Abstract
Avian influenza virus (AIV) subtype H9N2 has significantly threatened the poultry business in recent years by having become the predominant subtype in flocks of chickens, ducks, and pigeons. In addition, the public health aspects of H9N2 AIV pose a significant threat to humans. Early and rapid diagnosis of H9N2 AIV is therefore of great importance. In this study, a new method for the detection of H9N2 AIV based on fluorescence intensity was successfully established using CRISPR/Cas13a technology. The Cas13a protein was first expressed in a prokaryotic system and purified using nickel ion affinity chromatography, resulting in a high-purity Cas13a protein. The best RPA (recombinase polymerase amplification) primer pairs and crRNA were designed and screened, successfully constructing the detection of H9N2 AIV based on CRISPR/Cas13a technology. Optimal concentration of Cas13a and crRNA was determined to optimize the constructed assay. The sensitivity of the optimized detection system is excellent, with a minimum detection limit of 10° copies/μL and didn't react with other avian susceptible viruses, with excellent specificity. The detection method provides the basis for the field detection of the H9N2 AIV.
Key words: avian influenza virus, CRISPR/Cas13a, detection, RPA, SHERLOCK
Keypoints
CRISPR/Cas13a is more specific and reduces false positives.
Successful construction of CRISPR/Cas13a-based H9N2 AIV detection method.
The constructed detection system is optimized.
INTRODUCTION
Avian influenza, a devastating viral disease brought on by the avian influenza virus (AIV) (Long et al. 2019), poses a risk to human health as well as significant financial losses for the poultry sector (Liu et al. 2015; Wang et al. 2016). AIVs can be categorized into 18 HA and 11 NA subtypes based on variations in the surface glycoproteins hemagglutinin (HA) and neuraminidase (NA) (Tong et al. 2013). AIVs are classified into 2 main categories as Highly pathogenic avian influenza (HPAI) and Low pathogenic avian influenza (LPAI). The most lethal and contagious poultry diseases are primarily caused by the HPAI H5 and H7 viruses. Among these, HPAI H5N1 is typically regarded as the most economically significant virus. H7N9 and H9N2, although LPAI viruses, also hold substantial economic importance (Centre for Disease Control and Prevention, 2024). The AIV H9N2 subtype, first discovered in the United States of America in 1966, has subsequently spread throughout the world. Recent researches indicate that internal H9N2 AIV segments can produce zoonotic influenza viruses, including the highly pathogenic H10N8 and H7N9 (Gao et al. 2013; Chen et al. 2014). It has been observed that the virus can induce dreadful respiratory tract infections causing high mortalities and drop in egg production in laying hens (Naila et al. 2008). H9N2 avian influenza A virus appears to persist in chicks and continues to spread to other flocks. The gross pathological lesions induced by LPAI-H9N2 are generally mild hyperemia in the air sac and fibrinous exudate in the bifurcation of trachea, fibronecrotic cast in the tracheal lumen, mild hemorrhages and mild pulmonary congestion (Waheed et al. 2022; Bóna et al. 2023).
LPAI-H9N2 suppresses the host immunity in many ways to establish the infection. Recent studies have reported that PA-X protein of H9N2 suppresses the innate immunity of chicken bone marrow derived dendritic cells. Furthermore, H9N2 also downregulates the FcRY expression and suppresses the innate immunity of chicken (Qin et al. 2023; Sun et al. 2024). It has been observed that the severity of H9N2 induced disease gets higher when there is a co-infection with some other viral or bacterial pathogen. The co-infection of E. coli and LPAI H9N2 has resulted in severe clinical signs with higher mortalities and these 2 major pathogens can affect the broiler chickens much more than each other alone (Jaleel et al. 2017). Similarly, co-infections of LPAIV-H9N2 with other respiratory pathogens, such as infectious bronchitis virus (IBV), Mycoplasma gallisepticum, Staphylococcus aureus, Escherichia coli, and Ornithobacterium rhinotracheale, can exacerbate H9N2 infections, leading to high morbidity and mortality (Hassan et al. 2021).
Several diagnostic methods have been developed in the past for the detection of avian influenza subtype H9N2. PCR method is endorsed because of its advanced clinical diagnostic nature. It's difficult to isolate virus from putrefied samples but PCR has given promising detection results from those samples too. Numerous detection methods of conventional PCR and qPCR have been developed for the detection of AIV H9N2 (Siddique et al. 2008; Shabat et al. 2010). Although the conventional PCR methods are highly sensitive and specific in detection yet they have some flaws in sensitivity, simplicity, specificity and cost effectiveness which limit their usage for further research and clinical aspects. Furthermore, there have been successful attempts to develop ELISA-based detection methods for H9N2 avian influenza subtypes (Yang et al. 2016; Ming et al. 2019) but still there are some improvements required in the field of diagnosis so as to minimize the economic loss as well as health risk to birds and humans as well. In this study, we have made some contributions in the detection methods of avian influenza virusH9N2 subtype. We have developed a detection method for the H9N2 AIV that performs detection of nucleic acid based on a recombinase polymerase amplification (RPA)-CRISPR.
CRISPR-LwCas13a is an orthologue of Cas13aprotein from Leptotrichia wadei. It can be re-programmed using CRISPR RNA (crRNA) to only pick out the RNA that needs to be tested (Fozouni et al. 2021). After that, labeled RNA is degraded in vitro by activating the CRISPR-collateral-cleavage function of LwCas13a (East-Seletsky et al. 2016; Smargon et al. 2017). The RPA-CRISPR detection technique is additionally known as specialized High-sensitivity enzymatic reporter unlocking (SHERLOCK). This is a highly sensitive in vitro real-time nucleic acid detection platform based on RPA and CRISPR-mediated complementary cleavage of reporter gene RNA (Kellner et al. 2019). The RNA reporter serves as a cleavage substrate for Cas13a. Therefore, further transcription of the RPA amplicon into target RNA is required to initiate the cleavage event. It is noteworthy that after the RPA amplification, the DNA transcription will again amplify the number of copies of the crRNA target region. Cas13a is useful for detecting diagnostic samples with very low viral loads due its high sensitivity.
Combined with heating of nonextracted diagnostic samples to inactivate nucleases, RPA-CRISPR can be used to detect viral DNA or RNA from the body fluids of sick animals without the cumbersome process of DNA extraction (Chen et al. 2022). The field diagnosis applications of RPA-CRISPR may be increased when combined with a lateral flow strip (Myhrvold et al. 2018).
