Abstract
Betaine has been shown to enhance growth performance and increase breast muscle yield in ducks and broilers through various mechanisms, including the modification of DNA methylation. However, the impact of in ovo betaine injection on muscle growth in newly hatched goslings remains unclear. In this study, fifty eggs were injected with saline or betaine at 7.5 mg/egg prior to incubation, and the subsequent effects on breast muscle growth in the newly hatched goslings were investigated. Betaine significantly increased (P < 0.05) the hatch weight, breast muscle weight, and breast muscle index, accompanied by an augmentation in muscle bundle cross-sectional area. Concurrently, betaine significantly upregulated (P < 0.05) the expression levels of myogenic regulatory factors, including myogenin (MyoG) and paired box 7 (Pax7) both mRNA and protein, while downregulating (P < 0.05) the mRNA and protein levels of myostatin (MSTN). Histological analysis revealed a higher abundance of proliferating cell nuclear antigen (PCNA) and Pax7 immune-positive cells in the breast muscle of the betaine group, consistent with elevated PCNA and Pax7 mRNA and protein levels. Additionally, significantly increased (P < 0.05) contents of insulin-like growth factor 1 (IGF-1) and insulin-like growth factor 2 (IGF-2) were observed in the breast muscle of the betaine group, so was mRNA expression of IGF-1, IGF-2, and insulin-like growth factor 1 receptor (IGF-1R). Betaine also significantly in8creased (P < 0.05) global DNA methylation of the breast muscle, accompanied by enhanced mRNA and protein levels of methionine cycle and DNA methylation-related enzymes, Interestingly, the promoter regions of IGF-1, IGF-2, and IGF-1R genes were significantly hypomethylated (P < 0.05). Moreover, in ovo betaine injection significantly upregulated (P < 0.05) the protein level of farnesoid X receptor (FXR) in breast muscle and FXR binding to the promoter of IGF-2 gene. These findings suggest that in ovo betaine injection promotes breast muscle growth during embryonic development in goslings through the FXR-mediated IGF-2 pathway, ultimately improving hatch weight and breast muscle weight.
Key words: betaine, IGF-2, FXR, breast muscle, gosling
INTRODUCTION
Betaine, also known as trimethylglycine, is derived from dietary sources or through choline oxidation. It has been extensively utilized as a feed additive in animal nutrition to improve body composition and muscle yield in broilers and pigs. Vital for embryonic development, betaine exposure during this phase can modulate epigenetic modifications, exerting long-term effects on growth, metabolism, and overall health. Previous studies have demonstrated that maternal betaine supplementation throughout gestation significantly increased the birth weight of newborn piglets (Jia et al., 2015; Jin et al., 2018). Additionally, maternal betaine supplementation upregulates the gene expression of insulin-like growth factor 1 (IGF-1) in the liver (Jin et al., 2018) and insulin-like growth factor 2 (IGF-2) in the hippocampus of offspring piglets (Li et al., 2015) through modification of DNA methylation. Meanwhile, parental betaine supplementation increased hepatic IGF-2 gene expression in offspring goslings, associated with DNA hypomethylation in the IGF-2 gene promoter regions (Ma et al., 2024). In ovo betaine injection also modulates the growth and metabolism in chicken during both embryonic and postnatal stages (Hu et al 2015; Idriss et al., 2017; Abobaker et al., 2022). Considering the practical aspects of the poultry industry, in ovo injection offers several advantages over maternal betaine supplementation, including easy operation, precise timing, cost-effectiveness and high bioavailability. Furthermore, in ovo injection can be integrated into existing industrial practices using automated systems already established for vaccination and other treatments. However, it is note that the outcomes of in ovo injection can vary by species, strain, and injection parameters, necessitating the establishment of optimal procedures for reproducible results and broader commercial application. Therefore, it is intriguing to investigate the effects of in ovo betaine injection on the breast muscle growth in newly hatched goslings.
