finlay et al. discovered that listening to music aids in releasing negative emotions and managing discomfort arising from inflammation, chronic stress, and chronic pain (Finlay, 2014; Finn and Fancourt, 2018). Further analysis indicated that music therapy has potential value in alleviating the detrimental effects of diseases (Sung et al., 2010), particularly in the treatment of conditions such as liver depression and spleen deficiency, where five-element music has demonstrated remarkable therapeutic effects (Zhang et al., 2015). Despite extensive research on the impact of noise on organismal health, the detailed mechanisms underlying immune dysregulation remain unclear. The spleen, which serves as a central lymphoid organ, plays a crucial role in immune function and is primarily involved in regulating humoral immunity (Ingrao et al., 2013). Damage to these organs can disrupt the immune system, impair the reproductive performance of animals, and increase their susceptibility to infections, cancer, and autoimmune diseases (Hofmann et al., 2020). However, the immunomodulatory effects of music on noise-stimulated chicken spleens have not been reported. This study aimed to investigate the preventive effects of prolonged musical stimulation on noise-induced immune damage, including oxidative stress and apoptosis, in the chicken spleen. This study also aimed to provide an important foundation for the development of effective strategies to prevent noise-induced immune dysfunction. Histological ultrastructural analysis revealed that long-term exposure to music partially alleviated noise-induced pathological changes and notably attenuated noise-induced oxidative stress in splenic tissues. Oxidative stress is likely responsible for the abnormal pathological and structural alterations observed in the spleen. Noise significantly increases ROS levels in chickens, leading to severe oxidative stress. This is because ROS can oxidize and damage cell membrane lipids, proteins, and DNA, thereby causing oxidative damage. To demonstrate that long-term music exposure alleviate noise-induced oxidative stress, we evaluated the indicators of proteins and mRNAs associated with oxidative stress. Our results demonstrated that the activities of the antioxidant enzymes SOD, CAT, and GSH-Px in the spleen were reduced in the noise-exposed group, accompanied by a significant increase in the MDA content. In contrast, exposure to chronic music stimulation significantly increased the activities of these antioxidant enzymes while reducing the MDA content. Additionally, exposure to pure music elevates antioxidant stress levels in chickens. These findings suggest that noise exposure induces oxidative stress in chicken spleen tissues, whereas music can mitigate the oxidative stress induced by acute noise exposure. These findings are consistent with those of previous studies demonstrating the long-term mitigating effects of music exposure on oxidative stress (Nian et al., 2023). The activation of the Nrf2 pathway serves as a formidable defense mechanism against the detrimental effects of oxidative stress (Zhou et al., 2021). Consequently, Nrf2 has emerged as a pivotal therapeutic target not only for oxidative stress-related conditions but also for a diverse range of disorders. Furthermore, the inhibition of INOS and COX-2 has been proposed as an essential focal point in the mitigation of noise-induced effects through music in chickens. It can be hypothesized that music mitigates alterations in cytokine expression mediated by oxidative stress. The NS group exhibited a significant increase in the expression of Nrf2 and its downstream gene HO-1 at both the mRNA and protein levels in the nucleus, while the NSM group, which received music relief, demonstrated greater upregulation of HO-1 expression than the NS group. These results suggest that the beneficial effect of music can be partially attributed to its ability to stimulate the expression of cytoprotective genes following exposure to noise. When exposed to high noise levels, cells activate the NF-κB pathway as part of the stress response mechanism. This activation causes NF-κB to translocate to the nucleus, where it acts as a transcription factor to regulate the expression of genes involved in inflammation, the immune response, and cell survival. Our study revealed that following acute noise stimulation, the expression levels of IKK in the spleen tissue of the NS group were upregulated, leading to further separation of IKB and NF-κB P-65. This results in the translocation of NF-κB into the cell nucleus, subsequently promoting the expression of TNF-α, IL-1, IL-1β, and IFN-γ. However, after prolonged music intervention, we observed a decrease in the expression of IKK, TNF-α, IL-1, IL-1β, and IFN-γ. Although long-term exposure to music effectively reduced the damage caused by acute noise stimulation, we did not observe a complete reversal of this damage. These findings suggest that music may modulate the expression of inflammatory factors by regulating the NF-κB signaling pathway, although its impact on the immune response induced by acute noise remains limited.