The aim of this study was to develop a diagnostic method that is quick in detection, capable of identifying very small amounts of pathogenic DNA/RNA, cost-effective, favorable for field diagnosis and easy to use. This method will significantly satisfy the establishment of a highly sensitive RPA-CRISPR-based diagnosis approach for the quick identification of AIV H9N2 for laboratory and clinical use.
MATERIALS AND METHODS
Reagents and Instruments
The Escherichia coli BL21(DE3) pLysS Chemically Competent Cell was bought from TransGen Biotech (Beijing, China). pC013-Twinstrep-SUMO-huLwCas13a plasmid was purchased from miaolingbio (Wuhan, China). HisTrap Ni Sepharose excel chromatography column was obtained from GE Healthcare Life Sciences (MA). All DNA sequences used in this study were synthesized by Genewiz Biotechnology Co., Ltd. (Suzhou, China). HiScribe T7 Rapid High Yield RNA Synthesis Kit (CAT: E2050S) (Cambridge, MA) transcribes DNA to RNA. TwistAmp Basic Kit (CAT: TABAS03KIT) was bought from TwistDx Inc (Cambridge, Massachusetts, UK). Midi purification Kit was obtained from Tiangen Biotechnology Co., Ltd. (Beijing, China). The measurements of RNA concentration used a Nano Drop ND-1000 spectrophotometer (Thermo Fisher Scientific). Fluorescence signals were measured using an Applied Biosystems 7,500 (Life Technologies). All virus DNA/RNA were isolated and kept in the laboratory.
The Expression and Purification of LwCas13a Protein
The pC013-Twinstrep-SUMO-huLwCas13a plasmid was transformed into Escherichia coli BL21(DE3) pLysS Competent Cell, then inoculated at 1:100 to 1L LB media and shaken at 37℃, monitored the OD value at 600 nm every hour until cells reached an optical density of 0.4∼0.6. Isopropyl-beta-D-thiogalactopyranoside (IPTG) with final concentration of 500 μM was added to induce expression, and then cultured at 21℃ for 16 h. Cells were collected by centrifugation at 12000 rpm for 15 min at 4℃ and resuspended with 100 mg/mL lysate (20 mM Tris-HCl from Gentihold, LA, 500 mM NaCl from Tianjin Tianli Chemical, China, pH 8.0). Lysozyme (CAT: L8120) was added in the ratio of 1: 100 for ice treatment for 30 min, and then protease inhibitor (Phenylmethanesulfonyl fluoride, PMSF; CAT: ST507) was added in the ratio of 1: 100 for mixing and crushing. The cells were broken by ultrasonic breaker (Ningbo Xinzhi Biotech., China) on ice, with a power setting of 200W for 1s on and 2 s off, in a total of 10 min sonication time (time can be adjusted according to clarity). Cells were collected after centrifugation for 30 min at 4℃ and 12,000 rpm. Samples from each stage were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 10% polyacrylamide gel, 180 kDa marker (Vazyme CAT: MP102). The electrophoresis program consisted of 2 steps: step 1 (S1): 80 V for 30 min, step 2 (S2): 120 V for 50 min) (BIO-RAD). The results were verified by Western blotting with a primary antibody against 6×His (His-Tag monoclonal antibody from Proteintech, LA) and a secondary antibody of horseradish peroxidase (HRP)-labeled goat antimouse IgG (Thermo Fisher Scientific, MA).
The broken supernatant was diluted with binding buffer (20 mM sodium phosphate, 300 mM NaCl, 10 mM imidazole (CAT: S30365), pH 7.4) at the ratio of 1:1, and then filtered with a 0.22 μM filter. The filtered protein sample was purified by a HisTrap Ni Sepharose excel chromatography column. Collected elution components in each stage were analyzed by SDS-PAGE and verified by western-blot. The appropriate elution product was selected and digested with SUMO enzyme (Solarbio, Beijing, China) at 4℃. Miscellaneous protein was removed with 100 kDa ultrafiltration tube (UFC910096, Milliproe, Chicago) and concentrated. The protein solution was changed into storage buffer (Supplement 15mL of lysis buffer with 22.5μL of NP-40 lysis buffer from Aladdin, Shanghai, China), subpackaged and stored at −80℃.
Design and Preparation In Vitro of crRNA
The sequence of the H9N2 HA gene (A/Antropodides virgo/Baicheng/219/2013, GenBank: KM245331.1) was compared, and 2 conserved sequences were selected to design 2 crRNAs. At the same time, 1 crRNA was designed according to the conserved sequence of the H9N2 NA gene. ssDNA which can be transcribed into these 3 crRNAs was synthesized by the biological company (Sangon, Changchun, China) and all sequence information is listed in Table 1. The designed crRNA was obtained by in vitro transcription. Referring to in vitro transcription instructions, 1 μg ssDNA,10 μL NTP buffer mix, and 2 μL T7 RNA polymerase mix were added into a PCR tube. The mixture was made up to 30 μL with RNase-free water, then thoroughly mixed and briefly centrifuged before being incubated overnight at 37℃. To remove the template DNA, for each 30 µL of reaction, 45 µL of nuclease-free water, 2 µL of deoxyribonuclease I (RNase-free) was added, mixed and incubated at 37°C for 15 min. The transcribed RNA was purified and extracted by the Trizol method, and then the RNA concentration was determined by nanodrop.
Table 1.
The crRNA related sequence used in the experiment.
| Name | Sequence(5′–3′) |
|---|---|
| crRNA-1 | GAUUUAGACUACCCCAAAAACGAAGGGGACUAAAACGUUUGAGGUGUCUUUGACAUCUGUGUGA |
| crRNA-2 | GAUUUAGACUACCCCAAAAACGAAGGGGACUAAAACUAUUCUAACUACUAGUUUAGGUUCUAUAU |
| crRNA-3 | GAUUUAGACUACCCCAAAAACGAAGGGGACUAAAACGAACACGUCACCAUUACUGACUACCUUC |
| ssDNA-1 | TAATACGACTCACTATAGGGGATTTAGACTACCCCAAAAACGAAGGGGACTAAAACGTTTGAGGTGTCTTTGACATCTGTGTGA |
| ssDNA-2 | TAATACGACTCACTATAGGGGATTTAGACTACCCCAAAAACGAAGGGGACTAAAACTATTCTAACTACTAGTTTAGGTTCTATA |
| ssDNA-3 | TAATACGACTCACTATAGGGGATTTAGACTACCCCAAAAACGAAGGGGACTAAAACGAACACGTCACCATTACTGACTACCTTC |
Establishment and Optimization of Detection System
According to the RPA primer design principle of Twist Amp Basic Kit, 3 RPA primers were designed by using Primer 5.0 software and the best primer pair was screened. All sequence information is listed in Table 2. The purified Cas13a protein was applied to the established detection system and a negative control (NC) group was set up to determine whether the Cas13a protein has RNA cleavage activity. The detection system is shown in Table 3. The 3 crRNAs obtained by in vitro transcription were applied to the established detection system, and the fluorescence signal was monitored every 2 min for 1.5 h at 37°C. The best crRNA was determined by the fluorescence intensity. The LwCas13a protein and crRNA were diluted in a 2-fold gradient, and each concentration gradient was applied to the established detection system for 1.5 h at 37°C. The fluorescence signal was monitored every 2 min, and the optimal LwCas13a protein concentration and crRNA concentration were determined by the fluorescence intensity.