Skeletal muscle, comprising approximately 40% of total body weight (Frontera and Ochala, 2015), is crucial for meat production and is a key determinant of poultry productivity. In avian species, the period from embryo formation to hatch is pivotal, marked by myotube formation and subsequent differentiation into mature muscle fibers (Chen et al., 2013). At hatch, the total number of myofibers is fixed, posthatch skeletal muscle growth depends on the lengthening and thickening, known as hypertrophy, of existing myofibers (Chen et al., 2013). Regulation of skeletal muscle growth involves temporal expression of the myogenic regulatory factors, including myogenic determination factor I (MYOD), myogenic factor 5 (MYF5), myogenin (MYOG), myogenic regulatory factor 4 (MRF4), myostatin (MSTN), and paired-box transcription factors (Pax) (Zammit, 2017). An in vitro study reported that betaine could induce morphological changes and hypertrophy in neo myotubes by increasing Myf5, MyoD, and MyoG protein levels in murine C2C12 cells (Senesi et al., 2013). Moreover, the IGFs signaling pathway plays an important role in regulating myoblasts differentiation and hypertrophy, mediated by the mammalian target of rapamycin (mTOR) to promote protein synthesis (Saxton and Sabatini, 2017). Previous research in broiler chickens suggested that betaine promotes muscle growth by upregulating MyoD and MyoG gene expression and activating the IGFs signaling pathway (Chen et al., 2018). Nevertheless, the effect of in ovo betaine injection on these myogenic regulatory factors and the IGFs signaling pathway in newly hatched goslings remains unclear.
As a bile acids (BA) ligand-activated nuclear transcription factor (Chiang et al., 2009; Mancin et al., 2023), farnesoid X receptor (FXR) is widely expressed in various tissues, including skeletal muscle, liver, and kidney (Perino et al., 2021), and is involved in promoting the process of myocyte differentiation (Guo et al., 2021), enlarging myofibers (Benoit et al 2017), and increasing skeletal muscle mass (Qiu et al., 2022). Dietary BA supplementation has been shown to increase breast muscle yield in broilers through the FXR/IGF-2 pathway (Chen et al., 2024). However, the role of FXR in the effect of betaine on breast muscle growth and the underlying transcriptional mechanisms remains unknown.
Therefore, this study aims to investigate the effects of in ovo betaine injection on hatch weight and breast muscle mass in newly hatched goslings, elucidating the underlying mechanisms including the expression of genes/proteins involved in growth and methionine metabolism, as well as the DNA methylation status on the promoters of growth-related genes. Furthermore, the study further explore whether these changes are linked to FXR-mediated transcriptional regulation of IGF-2.
MATERIALS AND METHODS
Ethics Statement
The experimental protocol was approved by the Animal Ethics Committee of Nanjing Agricultural University with project number 31672512. The sampling procedures complied with the ‘‘Guidelines on Ethical Treatment of Experimental Animals’’ (2006) No. 398 set by the Ministry of Science and Technology, China.
Animals and Treatment
A total of fifty fertilized eggs laid by 46 wk of age Jiangnan white goose breeders (mean weight 166.4 ± 1.5 g) were obtained from a native farm and randomly divided into 2 groups: control (CON) and betaine (BET), each group containing 25 eggs. Before incubation, the CON group was injected with 100 μL saline, and the BET group was injected with 100 μL betaine (B2629, Sigma–Aldrich) saline solution. The dosage was determined based on reported previously betaine concentrations in chicken eggs (Zeisel et al., 2003; Hu et al., 2015; Idriss et al., 2017). Eggs were injected by advancing a Hamilton syringe into a hole in the middle of the long axis until the yolk membrane was penetrated (approximately 2 cm below the surface), using wax to seal, and then incubated. The incubator maintained a temperature of approximately 37.2 to 38.1°C, and the relative humidity was about 65 to 80%. After hatching, all goslings were weighed and killed by rapid decapitation which is considered to be acceptable for euthanasia of birds according to American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals: 2013 Edition. Samples including blood, liver, bile, and breast muscle were collected and stored at -80°C for subsequent analysis. Additionally, breast muscle samples were weighed to calculate the breast muscle index using the following equation: breast muscle index (%) = breast muscle weight/live body weight×100.
Biochemical Parameters in Serum and Liver
Serum samples were obtained by centrifuging blood samples at 3500 rpm for 10 min at 4°C, and the serum was stored at -20°C for further analysis of biochemical parameters. The concentrations of glucose (GLU, H108), triglyceride (TG, H201), total cholesterol (TCHO, H202), low-density lipoprotein cholesterol (LDL-C, H207), high-density lipoprotein cholesterol (HDL-C, H203), and total bile acid (TBA, H101T) in the serum were measured using an automatic biochemical analyzer (Hitachi 7020, Tokyo, Japan). The above commercial assay kits were purchased from Meinkang Biotech Co., LTD. (Ningbo, China), following the manufacturer's instructions.