Apoptosis is a tightly regulated process that plays a crucial role in maintaining tissue balance and eliminating excess or damaged cells. Under the influence of oxidative stress signals, pro-apoptotic proteins are activated, promoting the release of cytochrome C from mitochondria into the cytoplasm. Cytochrome c then interacts with other proteins to form an apoptosome, thereby activating caspase enzymes. Research suggests that oxidative stress can lead to mitochondrial and DNA damage, potentially triggering cell injury or apoptosis (Kujoth et al., 2005). Furthermore, the NS group exhibited a significantly greater percentage of apoptotic spleen cells than both the control and NSM groups. The results of the TUNEL assay revealed a substantial increase in the number of TUNEL-positive nuclei in the NS group, whereas the NSM and M groups displayed a significant reduction in the number of TUNEL-positive nuclei. Subsequently, we examined the expression levels of apoptosis-related genes and proteins and observed a decrease in the expression of these genes, except for the antiapoptotic gene Bcl-2. It is noteworthy that although there were still differences in the expression of apoptosis-related genes and proteins between the NSM group and the control group, music intervention still exhibited a positive mitigating effect in this scenario. However, the effect of noise on energy metabolism in the mitochondrial respiratory chain complex of chicken spleens requires further investigation. Taken together, these findings demonstrate that music protects against immune dysfunction by activating the Keap-1/Nrf2 signaling pathway while inhibiting NF-κB and proinflammatory cytokines in the chicken spleen. Additionally, our study provides evidence that noise can induce apoptosis in immune tissues, whereas music intervention can effectively alleviate these apoptotic effects. These findings have significant implications for understanding the pathophysiological mechanisms underlying noise-induced immune tissue damage and the potential therapeutic advantages of music therapy for ameliorating these effects. Musical intervention can alleviate these pathological changes to a certain extent, although it may not completely resolve them. These findings offer new insights and evidence for studying noise-induced immune system damage and the potential use of music as a therapeutic intervention. Furthermore, this study focused solely on the involvement of cellular apoptosis and the NF-κB signaling pathway in attenuating immune organ inflammation mediated by music during acute noise stimulation. Further research is needed to explore the interplay and interactions between these pathways to gain a better understanding of the preventive mechanisms by which music protects against noise. However, the potential regulatory mechanisms of other pathways, such as the endoplasmic reticulum stress pathway and the MAPK pathway, when modulated by oxidative stress during noise exposure remain unclear. To further demonstrate the sustained effects of music, we aimed to expand the scope of our experiments by including a larger number of test animals. By integrating molecular experiments with production data, we aimed to comprehensively understand the impact of music on mitigating the effects of acute noise stimulation on the immune system of chickens. This will enable us to provide targeted recommendations and strategies for agricultural production.
CONCLUSIONS
In conclusion, our results demonstrate that splenic oxidative stress, inflammation, and apoptosis can be induced by acute noise stimulation, resulting in splenic injury. Importantly, acute noise stimulation-induced inflammation and apoptosis in chicken spleens can be attenuated by long-term music through modulation of the Keap-1/Nrf2 and NF-κB pathways (Figure 6). This study contributes to the understanding of the mechanisms by which long-term music alleviates oxidative stress and inflammation and provides a new strategy for attenuating spleen injury induced by acute noise stimulation.
Figure 6.
Prolonged exposure to music-rich environments attenuates oxidative stress and apoptosis induced by acute noise stimulation by modulating oxidative-antioxidant responses, and inflammatory responses in the chicken spleen.
DISCLOSURES
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The study was supported by the National Natural Science Foundation of China (Grant No. 32172788, 31772645).
Author Contributions: Haowen Wang: Laboratory animal husbandry, data handling, and paper writing. Yulai Wang: Feeding of test animals and correction of articles. Haoran Zhang and Qingqing Chang: Changes to the articles with formatting problems. Jianhong Li provided experimental design ideas. Runxiang Zhang and Jun Bao: Provide article design ideas, page charges, experimental funding, and correct problems with the article.
Data Availability: If necessary, part of the data could be requested from the first author or the corresponding author with the author's consent.