Table 2.
The sequence of RPA primers used in the experiment.
| Name | Sequence(5′–3′) |
|---|---|
| RPA1-F | TAATACGACTCACTATAGGGCATCGGCTATCAATCAACAA |
| RPA1-R | GTCACAAGAAGGATTGCCATAGATTAGTCC |
| RPA2-F | TAATACGACTCACTATAGGGCGACAAAATGAACAAGCAGT |
| RPA2-R | TCACTTTATTATATAGATTGTTTACATTTG |
| RPA3-F | TAATACGACTCACTATAGGGCAGGAGTCGGAATGCGTTTG |
| RPA3-R | TGGGCTAATATTTATAATTTTCCCCTCTCT |
Table 3.
The SHERLOCK system.
| Component | Volume |
|---|---|
| RPA3-F(100μM) | 0.48 μL |
| RPA3-R(100μM) | 0.48 μL |
| Rehydration buffer | 59 μL |
| Lw Cas13a protein | 5.91 μL |
| crRNA | 4 μL |
| RNA reporter (5′-FAM-UUUUU-BHQ-3′) | 6 μL |
| RNase inhibitor | 2.73 μL |
| NTP buffer mix | 8 μL |
| T7 RNA polymerase mix | 2.5 μL |
| 1 M MgCl2 | 0.9 μL |
| MgAc | 5 μL |
| DNA | 5 μL |
| Total | 100 μL |
Evaluation Construction System
We used qPCR method to quantify the sample and drew a standard curve according to the cycle threshold value and copy number of the standard, and substituted the obtained CT value of the H9N2 AIV (GenBank: KM245331.1) genome into the standard curve to calculate the copy number. The optimized assay system was used to detect different avian susceptible viruses H5N1 AIV (GenBank: MK616077.1), Newcastle disease virus (NDV, La Sota strainGenBank: AJ629062.1), infectious bursal disease virus (IBDV, B87 strain GenBank: DQ906921.1), infectious bronchitis virus (IBV, LDT3-A strain GenBank: KR608272.1 and H120 strain GenBank: ON350837.1), H1N1(A/Puerto Rico/8/34) (GenBank: J04572.1) to determine their specificity and compared with conventional qPCR. H9N2 AIV samples with concentrations of 105, 104, 103, 102, 101 and 100 copies/μL were selected for the assay to determine the minimum detection limit of the optimized assay system and compared with conventional qPCR.
Biological Information and Statistical Analysis
In this experiment, the data of fluorescence detection were analyzed by GraphPad Prism8.0.1 software, the results are presented as the means ± S.E.M and were analysed by using t-tests or 1 way ANOVA;*P< 0.05; **P< 0.01; ***P< 0.001.
RESULTS
The Expression of LwCas13a Protein
In order to obtain the Cas13a protein, protein expression was carried out using a prokaryotic system. The LwCas13a protein is approximately 155 kDa, the negative control showed no band at 155 kDa after induction of expression, whereas BL21(DE3) receptor cells transformed with plasmid pC013-Twinstrep-SUMO-huLwCas13a showed a clear band at 155 kDa after induction of expression (Figure 1A). The protein was then analyzed for solubility and the results showed that the majority of the LwCas13a protein was located in the supernatant after fragmentation (Figure 1B). Since the protein carries a His-tag, and then WB validation was performed using a His-Tag monoclonal antibody. The results showed that a protein blot was produced at 155 kDa, confirming it as a Cas13a protein (Figure 1C). It demonstrated that soluble proteins can be correctly expressed in prokaryotic systems.
Figure 1.
The expression of Lw Cas13a protein. (A) The SDS-PAGE electrophoresis of Lw Cas13a protein induced expression (M:180 kDa Protein marker, a: BL21 empty bacteria, b: Cas13a+BL21,1: uninducted the whole bacteria solution,2: Concentrated 5 times of uninducted the whole bacteria solution, 3: The whole inducted bacterial liquid, 4: Concentrated 7 times of the whole inducted bacterial liquid, 5: Supernatant after crushing, 6: Precipitation after crushing). (B) The SDS-PAGE electrophoresis of Lw Cas13a protein solubility analysis (M:180 kDa Protein Marker,1: uninducted the whole bacteria solution, 2: The whole inducted bacterial liquid, 3: Supernatant after crushing,4: Precipitation after crushing). (C) The Western Blot of Lw Cas13a protein solubility analysis (M:180 kDa Protein Marker,1: uninducted the whole bacteria solution, 2: The whole inducted bacterial liquid, 3: Supernatant after crushing, 4: Precipitation after crushing).
The Purification and Enzymatic Cleavage of LwCas13a Protein
The expression product was purified by employing a HisTrap Ni Sepharose excel chromatography column to isolate the single Cas13a protein after removing the heteroprotein. The results showed that the 120 mM imidazole eluted product was free of stray bands except for Cas13a protein and the purification was excellent (Figure 2A). The purified Cas13a protein was subsequently validated using western blot (WB), and the results showed that a protein blot was produced at 155 kDa (Figure 2B). Electrophoresis of the protein after SUMO digestion showed that the Cas13a protein band was reduced by approximately 30 kDa, in line with the theoretical size (Figure 2C). The Cas13a protein loses its His-tag after enzymatic digestion, therefore didn't produce a protein blot in western blot experiment using the His-tag monoclonal antibody (Figure 2D).
Figure 2.