The contents of TBA in the liver were extracted using a previously method (Sato et al., 2008). Briefly, 50 mg of the liver was homogenized with 75% ethanol, incubated at 50°C water for 2 h, and then centrifuged at 7,000 rpm for 10 min. Hepatic TCHO content was determined using the chloroform-methanol extraction method. Approximately 50 mg of liver was homogenized with chloroform: methanol (2:1 v/v) and then centrifuged at 8000 rpm for 10 min. The extracts and bile samples were used to measuring the TBA and TCHO contents with an automatic biochemical analyzer (Hitachi 7020, Tokyo, Japan).
Histological Evaluation of Breast Muscle
Fresh breast muscle samples were fixed overnight in 4% paraformaldehyde and then embedded in paraffin. Muscle sections (5 μm) were stained with hematoxylin and eosin (H&E). Each histological section was imaged with the optical microscope (Olympus-BX53, Tokyo, Japan) and photographed. Five different images from each sample section were randomly selected to measure the bundle cross-sectional areas (μm2) and number of muscle bundles (per µm2) in breast muscle using Image J software.
Immunohistochemical and Immunofluorescence Staining of Breast Muscle
The immunohistochemical staining of proliferating cell nuclear antigen (PCNA, BS5842, Bioworld, diluted 1:1,000) and paired box 7 (Pax7, sc-81648, Santa Cruz, diluted 1:1,000) immunofluorescence in breast muscle was conducted by Wuhan Powerful Biology Co., Ltd. The images were captured under the optical microscope (Olympus-BX53, Tokyo, Japan) and fluorescence microscope (DM16000B, Leica, Germany), respectively. PCNA and Pax7 positive staining cells were counted using an Image-Plus 6.0 software from 5 randomly selected microscopic fields in each section (3 samples per group).
Elisa for IGF-1 and IGF-2 Concentrations in Serum and Liver
IGF-1 (YB-IGF1-Go) and IGF-2 (YB-IGF2-Go) concentrations in breast muscle extracts and serum samples using commercial Elisa kits, following the manufacturer's instructions. The intra- and inter-assay coefficients of variations of all the kits were 9% and 11%, respectively.
RNA Isolation and Real-time PCR for mRNA Quantification
Total RNA was isolated from breast muscle samples using TRIzol reagent (TSP401, Tsingke Biotech Co., Ltd, Nanjing, China), and reverse transcription into cDNA was performed according to the manufacturer's protocol (RK20429, ABclonal Technology Co., Wuhan, China). Real-time PCR was performed with QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems Foster City, CA), using peptidylprolyl isomerase A (PPIA) as an internal control to normalize the technical variations. The mRNA sequences of all target genes were exported from the GenBank database on the NCBI website, and primer design was performed using the Primer-blast tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast) on the NCBI website. Primer sequences (Table S1) were synthesized by Tsingke Biotechnology Co., Ltd. (Nanjing, China). Data were analyzed using the method of 2-ΔΔCT.
Total Protein Extraction and Western Blotting
Total protein was extracted from frozen liver samples using RIPA buffer added protease inhibitor cocktail (B14001, Selleckchem). Protein concentration was measured by using the BCA Protein Assay kit (DQ111-01, TransGen, Beijing, China). Forty micrograms of protein were used for electrophoresis on a 6-10% SDS-PAGE gel. Western blot analysis for FXR (A24015, Abclonal, diluted 1:1,000), Pax7 (sc-81648, Santa Cruz, diluted 1:1,000), MyoG (ab1835, Abcam, diluted 1:1,000), MSTN (19142-1-AP, Proteintech, diluted 1:1,000), Myf5 (ab125301, Abcam, diluted 1:1,000), AKT (AP0059, Bioworld, diluted 1:1,000), p-AKT (B34009, Bioworld, diluted 1:1,000), mTOR (2938, Cell Signaling Technology, diluted 1:1,000), p-mTOR (3398, Cell Signaling Technology, diluted 1:1,000), PCNA (BS5842, Bioworld, diluted 1:1,000), betaine-homocysteine methyltransferase (BHMT, 15965-1-AP, Proteintech, diluted 1:1,000), methionine adenosyltransferase 2 B (MAT2B,15952-1-AP, Proteintech, diluted 1:1,000), glycine N-methyltransferase (GNMT, 18790-1-AP, Proteintech, diluted 1:1,000), DNA (cytosine-5-)- methyltransferase 1 (DNMT1, 24206-1-AP, Proteintech, diluted 1:1,000), DNA (cytosine-5-)- methyltransferase 3A (DNMT3A, A16834, Abclonal, diluted 1:1,000) were performed following the manufacturer's instructions. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, MB001H, Bioworld, diluted 1:10,000) was used as an internal reference. Images were captured by VersaDoc 4,000MP system (Bio-Rad) and the band density was analyzed with Quantity One software (Bio-Rad).