REFERENCES
- Alworth L.C., Buerkle S.C. The effects of music on animal physiology, behavior and welfare. Lab Anim. 2013;42:54–61. doi: 10.1038/laban.162. [DOI] [PubMed] [Google Scholar]
- Arranz L., De Castro N.M., Baeza I., Mate I., Viveros M.P., De I.F.M. Environmental enrichment improves age-related immune system impairment: long-term exposure since adulthood increases life span in mice. Rejuv. Res. 2010;13:415–428. doi: 10.1089/rej.2009.0989. [DOI] [PubMed] [Google Scholar]
- Bi Y., Wei H., Chai Y., Wang H., Xue Q., Li J. Intermittent mild cold acclimation ameliorates intestinal inflammation and immune dysfunction in acute cold-stressed broilers by regulating the tlr4/myd88/nf-κb pathway. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2024.103637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brod S., Gobbetti T., Gittens B., Ono M., Perretti M., D'Acquisto F. The impact of environmental enrichment on the murine inflammatory immune response. JCI Insight. 2017;2:e90723. doi: 10.1172/jci.insight.90723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V., Pittet M.J. The spleen in local and systemic regulation of immunity. Immunity. 2013;39:806–818. doi: 10.1016/j.immuni.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo J.L., Gil M.G., Dávila S.G. Effects of specific noise and music stimuli on stress and fear levels of laying hens of several breeds. Appl. Anim. Behav. Sci. 2005;91:75–84. [Google Scholar]
- Chang Q., Li C., Zhao S., Wang H., Li J., Zhang R., Bao J. Research note: effects of environmental sound stimulus on behavioral responses, cortisol levels, and horizontal immunity of transferred pullets. Poult Sci. 2024;103 doi: 10.1016/j.psj.2024.103689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury S., Nag T.C., Jain S., Wadhwa S. Role of sound stimulation in reprogramming brain connectivity. J. Biosci. 2013;38:605–614. doi: 10.1007/s12038-013-9341-8. [DOI] [PubMed] [Google Scholar]
- Chen D., Jia G., Zhang Y., Mao H., Zhao L., Li W., Chen Y., Ni Y. Sox2 overexpression alleviates noise-induced hearing loss by inhibiting inflammation-related hair cell apoptosis. J Neuroinflammation. 2022;19:59. doi: 10.1186/s12974-022-02414-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhou Z., Min W. Mitochondria, oxidative stress and innate immunity. Front. Physiol. 2018;9:1487. doi: 10.3389/fphys.2018.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiber A., Kröller-Schön S., Frenis K., Oelze M., Kalinovic S., Vujacic-Mirski K., Kuntic M., Jimenez M.T.B., Helmstädter J., Steven S., Korac B., Münzel T. Environmental noise induces the release of stress hormones and inflammatory signaling molecules leading to oxidative stress and vascular dysfunction—Signatures of the internal exposome. Biofactors. 2019;45:495–506. doi: 10.1002/biof.1506. [DOI] [PubMed] [Google Scholar]
- Demirel R., Mollaoğlu H., Yeşilyurt H., Üçok K., Ayçiçek A., Akkaya M., Genç A., Uygur R., Doğan M. Noise induces oxidative stress in rat. Eur. J. Gen. Med. 2009;6:20–24. [Google Scholar]
- Finlay K.A. Music-induced analgesia in chronic pain: Efficacy and assessment through a primary-task paradigm. Psychol. Music. 2014;42:325–346. [Google Scholar]
- Finn S., Fancourt D. The biological impact of listening to music in clinical and nonclinical settings: a systematic review. Prog. Brain Res. 2018;237:173–200. doi: 10.1016/bs.pbr.2018.03.007. [DOI] [PubMed] [Google Scholar]
- Garofalo S., D'Alessandro G., Chece G., Brau F., Maggi L., Rosa A., Porzia A., Mainiero F., Esposito V., Lauro C., Benigni G., Bernardini G., Santoni A., Limatola C. Enriched environment reduces glioma growth through immune and non-immune mechanisms in mice. Nat. Commun. 2015;6:6623. doi: 10.1038/ncomms7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröschel M., Basta D., Ernst A., Mazurek B., Szczepek A.J. Acute noise exposure is associated with intrinsic apoptosis in murine central auditory pathway. Front. Neurosci. 2018;12:312. doi: 10.3389/fnins.2018.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahad O., Jimenez M.T.B., Kuntic M., Frenis K., Steven S., Daiber A., Münzel T. Cerebral consequences of environmental noise exposure. Enviro. Int. 2022;165 doi: 10.1016/j.envint.2022.107306. [DOI] [PubMed] [Google Scholar]