The purification and enzymatic cleavage of Lw Cas13a protein. (A) The SDS-PAGE electrophoresis of Lw Cas13a Protein purification (M:180 kDa Protein marker,1: Effluent,2:25 mM imidazole elution product,3:120 mM imidazole elution product,4:250 mM imidazole elutes the product). (B) The WB of Lw Cas13a Protein purification (M:180 kDa Protein marker,1: Effluent,2:25 mM imidazole elution product,3:120 mM imidazole elution product, 4:250 mM imidazole elutes the product). (C) The SDS-PAGE electrophoresis of Lw Cas13a Protein enzymatic cleavage (M:180 kDa Protein marker,1,2: Digested Lw Cas13a protein,3: Purified Lw Cas13a protein). (D) The Western Blot of Lw Cas13a Protein enzymatic cleavage (M:180 kDa Protein marker,1,2: Digested Lw Cas13a protein,3: Purified Lw Cas13a protein).
Establishment of the SHERLOCK System
In the screening process for optimal primer pairs, RT-RPA experiments were conducted individually using different RPA primer pairs. The findings revealed that RT-RPA1 and RT-RPA2 amplification products exhibited nonspecific bands, whereas the RT-RPA3 amplification product solely displayed the target band, indicating higher specificity. Additionally, RT-RPA3 exhibited the highest amplification efficiency, thus making it the prime choice for subsequent testing (Figure 3A). When LwCas13a protein was applied to the SHERLOCK system, the fluorescence intensity of the LwCas13a protein group started to show an increasing trend at 25 min, and the final fluorescence intensity of the LwCas13a protein experimental group was at a high level after 3 h of reaction, but the negative control group had not been producing fluorescence intensity. These data indicated that the purified LwCas13a protein had excellent RNA enzymatic activity (Figure 3B,C). To screen the best crRNA, the SHERLOCK experiment was carried out using these 3 crRNAs respectively. All 3 crRNAs exhibited fluorescence intensity compared with the control group, in addition to crRNA3 which had reached high levels of fluorescence intensity at an earlier time (Figure 3D). Compared with the crRNA1 and crRNA2 groups, the final fluorescence intensity was highest in the crRNA3 group and developed a significant difference from the other 2 groups (Figure 3E). Above all, SHERLOCK detection system has been established.
Figure 3.
The creation of the SHERLOCK system. (A) The 1-step RT-RPA nucleic acid electrophoresis results (M:2000bp DNA marker,1: 1-step RT-RPA1 amplification product,2: 1-step RT-RPA2 amplification product,3: 1-step RT-RPA3 amplification product). (B) Real-time fluorescence intensity curve of activity validation of Lw Cas13a protein. (C) The final fluorescence intensity comparison chart of activity validation of Lw Cas13a protein. (D) Real-time fluorescence intensity curve of best crRNA selection. (E) The final fluorescence intensity comparison chart of best crRNA selection.
Optimization of the SHERLOCK System
In order to optimize the constructed SHERLOCK reaction system, 7 different concentrations of LwCas13a protein groups were set up, and these 7 different concentrations of LwCas13a protein were used for SHERLOCK experiments, while the group without LwCas13a protein was set up as a negative control group. The results showed that the final fluorescence intensity of the reaction reached the highest level when the concentration of LwCas13a protein was 12.5 ng/μL, which was different from the rest of the groups. Therefore, the optimal LwCas13a protein concentration in the constructed SHERLOCK reaction system was 12.5 ng/μL, which was chosen for subsequent experiments (Figure 4A). To optimize the constructed SHERLOCK reaction system, 6 different concentrations of crRNA groups were set up, and these 6 different concentrations of crRNA were used to perform SHERLOCK experiments respectively, while the no-crRNA group was set up as a negative control group. The results showed that when the crRNA concentration was 1.875 ng/μL, the final fluorescence intensity of the reaction was the highest level. After statistical analysis, when the crRNA concentration was 0.9375 ng/μL, the final fluorescence intensity of the reaction was not significantly different from the 1.875 ng/μL group, while it was extremely different from the 0.46875 ng/μL group. Therefore, the optimal crRNA concentration in the constructed SHERLOCK reaction system was 0.9375 ng/μL, which was selected for subsequent experiments (Figure 4B). These data indicated that the constructed system has been successfully optimized.
Figure 4.
The optimisation of the SHERLOCK system. (A) The best reaction concentration of Lw Cas13a protein concentration. (B) The best reaction concentration of crRNA concentration.
The Sensitivity of the SHERLOCK System
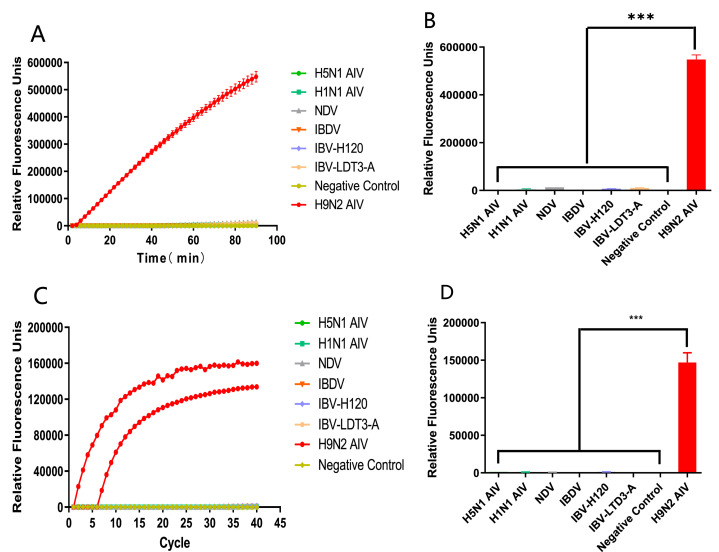
After determining the H9N2 genome copy number, the concentration was adjusted and applied to the SHERLOCK system to determine the minimum detection limit of the detection system. The sensitivity test results of the CRISPR/Cas13a-based H9N2 subtype AIV assay showed that the minimum detection limit of the assay was 10° copies/μL and the sensitivity of the assay was good. At 40 min, the fluorescence intensity of all groups except the 105 copies/μL group reached a high level, while the NC group had no fluorescence intensity (Figure 5A). The final fluorescence intensities of the experimental groups were significantly different from those of the NC group after 90 min, with the 103 copies/μL group having the highest final fluorescence intensity (Figure 5B). The traditional qPCR method was also adopted for sensitivity testing, the results showed that the minimum diagnostic capacity was the same as the CRISPR/Cas13a-based AIV subtype H9N2 detection assay i.e., 10° copies/μL. The final fluorescence intensity of all reactions in the experimental group was higher than that of the NC group (Figure 5C) but it took longer to generate fluorescence intensity in the 101 and 100 copies/μL group by the conventional qPCR method (Figure 5D). These results indicated that the sensitivity of the H9N2 AIV detection system constructed based on CRISPR/Cas13a technology is excellent.