Methylated DNA Dot Blotting
DNA was extracted from frozen breast muscle sample diluted to 250 ng/µL and denatured at 95°C for 10 min. The samples were immediately placed on ice for 5 min, and 1 μL (250 ng) from each sample was blotted on the Hybond-N+ membrane (GE Healthcare, Piscataway, NJ). The DNA was cross-linked in a UV Stratalinker, blocked in 5% nonfat milk for 1 h, followed by incubation with an anti-5 mc antibody (ab10805, Abcam, diluted 1:1,000) overnight. The membrane was washed 3 times and incubated with a secondary antibody at room temperature for 2 h. The signals were visualized by an imaging system (Bio-Rad), and the dot density was analyzed with Quantity One software (Bio-Rad), with staining of 0.02% methylene blue (in 0.3 mol/L sodium acetate, pH = 5.2) as control.
Methylated DNA Immunoprecipitation
The genomic DNA extracted from breast muscle samples was purified and fragmented to an average size of 300 to 500 base pairs (bp) using ultrasonication. Subsequently, 1 μg of the fragmented DNA was heat-denatured and subjected to immunoprecipitated with an anti-5 mc antibody (ab10805, Abcam, diluted 1:1,000). A portion of the denatured DNA was preserved as input DNA. The immune complexes were captured by pretreated protein G agarose beads (sc-2003, Santa Cruz Biotechnology, Inc., CA). Following purification, The immunoprecipitated DNA was utilized as templates for amplifying the promoter sequences of IGF-1, IGF-2, and IGF-1R genes through real-time PCR, with specific primers are listed in Supplementary Table S1.
Chromatin Immunoprecipitation Assay
Chromatin immunoprecipitation (ChIP) was performed following the protocal previously described (Feng et al., 2021). Briefly, 200 mg frozen breast samples were washed with PBS containing a protease inhibitor cocktail (P1010, Beyotime, Shanghai, China). After cross-linking in 1% formaldehyde, the reaction was stopped with 2.5 mol/L glycine. The pellets were lysed and the chromatin was sonicated to an average length of approximately 300 bp. The DNA-protein complex was then diluted in ChIP dilution buffer and incubated overnight at 4°C with 2 μg of FXR antibody (sc-25309X, Santa Cruz, CA). Samples with normal IgG or without the addition of FXR antibody served as negative controls. Protein G agarose beads (sc-2003, Santa Cruz, CA) were employed to capture immunoprecipitated chromatin complexes. Subsequent to reverse cross-linking at 65°C for 5 h to release DNA fragments from the immunoprecipitated complex and DNA fragments were purified. The putative FXREs in the IGF-2 promoter were predicted using the online software hTFtarget (http://bioinfo.life.hust.edu.cn/hTFtarget/#!/prediction). The purified DNA fragments were used as templates for real-time PCR, with the sequences of specific primers used to amplify the putative FXREs are listed in Table S1.
Statistical Analysis
Comparisons between the 2 groups were analyzed using independent-samples t-test with SPSS 20.0 software (SPSS Inc., Chicago, IL). Data are presented as means ± SEM. Pearson correlation analysis was performed for correlation analysis. Differences were considered statistically significant when P < 0.05, ** P < 0.01.
RESULTS
Plasma and Liver Biochemical Parameters
In ovo betaine injection significantly reduced serum TG concentration. Additionally, there was a tendency towards decreased TCHO concentration in the liver (P = 0.061). However, the concentration of TBA in the liver and bile were significantly increased (P < 0.05) in the BET group compared to the CON group (Table 1).
Table 1.
Effect of in ovo betaine on serum and liver biochemical indexes of newly hatched goslings.
| Parameters | CON (n = 16) | BET (n = 15) | P-value |
|---|---|---|---|
| TG (mmol/L) | 2.61 ± 0.17 | 2.14 ± 0.13 | 0.040 |
| GLU (mmol/L) | 11.25 ± 0.22 | 10.88 ± 0.26 | 0.291 |
| TCHO (mmol/L) | 8.75 ± 0.42 | 7.89 ± 0.45 | 0.177 |
| TBA (μmol/L) | 21.70 ± 1.23 | 25.34 ± 1.79 | 0.098 |
| LDL-C (mmol/L) | 0.84 ± 0.13 | 0.85 ± 0.09 | 0.944 |
| HDL-C (mmol/L) | 2.38 ± 0.20 | 2.75 ± 0.20 | 0.210 |
| Hepatic TCHO (mg/g) | 10.88 ± 0.29 | 9.79 ± 0.48 | 0.061 |
| Hepatic TBA (μmol/g) | 0.28 ± 0.02 | 0.37 ± 0.23 | 0.015 |
| Bile TCHO (mmol/L) | 1.86 ± 0.02 | 1.64 ± 0.25 | 0.519 |
| Bile TBA (μmol/L) | 52.42 ± 4.59 | 66.15 ± 5.03 | 0.041 |
Abbreviations: GLU: glucose; HDL-C: High-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TBA: total bile acid; TCHO: total cholesterol; TG: triglyceride.