- Hofmann T., Schmucker S.S., Bessei W., Grashorn M., Stefanski V. Impact of housing environment on the immune system in chickens: a review. Animals. 2020;10:1138. doi: 10.3390/ani10071138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingrao F., Rauw F., Lambrecht B., van den Berg T. Infectious bursal disease: a complex host-pathogen interaction. Dev. Comp. Immunol. 2013;41:429–438. doi: 10.1016/j.dci.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Kight C.R., Swaddle J.P. How and why environmental noise impacts animals: an integrative, mechanistic review. Ecol. Lett. 2011;14:1052–1061. doi: 10.1111/j.1461-0248.2011.01664.x. [DOI] [PubMed] [Google Scholar]
- Kujoth G.C., Hiona A., Pugh T.D., Someya S., Panzer K., Wohlgemuth S.E., Hofer T., Seo A.Y., Sullivan R., Jobling W.A., Morrow J.D., Van Remmen H., Sedivy J.M., Yamasoba T., Tanokura M., Weindruch R., Leeuwenburgh C., Prolla T.A. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- Kupcikova Z., Fecht D., Ramakrishnan R., Clark C., Cai Y.S. Road traffic noise and cardiovascular disease risk factors in UK Biobank. Eur. Heart J. 2021;42:2072–2084. doi: 10.1093/eurheartj/ehab121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G., Hannan A.J., Macrì S., Solinas M., Jaber M. Effects of enriched environment on animal models of neurodegenerative diseases and psychiatric disorders. Neurobiol. Dis. 2008;31:159–168. doi: 10.1016/j.nbd.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Li J., Li X., Liu H., Li J., Han Q., Wang C., Zeng X., Li Y., Ji W., Zhang R., Bao J. Effects of music stimulus on behavior response, cortisol level, and horizontal immunity of growing pigs. J. Anim. Sci. 2021;99:skab043. doi: 10.1093/jas/skab043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirmohammadi S., Khanjani N., Nazarkhani F., Abediankenari S., Yazdani J., Tilaki R.A.D. The effect of noise and dust exposure on oxidative stress among livestock and poultry feed industry workers. Toxicol. Ind. Health. 2020;36:908–915. doi: 10.1177/0748233720962253. [DOI] [PubMed] [Google Scholar]
- Nian H.Y., Zhang R.X., Ding S.S., Wang Y.L., Li J.F., Liu H.G., Li J.H., Li X., Bao J. Emotional responses of piglets under long-term exposure to negative and positive auditory stimuli. Domest. Anim. Endocrinol. 2023;82 doi: 10.1016/j.domaniend.2022.106771. [DOI] [PubMed] [Google Scholar]
- Papadakakis A., Sidiropoulou K., Panagis G. Music exposure attenuates anxiety-and depression-like behaviors and increases hippocampal spine density in male rats. Behav. Brain Res. 2019;372 doi: 10.1016/j.bbr.2019.112023. [DOI] [PubMed] [Google Scholar]
- Qi S., Xin Y., Guo Y., Diao Y., Kou X., Luo L., Yin Z. Ampelopsin reduces endotoxic inflammation via repressing ROS-mediated activation of PI3K/Akt/NF-κB signaling pathways. Int. Immunopharmacology. 2012;12:278–287. doi: 10.1016/j.intimp.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Rattazzi L., Piras G., Brod S., Smith K., Ono M., D'Acquisto F. Impact of enriched environment on murine T cell differentiation and gene expression profile. Front. Immunol. 2016;7:381. doi: 10.3389/fimmu.2016.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reybrouck M., Vuust P. Neural correlates of music listening: does the music matter? Brain Sci. 2021;11 doi: 10.3390/brainsci11121553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemer S., Heritier C., Windschnurer I., Pratsch L., Arhant C., Affenzeller N. A review on mitigating fear and aggression in dogs and cats in a veterinary setting. Animals. 2021;11:158. doi: 10.3390/ani11010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenburg T., et al. Light during incubation and noise around hatching affect cognitive bias in laying hens. Proceedings of the 51st Congress of the International Society for Applied Ethology (ISAE); Aarhus, Denmark; 2017. [Google Scholar]
- Saghari H., Sheibani V., K E., Rehman N.U. Music alleviates learning and memory impairments in an animal model of post-traumatic stress disorder. Biointerface Res. Appl. Chem. 2021;11:7775–7784. [Google Scholar]
- Saha S., Buttari B., Panieri E., Profumo E., Saso L. An overview of nrf2 signaling pathway and its role in inflammation. Molecules. 2020;25:5474. doi: 10.3390/molecules25225474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesti F., Tsitsilonis O.E., Kotsinas A., Trougakos I.P. Oxidative stress-mediated biomolecular damage and inflammation in tumorigenesis. In. Vivo. 2012;26:395–402. [PubMed] [Google Scholar]