Figure 5.
The sensitivity of the SHERLOCK system. (A) Real-time fluorescence intensity curve of the build CRISPR/Cas13a system sensitivity test. (B) Comparison chart of final fluorescence intensity of the build CRISPR/Cas13a system sensitivity test. (C) Real-time fluorescence intensity curve of conventional qPCR sensitivity test. (D) Comparison chart of final
The Specificity of the SHERLOCK System
To determine the specificity of the test system, 7 avian susceptible viruses were tested separately using the SHERLOCK system. The specificity test results of the constructed CRISPR/Cas13a system were the same as the conventional qPCR, which showed that the H5N1 AIV, H1N1 AIV, NDV, IBDV, and IBV groups showed almost no increase in real-time fluorescence compared with the NC group, while the H9N2 AIV group produced a fluorescence signal in a very short period of time and remained on the rise (Figure 6A,C). The final fluorescence intensity of H5N1 AIV, H1N1 AIV, NDV, IBDV, and IBV groups were not significantly different from the NC group, while the final fluorescence intensity of H9N2 AIV group was significantly different from the NC group (Figure 6B,D). This indicated that the specificity of the H9N2 AIV detection system constructed based on CRISPR/Cas13a technology was excellent.
Figure 6.
The specificity of the SHERLOCK system. (A) Real-time fluorescence intensity curve of the build CRISPR/Cas13a system specificity test. (B) Comparison chart of final fluorescence intensity of the build CRISPR/Cas13a system specificity test. (C) Real-time fluorescence intensity curve of conventional qPCR specificity test. (D) Comparison chart of final fluorescence intensity of conventional qPCR specificity test.
DISCUSSION
The avian influenza virus (AIV), which causes the fatal infection, endangers human health and causes the poultry industry to suffer large financial losses (Liu et al. 2015; Wang et al. 2016). Despite the fact that H9N2 AIV infection does not have evident clinical signs or a high death rate, the predominance of the virus in the poultry industry leads to significant economic losses since it affects the production of eggs, causes body weight to drop, and co-infects birds with other infections (Nagy, Mettenleiter and Abdelwhab 2017). Additionally, H9N2 AIV infection causes short-term immunosuppression, which might make other concomitant or secondary illnesses worse (Jaleel et al. 2017; Hassan et al. 2021; Qin et al. 2023; Sun et al. 2024). H9N2 has multiple hosts including birds, pigs, ferrets, horses, dogs, minks and humans (Liu et al. 2016). It has been diagnosed in several avian species e.g., chickens, pigeons, and domestic waterfowl in China (Dong et al. 2022). It's important to note that human H9N2 AIV infections have been detected all across the world and have been rising recently. Research has shown that all H9N2 AIV obtained from humans are of avian origin and genetically related to those that are now widespread in nearby poultry (Song and Qin 2020).
There have been many diagnostic methods developed for the detection of avian influenza virus subtype H9N2. AIV diagnostic tests often rely on the identification of a particular antibody or the virus itself. The viral isolation test is the most popular, although it is laborious and time-consuming (Wu et al. 2019). PCR is endorsed for determining and validating the skeptical instances in clinical diagnosis. PCR methods are very helpful for the identification of materials that cannot be used for viral isolation owing to putrefaction. Several traditional PCR and qPCR techniques for H9N2 AIV detection have been developed in earlier research (Siddique et al. 2008; Shabat et al. 2010). PCR techniques, on the other hand, rely on the detection of viral gene sequences, which are subject to viral genetic modification. Epidemiological data really show that AIV is always changing due to mutation or assortment. Additionally, PCR requires time-consuming laboratory operations and expensive, specialized analysis equipment (Hemmati et al. 2015). The development of ELISA-based detection techniques for H9N2 avian influenza subtypes has also been successful, although the use of these approaches for diagnosing acute infections is limited by the requirement that specimens be obtained as soon as possible after the onset of disease. Serological diagnosis is also less useful for surveillance since it cannot distinguish vaccinated from infected animals (Hui et al. 2015; Yang et al. 2016; Ming et al. 2019). Recent years have also seen the development of several highly sophisticated and diagnostically helpful contemporary detection procedures. The subtype H9 influenza A virus has been detected in chicken feces using a quick fluorescent diagnostic technique, although this test has the disadvantage of also requiring high-end laboratory equipment, making it impractical for field diagnosis (Tuong et al. 2021; Lin and Gu 2022). An elaborate, targeted, and quick assay that may aid in the prompt and reliable identification of H9N2 AIV was urgently needed in light of the disease's severe impacts and the shortcomings of previously employed diagnostic techniques.
In this study, we have made some contributions to the detection methods of the avian influenza virus H9N2 subtype. Recombinase polymerase amplification assay has been applied for the diagnostic purpose. The CRISPR-LwCas13a and Cas13a from Leptotrichia wadei are ortholog to each other. CRISPR RNA (crRNA) can be reprogrammed to exclusively target the RNA that needs to be tested (Abudayyeh et al. 2016). Then, tagged RNA is broken down in vitro by triggering the action of LwCas13a, a component of the CRISPR-collateral-cleavage system (Smargon et al. 2017). The RPA-CRISPR diagnostic technique is also known as Specialist High-sensitivity Enzymatic Reporter unlocking (SHERLOCK). It is a single-copy sensitive in vitro method for determining nucleic acids based on collateral splitting of a reporter RNA using RPA and CRISPR (Kellner et al. 2019). Since the RNA reporter serves as a substrate for Cas13a cleavage, further transcription of the RPA amplicon into target RNA is required to initiate the cleavage process. In particular, the DNA transcription will amplify the number of copies of the crRNA target region a second time after the RPA amplification. Previous studies have shown that the minimum sensitivity limit of RT-qPCR is 100 copies/μL which is the same as compared to our detection assay (Chen et al. 2013) but our study found that the fluorescence activity took longer for RT-qPCR to develop for 100 and 101 copies per reaction as compared to the CRISPR- Cas 13a (Yang et al. 2016; Tuong et al. 2021). This means that our assay can detect low viral loads faster than traditionally used RT-qPCR methods. Specificity of the RPA-CRISPR was also checked and compared with RT-qPCR and it was noted that fluorescence activity was generated in a very short period of time and it stayed on the rise (Heine et al. 2015; Zhang et al. 2019; Le et al. 2020). The whole experiment was carried out under the condition of normal temperature, and the reaction conditions are relatively simple. Cas13a has good signal amplification and high cleavage efficiency, so it has high sensitivity. Cas13a only allows single base mismatch, so it has high specificity and low false detection rate (MacGregor et al. 2023). Therefore, it will not be wrong to say that the RPA-CRISPR as a diagnostic method is better than traditionally used RT-qPCR methods in sensitivity and specificity.