Histological Traits and Expression of Genes Associated with Myogenic Regulatory in Breast Muscle
In ovo betaine injection significantly increased (P < 0.05) body weight (Fig. 1A) and relative body weight (Figure 1B) at hatch. Concurrently, goslings in the BET group exhibited higher (P < 0.05) breast muscle weight (Figure 1C) and breast muscle index (Figure 1D), which correlated with a significant increase in the cross-sectional area of muscle bundles (Figure 1E-G), while a decreasing tendency (P = 0.075) was observed in density of muscle bundle (Figure 1E, F, H). Furthermore, in ovo betaine injection significantly upregulated (P < 0.05) MyoG and Pax7 expression at both mRNA (Figure 1H) and protein (Figure 1I) levels, while MSTN was significantly downregulated (Figure 1H, I).
Figure 1.
In ovo betaine injection on histomorphology and expression of myogenesis-related genes in breast muscle of newly hatched goslings. (A) Body weight; (B) Relative body weight; (C) Breast muscle weight; (D) Breast muscle index; (E) HE staining of breast muscle sections (200×), scale bar = 50 μm;(F) Muscle bundle area analysis; (G) Muscle bundle density analysis; (H) mRNA expression of myogenesis related genes; (I) Expression of myogenesis-related protein. Data are means ± SEM (Figure A - D and H, CON: n = 16, BET: n = 15; Figure E - G, n = 5; Figure I, n = 7), * P < 0.05, **P < 0.01.
Proliferation Level of Satellite Cells in Breast Muscle
In ovo betaine injection significantly increased (P < 0.05) the area of PCNA immunopositive cells (Figure 2A, B), together with a significant upregulation of PCNA at both mRNA (Figure 2C) and protein levels (Figure 2D). Immunofluorescent staining showed higher (P < 0.05) intensity of Pax7 immunopositive cells in the breast muscle of the BET group (Figure 2E, F), with a positive correlation between the area of PCNA immunopositive cells and the intensity of Pax7 immunopositive cells (r2 = 0.761, P < 0.05, Figure 2 G).
Figure 2.
In ovo betaine injection on the number of satellite cells in breast muscle of newly hatched goslings. (A) Immunohistochemical staining for PCNA (400×), scale bar = 20 μm (n = 3); (B) Immunopositive area analysis of PCNA; (C) mRNA expression of PCNA (CON: n = 16, BET: n = 15); (D) Protein expression of PCNA (n = 7); (E) Pax7 expression was assessed by immunofluorescence (200×), and the nucleus was stained with DAPI, scale bar = 50 μm (n = 3); (F) Analysis of positive expression rate of Pax7; (G) Correlation analysis was performed between immunopositive area of PCNA and positive expression rate of Pax7. Data are means ± SEM. *P < 0.05, **P < 0.01.
IGFs Contents and Gene Expression Associated with IGFs Signalling Pathway in Breast Muscle
In ovo betaine injection significantly increased (P < 0.05) the contents of IGF-1 (Figure 3A) and IGF-2 (Figure 3B) in breast muscle, consistent with significantly enhanced the mRNA expression of IGF-1, IGF-2 and IGF-1R genes (Figure 3C). No differences were detected in the mRNA expression of IGF-binding proteins (Figure 3D). The downstream signaling protein, such as phosphorylated mTOR, was significantly higher (P < 0.05) in the BET group (Figure 3E). Moreover, the p-AKT/AKT and p-mTOR/ mTOR ratios were significantly enhanced (P < 0.05) in the BET group (Figure 3E).
Figure 3.
Effect of in ovo betaine injection on the expression of IGFs and signaling pathway related-genes in breast muscle of newly hatched goslings. (A) IGF-1 content; (B) IGF-2 content; (C) mRNA expression of growth-related genes; (D) mRNA expression of IGF-binding protein; (E) Protein expression of AKT/mTOR signaling pathway. Data are means ± SEM (Figure A - D, CON: n = 16, BET: n = 15; Figure E: n = 7). *P < 0.05, **P < 0.01.