- Shukla M., Mani K.V., Deepshikha S.S., Kapoor N. Moderate noise associated oxidative stress with concomitant memory impairment, neuro-inflammation and Neurodegeneration. Brain Behav. Immu. Health. 2020;5 doi: 10.1016/j.bbih.2020.100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H.C., Chang A.M., Lee W.L. A preferred music listening intervention to reduce anxiety in older adults with dementia in nursing homes. J. Clin. Nurs. 2010;19:1056–1064. doi: 10.1111/j.1365-2702.2009.03016.x. [DOI] [PubMed] [Google Scholar]
- Su Y., Li T., He X., Sun H., Li J. PI3K/AKT pathway modulation and cold acclimation alleviation concerning apoptosis and necroptosis in broiler thymus. Poult Sci. 2024;103 doi: 10.1016/j.psj.2024.103634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaut M.H. Music as therapy in early history. Prog. Brain Res. 2015;217:143–158. doi: 10.1016/bs.pbr.2014.11.025. [DOI] [PubMed] [Google Scholar]
- Trappe H.-J. The effects of music on the cardiovascular system and cardiovascular health. Heart. 2010;96:1868. doi: 10.1136/hrt.2010.209858. [DOI] [PubMed] [Google Scholar]
- Turner J.G., Parrish J.L., Hughes L.F., Toth L.A., Caspary D.M. Hearing in laboratory animals: strain differences and nonauditory effects of noise. Comp. Med. 2005;55:12–23. [PMC free article] [PubMed] [Google Scholar]
- Wachi M., Koyama M., Utsuyama M., Bittman B.B., Kitagawa M., Hirokawa K. Recreational music-making modulates immunological responses and mood states in older adults. J. Med. Dent. Sci. 2009;56:79–90. [PubMed] [Google Scholar]
- Wang H., Chai Y., Xu Y., Wang Y., Li J., Zhang R., Bao J. Long-term music stimulating alleviated the inflammatory responses caused by acute noise stress on the immune organs of broilers by nf-κb signaling pathway. Ecotoxicol. Environ. Saf. 2024;273 doi: 10.1016/j.ecoenv.2024.116131. [DOI] [PubMed] [Google Scholar]
- Wei H., Li T., Zhang Y., Liu X., Gong R., Bao J., Li J. Cold stimulation causes oxidative stress, inflammatory response and apoptosis in broiler heart via regulating nrf2/ho-1 and nf-κb pathway. J. Therm. Biol. 2023;116 doi: 10.1016/j.jtherbio.2023.103658. [DOI] [PubMed] [Google Scholar]
- Wei H., Zhang Y., Li T., Zhang S., Yin J., Liu Y., Xing L., Bao J., Li J. Intermittent mild cold stimulation alleviates cold stress-induced pulmonary fibrosis by inhibiting the TGF-beta1/Smad signaling pathway in broilers. Poult Sci. 2024;103 doi: 10.1016/j.psj.2023.103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman J.C., Walters J.R. Noise and stress: a comprehensive approach. Environ. Health Persp. 1981;41:291–309. doi: 10.1289/ehp.8141291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaatar M.T., Alhakim K., Enayeh M., Tamer R. The transformative power of music: Insights into neuroplasticity, health, and disease. Brain Behav Immun Health. 2024;35:100716. doi: 10.1016/j.bbih.2023.100716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Cai Z., Yu Y., Wu L., Zhang Y. Effect of lixujieyu recipe in combination with five elements music therapy on chronic fatigue syndrome. J. Tradit. Chin. Med. 2015;35:637–641. doi: 10.1016/s0254-6272(15)30152-7. [DOI] [PubMed] [Google Scholar]
- Zhou F., Zou X., Zhang J., Wang Z., Yang Y., Wang D. Jian-pi-yi-shen formula ameliorates oxidative stress, inflammation, and apoptosis by activating the nrf2 signaling in 5/6 nephrectomized rats. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.630210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Wang K., Jia X., Fu C., Yu H., Wang Y. Antioxidant peptides, the guardian of life from oxidative stress. Med. Res. Rev. 2024;44:275–364. doi: 10.1002/med.21986. [DOI] [PubMed] [Google Scholar]
Further readings
- Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., Li Y., Wang X., Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B.H., Guo W., Wang P.Y., Henderson D., Jiang S.C. Intense noise-induced apoptosis in hair cells of guinea pig cochleae. Acta Oto-laryngol. 2000;120:19–24. [PubMed] [Google Scholar]
- Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruvengadam M., Venkidasamy B., Subramanian U., Samynathan R., Ali S.M., Rebezov M., Girish S., Thangavel S., R.j Dhanapal A., Fedoseeva N., Lee J., Chung I.-M. Bioactive compounds in oxidative stress-mediated diseases: targeting the nrf2/are signaling pathway and epigenetic regulation. Antioxidants. 2021;10:1859. doi: 10.3390/antiox10121859. [DOI] [PMC free article] [PubMed] [Google Scholar]