Cas13a has advantages for identifying samples with extremely low viral loads due to its excellent sensitivity. RPA-CRISPR can immediately identify the DNA/RNA of virus in bodily fluids collected from diseased animals without the tedious process of extracting the DNA (Gootenberg et al. 2017) when combined with heating unextracted diagnostic samples to annihilate nucleases. RPA-CRISPR has obvious advantages: short reaction time, high error tolerance, simple primer design and support for multiple amplification reactions (Xu et al. 2021; Baerwald et al., 2023). RPA-CRISPR significantly satisfies the need to establish a highly sensitive RPA-CRISPR-based diagnosis approach for the quick identification of H9N2 AIV for both laboratory and clinical use. RPA-CRISPR is more sensitive than traditional CRISPR, yet it has several drawbacks. The availability of ideal primers is necessary for the RPA amplification component. However, there is no primer design tool available to predict the RPA reaction's amplification performance. Therefore, before RPA-CRISPR sample analysis, rigorous primer screening should be carried out (Ren et al. 2021; Xu et al. 2021; Munawar 2022).
In summary, it can be established that RPA-CRISPR assay is advanced method of diagnosis for clinical and field applications as compared to the other conventional PCR methods. It is also superior in sensitivity and specificity as compared to previous methods and it requires short time and has less complications. It has few limitations too which can be improved in the near future and gives an insight to scientists and researchers to come up with solutions.
CONCLUSION
The RPA-CRISPR assay represents a significant advancement in diagnostic methods for both clinical and field applications, surpassing conventional PCR methods in sensitivity and specificity. This assay offers quicker results and involves fewer complications. Although it has some limitations, these can be addressed through ongoing research, providing valuable opportunities for scientists and researchers to develop solutions. To further enhance the RPA-CRISPR assay, future efforts should focus on optimizing assay components to improve reagent stability and efficiency, and integrating the technology with portable, user-friendly devices for field use. Expanding the range of detectable pathogens or genetic markers will increase the assay's versatility, while reducing production costs will make the technology more accessible.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ACKNOWLEDGMENTS
This research has been supported by National Key R&D Program of China (2023YFD1800302), the National Natural Science Foundation of China (32373023, 31972696, 32072888 and U21A20261), the China Agriculture Research System of MOF and MARA (CARS-35), the Science and Technology Development Program of Jilin Province (20240101216JC, 20210202102NC and YDZJ202102CXJD029).
Author Contributions: WTY and CFW designed the research. SSC, YLY, HYW and TKG performed the research. TKG and RMA analyzed the data. WTY, CFW and GLY supported the research. SSC and RMA wrote the manuscript. GLY, HBH, YLJ, JZW, XC, NW, and YZ provided useful feedback for manuscript writing. All authors contributed to the article and approved the submitted version. All authors read and approved the manuscript.
Ethics approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Data availability: The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Contributor Information
Wen-Tao Yang, Email: yangwentao@jlau.edu.cn.
Chun-Feng Wang, Email: wangchunfeng@jlau.edu.cn.
REFERENCES
- Abudayyeh O.O., Gootenberg J.S., Konermann S., Joung J., Slaymaker I.M., Cox D.B.T., Shmakov S., Makarova K.S., Semenova E., Minakhin L., Severinov K., Regev A., Lander E.S., Koonin E.V., Zhang F. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science. 2016;353:aaf5573. doi: 10.1126/science.aaf5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerwald M.R., Funk E.C., Goodbla A.M., Campbell M.A., Thompson T., Meek M.H., Schreier A.D. Rapid CRISPR-Cas13a genetic identification enables new opportunities for listed Chinook salmon management. Mol. Ecol. Resour. 2023,;14:3055–3067. doi: 10.1111/1755-0998.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Shabat M., Meir R., Haddas R., Lapin E., Shkoda I., Raibstein I., Perk S., Davidson I. Development of a real-time TaqMan RT-PCR assay for the detection of H9N2 avian influenza viruses. J. Virol. Methods. 2010;168:72–77. doi: 10.1016/j.jviromet.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Bóna M., Kiss I., Dénes L., Szilasi A., Mándoki M. Tissue tropism of H9N2 low-pathogenic avian influenza virus in broiler chickens by immunohistochemistry. Animals (Basel) 2023;13:1052. doi: 10.3390/ani13061052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centre for Disease Control and Prevention. 2024. ‘Influenza type A viruses’, Accessed February 01, 2024. https://www.cdc.gov/flu/avianflu/influenza-a-virus-subtypes.htm.