Global DNA Methylation and Expression of Methionine Metabolic Genes in Breast Muscle
In ovo betaine injection significantly increased the global DNA methylation level in breast muscle (Figure 4A). Concurrently, the mRNA expression of methionine metabolic genes, AHCYL, and GNMT were significantly elevated (P < 0.05) in the betaine group compared with the control (Figure 4B). The protein expression of GNMT and DNMT1 was also significantly increased (P < 0.05) in the betaine group compared with the control (Figure 4B).
Figure 4.
Effect of in ovo betaine injection on the expression of methionine metabolic genes in breast muscle of newly hatched goslings. (A) Global DNA 5mC level was detected by dot blot (n = 6); (B) mRNA expression of methionine metabolic genes (CON: n = 16, BET: n = 15); (C) Protein expression of BHMT, GNMT, MAT2B, DNMT1 and DNMT3A (n = 7). Data are means ± SEM. *P < 0.05. **P < 0.01.
DNA Methylation Status on the Promoter of Affected Genes in Breast Muscle
The methylation status of the IGF-2 gene promoter was found to be significantly hypomethylated (P < 0.05) in breast muscle of the betaine group (Figure 5A), while no alterations were detected in the methylation status of IGF-2 exons in the MeDIP analysis (Figure 5A). Similarly, the IGF-1 promoter and exon 2 were significantly hypomethylated (P < 0.05) in the betaine group (Figure 5B). At the same time, the IGF-1R gene promoter was also detected to be hypomethylated (P < 0.05) (Figure 5C).
Figure 5.
Effect of in ovo betaine injection on DNA methylation at the promoter of IGFs-related genes. (A) DNA methylation level on the promoter and exon regions of the IGF-2 gene; (B) DNA methylation level on the promoter and exon regions of the IGF-1 gene; (C) DNA methylation level on the promoter region of the IGF-1R gene. Data are means ± SEM (Figure A - C: n = 6). *P < 0.05.
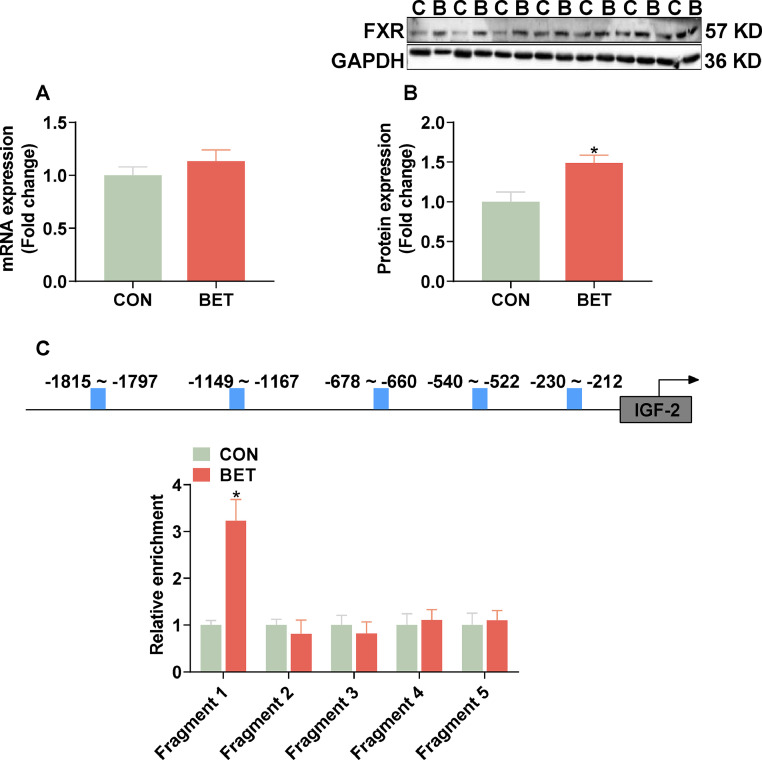
Expression of FXR Gene and its Binding Levels to the Promoter of IGF-2 Gene
The mRNA expression of the FXR gene in the BET group was not changed (Figure 6A), while the protein expression of FXR was significantly up-regulated (Figure 6B). Additionally, ChIP-PCR analysis showed a significant increase (P < 0.05) in FXR binding to the promoter of the IGF-2 gene in breast muscle of the betaine group (Figure 6C).
Figure 6.