- Chen H., Yuan H., Gao R., Zhang J., Wang D., Xiong Y., Fan G., Yang F., Li X., Zhou J., Zou S., Yang L., Chen T., Dong L., Bo H., Zhao X., Zhang Y., Lan Y., Bai T., Dong J., Li Q., Wang S., Zhang Y., Li H., Gong T., Shi Y., Ni X., Li J., Zhou J., Fan J., Wu J., Zhou X., Hu M., Wan J., Yang W., Li D., Wu G., Feng Z., Gao G.F., Wang Y., Jin Q., Liu M., Shu Y. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: a descriptive study. Lancet. 2014;383:714–721. doi: 10.1016/S0140-6736(14)60111-2. [DOI] [PubMed] [Google Scholar]
- Chen J., Huang Y., Xiao B., Deng H., Gong K., Li K., Li L., Hao W. Development of a RPA-CRISPR-Cas12a assay for rapid, simple, and sensitive detection of mycoplasma hominis. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.842415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Liu T.C., Cai L.J., Du H.Y., Li M. A one-step RT-PCR array for detection and differentiation of zoonotic influenza viruses H5N1, H9N2, and H1N1. J. Clin. Lab. Anal. 2013;27:450–460. doi: 10.1002/jcla.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J.Z., Zhou Y., Pu J., Liu L.T. Status and challenges for vaccination against avian H9N2 influenza virus in China. Life-Basel. 2022;12:1326. doi: 10.3390/life12091326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East-Seletsky A., O'Connell M.R., Knight S.C., Burstein D., Cate J.H.D., Tjian R., Doudna J.A. Two distinct RNase activities of CRISPR-C2c2 enable guide-RNA processing and RNA detection. Nature. 2016;538:270–273. doi: 10.1038/nature19802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozouni P., Son S., Diaz de Leon Derby M., Knott G.J., Gray C.N., D'Ambrosio M.V., Zhao C., Switz N.A., Kumar G.R., Stephens S.I., Boehm D., Tsou C.L., Shu J., Bhuiya A., Armstrong M., Harris A.R., Chen P.Y., Osterloh J.M., Meyer-Franke A., Joehnk B., Walcott K., Sil A., Langelier C., Pollard K.S., Crawford E.D., Puschnik A.S., Phelps M., Kistler A., DeRisi J.L., Doudna J.A., Fletcher D.A., Ott M. Amplification-free detection of SARS-CoV-2 with CRISPR-Cas13a and mobile phone microscopy. Cell. 2021;184:323-33 e9. doi: 10.1016/j.cell.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R., Cao B., Hu Y., Feng Z., Wang D., Hu W., Chen J., Jie Z., Qiu H., Xu K., Xu X., Lu H., Zhu W., Gao Z., Xiang N., Shen Y., He Z., Gu Y., Zhang Z., Yang Y., Zhao X., Zhou L., Li X., Zou S., Zhang Y., Li X., Yang L., Guo J., Dong J., Li Q., Dong L., Zhu Y., Bai T., Wang S., Hao P., Yang W., Zhang Y., Han J., Yu H., Li D., Gao G.F., Wu G., Wang Y., Yuan Z., Shu Y. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- Gootenberg J.S., Abudayyeh O.O., Lee J.W., Essletzbichler P., Dy A.J., Joung J., Verdine V., Donghia N., Daringer N.M., Freije C.A., Myhrvold C., Bhattacharyya R.P., Livny J., Regev A., Koonin E.V., Hung D.T., Sabeti P.C., Collins J.J., Zhang F. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science. 2017;356:438–442. doi: 10.1126/science.aam9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan K.E., El-Kady M.F., El-Sawah A.A.A., Luttermann C., Parvin R., Shany S., Beer M., Harder T. Respiratory disease due to mixed viral infections in poultry flocks in Egypt between 2017 and 2018: Upsurge of highly pathogenic avian influenza virus subtype H5N8 since 2018. Transbound. Emerg. Dis. 2021;68:21–36. doi: 10.1111/tbed.13281. [DOI] [PubMed] [Google Scholar]
- Heine H.G., Foord A.J., Wang J.N., Valdeter S., Walker S., Morrissy C., Wong F.Y.K., Meehan B. Detection of highly pathogenic zoonotic influenza virus H5N6 by reverse-transcriptase quantitative polymerase chain reaction. Virol. J. 2015;12:18. doi: 10.1186/s12985-015-0250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmati R., Hosseinkhani S., Sajedi R.H., Azad T., Tashakor A., Bakhtiari N., Ataei F. Luciferin-regenerating enzyme mediates firefly luciferase activation through direct effects of D-cysteine on luciferase structure and activity. Photochem. Photobiol. 2015;91:828–836. doi: 10.1111/php.12430. [DOI] [PubMed] [Google Scholar]
- Hui K.M., Li X.J., Pan L., James Li X. Advances in global health through sensing technologies. SPIE; 2015. A Homogeneous biochemiluminescent assay for detection of influenza, in Proc. SPIE 9490. [Google Scholar]
- Jaleel S., Younus M., Idrees A., Arshad M., Khan A.U., Ehtisham-Ul-Haque S., Zaheer M.I., Tanweer M., Towakal F., Munibullah M.Y.T., Sohail M.L., Umar S. Pathological alterations in respiratory system during co-infection with low pathogenic avian influenza virus (H9N2) and Escherichia coli in broiler chickens. J. Vet. Res. 2017;61:253–258. doi: 10.1515/jvetres-2017-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner M.J., Koob J.G., Gootenberg J.S., Abudayyeh O.O., Zhang F. SHERLOCK: Nucleic acid detection with CRISPR nucleases. Nat. Protoc. 2019;14:2986–3012. doi: 10.1038/s41596-019-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T.B., Kim H.K., Na W., Le V.P., Song M.S., Song D., Jeong D.G., Yoon S.W. Development of a multiplex RT-qPCR for the detection of different clades of avian influenza in poultry. Viruses. 2020;12:100. doi: 10.3390/v12010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X.X., Gu J. A biochemiluminescent assay for rapid diagnosis of influenza. Clin. Experiment. Med. 2022;22:577–581. doi: 10.1007/s10238-021-00778-w. [DOI] [PubMed] [Google Scholar]
- Liu M.B., Li X.D., Yuan H., Zhou J.F., Wu J.W., Bo H., Xia W., Xiong Y., Yang L., Gao R.B., Guo J.F., Huang W.J., Zhang Y., Zhao X., Zou X.H., Chen T., Wang D.Y., Li Q., Wang S.W., Chen S.G., Hu M.H., Ni X.S., Gong T., Shi Y., Li J.X., Zhou J., Cai J., Xiao Z.K., Zhang W., Sun J., Li D.X., Wu G.Z., Feng Z.J., Wang Y., Chen H.Y., Shu Y.L. Genetic diversity of avian influenza A (H10N8) virus in live poultry markets and its association with human infections in China. Sci. Rep. 2015;5:7632. doi: 10.1038/srep07632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.F., Lai H.Z., Li L., Liu Y.P., Zhang W.Y., Gao R., Huang W.K., Luo Q.F., Gao Y., Luo Q., Xie X.Y., Xu J.H., Chen R.A. Endemic variation of H9N2 avian influenza virus in China. Avian Dis. 2016;60:817–825. doi: 10.1637/11452-061616-Reg. [DOI] [PubMed] [Google Scholar]
- Long J.S., Mistry B., Haslam S.M., Barclay W.S. Host and viral determinants of influenza A virus species specificity. Nat. Rev. Microbiol. 2019;17:67–81. doi: 10.1038/s41579-018-0115-z. [DOI] [PubMed] [Google Scholar]
- MacGregor S.R., McManus D.P., Sivakumaran H., Egwang T.G., Adriko M., Cai P., Gordon C.A., Duke M.G., French J.D., Collinson N., Olveda R.M., Hartel G., Graeff-Teixeira C., Jones M.K., You H. Development of CRISPR/Cas13a-based assays for the diagnosis of schistosomiasis. E. Bio. Med. 2023;94 doi: 10.1016/j.ebiom.2023.104730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming F., Cheng Y.Q., Ren C.W., Suolang S.Z., Zhou H.B. Development of a DAS-ELISA for detection of H9N2 avian influenza virus. J. Virol. Methods. 2019;263:38–43. doi: 10.1016/j.jviromet.2018.10.014. [DOI] [PubMed] [Google Scholar]
- Munawar M.A. Critical insight into recombinase polymerase amplification technology. Exp. Rev. Mol. Diagnos. 2022;22:725–737. doi: 10.1080/14737159.2022.2109964. [DOI] [PubMed] [Google Scholar]
- Myhrvold C., Freije C.A., Gootenberg J.S., Abudayyeh O.O., Metsky H.C., Durbin A.F., Kellner M.J., Tan A.L., Paul L.M., Parham L.A., Garcia K.F., Barnes K.G., Chak B., Mondini A., Nogueira M.L., Isern S., Michael S.F., Lorenzana I., Yozwiak N.L., MacInnis B.L., Bosch I., Gehrke L., Zhang F., Sabeti P.C. Field-deployable viral diagnostics using CRISPR-Cas13. Science. 2018;360:444–448. doi: 10.1126/science.aas8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A., Mettenleiter T.C., Abdelwhab E.M. A brief summary of the epidemiology and genetic relatedness of avian influenza H9N2 virus in birds and mammals in the Middle East and North Africa. Epidemiol. Infect. 2017;145:3320–3333. doi: 10.1017/S0950268817002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naila, S., N. Khalid, A. Zaheer, and Salman Akbar %J International Journal of Poultry Science Malik. 2008. 'Evaluation of RT-PCR for the detection of influenza virus serotype H9N2 among broiler chickens in Pakistan', 7: 1122-27.
- Qin T., Chen Y., Huangfu D., Miao X., Yin Y., Yin Y., Chen S., Peng D., Liu X. PA-X protein of H9N2 subtype avian influenza virus suppresses the innate immunity of chicken bone marrow-derived dendritic cells. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M.S., Mei H., Zhou J.J., Zhou M., Han H.Y., Zhao L. Early diagnosis of rabies virus infection by RPA-CRISPR techniques in a rat model. Arch. Virol. 2021;166:1083–1092. doi: 10.1007/s00705-021-04970-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique N., Naeem K., Ahmed Z., Malik S.A. Evaluation of RT-PCR for the detection of influenza virus serotype H9N2 among broiler chickens in Pakistan. Int. J. Poult. Sci. 2008;7:1122–1127. [Google Scholar]
- Smargon A.A., Cox D.B.T., Pyzocha N.K., Zheng K.J., Slaymaker I.M., Gootenberg J.S., Abudayyeh O.A., Essletzbichler P., Shmakov S., Makarova K.S., Koonin E.V., Zhang F. Cas13b is a type VI-B CRISPR-associated RNA-guided RNase differentially regulated by accessory proteins Csx27 and Csx28. Mol. Cell. 2017;65:618-630 e7. doi: 10.1016/j.molcel.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W.J., Qin K. Human-infecting influenza A (H9N2) virus: A forgotten potential pandemic strain? Zoo. Public Health. 2020;67:203–212. doi: 10.1111/zph.12685. [DOI] [PubMed] [Google Scholar]
- Sun Z., Zhang W., Li J., Yang K., Zhang Y., Li Z. H9N2 avian influenza virus downregulates FcRY expression in chicken macrophage cell line HD11 by activating the JNK MAPK pathway. Int. J. Mol. Sci. 2024;25:2650. doi: 10.3390/ijms25052650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S.X., Zhu X.Y., Li Y., Shi M., Zhang J., Bourgeois M., Yang H., Chen X.F., Recuenco S., Gomez J., Chen L.M., Johnson A., Tao Y., Dreyfus C., Yu W.L., McBride R., Carney P.J., Gilbert A.T., Chang J., Guo Z., Davis C.T., Paulson J.C., Stevens J., Rupprecht C.E., Holmes E.C., Wilson I.A., Donis R.O. New world bats harbor diverse influenza A viruses. Plos Pathogens. 2013;9 doi: 10.1371/journal.ppat.1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuong H.T., Jeong J.H., Choi Y.K., Park H., Baek Y.H., Yeo S.J. Development of a rapid fluorescent diagnostic system to detect subtype H9 influenza A virus in chicken feces. Int. J. Mol. Sci. 2021;22:8823. doi: 10.3390/ijms22168823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed S.F., Aslam A., Khan M.R., Ashraf K., Anjum A. A perspective of the prevalent H9N2 virus with a special focus on molecular and pathological aspects in commercial broiler chicken in Punjab, Pakistan. Braz. J. Biol. 2022;84 doi: 10.1590/1519-6984.261849. [DOI] [PubMed] [Google Scholar]
- Wang H.M., Zhang Z.J., Chen Z.Q., Zhang Y.R., Lv Q., An X.P., Tong Y.G., Carr M.J., Sun S.H., Shi W.F. High genetic diversity and frequent genetic reassortment of avian influenza A(H9N2) viruses along the East Asian-Australian migratory flyway. Infect. Gen. Evol. 2016;39:325–329. doi: 10.1016/j.meegid.2016.02.013. [DOI] [PubMed] [Google Scholar]
- Wu S.J., Lin X.X., Hui K.M., Yang S., Wu X.L., Tan Y.C., Li M.M., Qin A.Q., Wang Q.X., Zhao Q., Ding P.F., Shi K.S., Li X.J. A biochemiluminescent sialidase assay for diagnosis of bacterial vaginosis. Sci. Rep. 2019;9:20024. doi: 10.1038/s41598-019-56371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.L., Duan J.X., Chen J.M., Ding S.J., Cheng W. Recent advances in rolling circle amplification-based biosensing strategies-a review. Anal. Chimica. Acta. 2021;1148 doi: 10.1016/j.aca.2020.12.062. [DOI] [PubMed] [Google Scholar]
- Yang J., Dai X., Chen H., Teng Q., Li X., Rong G., Yan L., Liu Q., Li Z. Development of blocking ELISA for detection of antibodies against H9N2 avian influenza viruses. J. Virol. Methods. 2016;229:40–47. doi: 10.1016/j.jviromet.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Zhang Z.J., Liu D., Hu J., Sun W.Q., Liu K.T., Li J., Xu H.X., Liu J., He L.H., Jiang D.X., Gu M., Hu S.L., Wang X.Q., Liu X.W., Liu X. Multiplex one-step real-time PCR assay for rapid simultaneous detection of velogenic and mesogenic Newcastle disease virus and H5-subtype avian influenza virus. Arch. Virol. 2019;164:1111–1119. doi: 10.1007/s00705-019-04180-6. [DOI] [PubMed] [Google Scholar]