Effect of in ovo betaine injection on FXR binding at the promoter of IGF-2 gene in breast muscle of newly hatched goslings. (A) mRNA expression of FXR; (B) Protein expression of FXR; (C) FXR binding to promoter region of the IGF-2 gene. Data are means ± SEM (Figure A - B, CON: n = 16, BET: n = 15; Figure C: n = 4). *P < 0.05.
DISCUSSION
Eggs contain a substantial amount of betaine, which is regarded as an essential nutrient for the embryonic and fetal development (Zeisel et al., 2003; Lever and Slow., 2010; Hu et al., 2015). Previous studies have revealed that betaine exposure before incubation did not affect body weight, but significantly increased the serum and hepatic contents of total cholesterol in newly-hatched chicks (Hu et al., 2015). In contrast, our study found that the TBA contents in liver and bile were significantly increased. Avian embryos are completely dependent on the nutrients derived from the yolk, which is rich in lipids (van der Wagt et al., 2020). Bile acid, serving as emulsifier of fats, may facilitate the digestion and utilization of fats during embryonic development in gosling, thereby promoting growth and development (Ge et al., 2019; Chen et al., 2024). Moreover, bile acid supplementation in a high-fat diet significantly increased the body weight and breast mass of broilers (Chen et al., 2024). In the present study, in ovo administration of betaine led to a significant increase in hatch weight and relative body weight in newly hatched goslings. In addition, higher breast muscle weight and breast muscle index were associated with an increased cross-sectional area of the breast muscle bundle. This suggests that betaine may exert growth-promoting effects on the embryonic development of goslings. Since the myofiber number remains constant after birth, generating more myofibers during embryonic development enhances the potential of the muscle production in the posthatch period (Muyyarikkandy et al., 2023). Similarly, we found a significant increase in the cross-sectional area of breast muscle bundles in the betaine-treated group, which may be associated with higher number of myofibers, potentially enhancing the capacity of meat yield in the postnatal period.
Skeletal muscle growth is predominantly regulated by various key hormones, including thyroid hormone, growth hormone and IGF1/2 (Fuentes et al., 2013; Bloise et al., 2018). Skeletal muscle functions as an autocrine and paracrine organ by secreting IGF1/2, which stimulates the proliferation and differentiation of satellite cells, promotes protein synthesis, and ultimately augments muscle mass (Velloso, 2008; Chen et al., 2018). Previous in vitro and in vivo studies demonstrated that betaine enhances muscle growth via the IGF-1-Akt signaling pathway (Apicella et al., 2013; Senesi et al., 2013). Moreover, betaine supplementation increased mRNA expression of IGF-1, aligning with higher IGF-1 content in the breast muscle of broilers (Chen et al., 2018). Similarly, single-base editing of the IGF-2 gene could improve birth weight and meat production in pigs (Duo et al., 2023). Consistent with these findings, in this study, the upregulation of IGF-1, IGF-2, and IGF-1R gene expression were observed, along with higher IGF-1 and IGF-2 contents in the breast muscle of betaine-treated goslings. Meanwhile, we found that significant increase in the ratio of p-AKT/AKT and p-mTOR/mTOR in the betaine group, indicating that betaine could activate the downstream pathway of IGFs and then promote breast muscle protein synthesis.
Skeletal muscle growth is influenced by myogenic regulatory factors and satellite cells. MyoG plays a crucial role in the fusion and differentiation of myoblasts, facilitating myofiber formation and maturation (Chargé and Rudnicki, 2004). Conversely, MSTN acts as a negative regulator of muscle growth by inhibiting the proliferation and differentiation of myoblasts (Chen et al., 2021). In this study, the MyoG was upregulated for both mRNA and protein levels in the betaine-treated group, while downregulated MSTN expression, both at mRNA and protein levels. Similar result was reported that betaine supplementation linearly decreased the mRNA expression of MSTN in the breast muscle of Cherry Valley ducks (Chen et al., 2019). These suggest that betaine promotes myoblast fusion and differentiation during goose embryonic development, leading to an increase in the number of myofibers in newly hatched goslings. The number of myofiber nuclei and myofiber protein accumulation during the postnatal period primarily depends on satellite cell proliferation (Huang et al., 2013). However the proportion of satellite cells decreases rapidly with age (Halevy et al., 2006). Early feed restriction exhibited lower satellite cell proliferation in the gastrocnemius, inhibiting myofiber hypertrophy, and ultimately decreasing the body weight of broilers (Li et al., 2007). Additionally, IGF-1 promotes myofiber hypertrophy and protein synthesis by activating satellite cell proliferation, thereby increasing skeletal muscle weight in animals (Liu et al., 2012; Bai et al., 2016). These findings underscore the pivotal role of satellite cells in early postnatal muscle growth. In the current study, both mRNA and protein expression of PCNA and Pax7 were significantly enhanced in the breast muscle of the betaine group, which was further confirmed by immunohistochemical staining of PCNA and immunofluorescent staining of Pax7 results. Collectively, these results suggest that in ovo betaine injection could stimulate satellite cell proliferation in the breast muscle of goslings. At the same time, the higher contents of IGF1/2 in breast muscle may contribute to stimulating satellite cell proliferation to some extent.
In this study, in ovo betaine injection significantly increased the expression of methionine metabolism and methyl transfer genes GNMT and DNMT1 at protein levels in breast muscle of the betaine group, which in line with a previous study reported that maternal betaine supplementation upregulated the AHCYL, GNMT and DNMT1 genes expression in the adrenal of offspring cockerels (Abobaker et al., 2022). Generally, the supply of methyl donors enhances methionine metabolism, leading to the upregulation of DNMT1, and thus resulting in global genomic hypermethylation (Biniszkiewicz et al., 2002; Mahmoud and Ali, 2019). Indeed, the upregulation of DNMT1 in this study coincide with global genomic DNA hypermethylation in breast muscle of the betaine-treated goslings. However, the methylation status of the promoters or regulatory regions in response to methyl donor availability is complex fashion and gene-specific. Concurrently, The methylation status of the promoter or regulatory region of specific gene may exhibit distinct reactions compared to the global genomic DNA (Kovacheva et al., 2007). In fact, existing finding reported that the sterol regulator element-binding protein 2 (SREBP2) gene promoter exhibited hypermethylation, while that of cholesterol-7alpha-hydroxylase (CYP7A1) gene was hypomethylated in the liver of betaine-treated chickens (Hu et al., 2020). Moreover, gestational choline deficiency in rats induces hypomethylation of the hepatic DNMT1 gene in fetuses, ultimately resulting in hypermethylation of the genome as well as the IGF-2 gene (Kovacheva et al., 2007). We previously reported maternal betaine supplementation increased IGF-1 and IGF-2 gene expression in offspring rats, pigs, and goslings by affecting DNA methylation on the promoter (Li et al., 2015; Yang et al., 2018; Ma et al., 2024). Similarly, the promoter of IGF-1, IGF-2, and IGF-1R genes in the breast muscle of betaine-treated goslings were found to be hypomethylated, which is consistent with their gene expression levels.
The transcriptional regulatory mechanisms of IGF-2 in response to betaine were investigated in this study. Our findings revealed that betaine exposure increased hepatic and bile content of total bile acid, consistent with previous publications that dietary betaine supplementation increased hepatic total bile acid synthesis in rats (Li et al., 2020). FXR, functioning as a bile acid-activated transcription factor, regulates gene transcription by binding to the FXRE binding site in the regulatory regions of target genes (Shin and Wang, 2019). In an earlier study reported that depletion of gut microbiota suppressed FXR-mediated signaling in the ileum, resulting in reduced in skeletal muscle mass (Qiu et al., 2021). Furthermore, Dietary bile acid in broilers was found to increase breast muscle mass, which was associated with higher enrichment of FXR on the IGF-2 gene promoter (Chen et al., 2024). In this study, in ovo betaine injection enhanced the FXR protein expression in breast muscle. Moreover, 5 putative FXR binding sites was predicted in the promoter region of IGF-2, which were further investigated using ChIP-PCR. Indeed, FXR binding to the IGF-2 promoter was found to be increased in breast muscle of the betaine group. These results indicate that FXR is involved in the transcriptional regulation of IGF-2 gene expression in the breast muscle of goslings. Additionally, we speculated that the hypomethylation status of the IGF-2 promoter may facilitate FXR binding to it. However, the above findings are solely based on the associations, more in-depth studies are required to elucidate such links indeed exist in the scenario of betaine treatment by using in vitro models.
CONCLUSIONS
Taken together, we demonstrate that in ovo betaine injection promotes embryonic development, resulting in increased hatch weight and breast muscle mass in newly hatched goslings. These improvements may be mediated through the FXR/IGF2 pathway. This study helps understand the molecular mechanisms of betaine regulates the skeletal muscle growth in goose.
DISCLOSURES
There is no conflict of interests in the submission of this manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (2022YFD1300401), the National Natural Science Foundation of China (32272962, 31972638).
Author contributions: The manuscript is our original unpublished work and not submitted to any other journals for publication, in whole or in part. All authors listed have read and approved this manuscript of the paper.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2024.104075.
Appendix. Supplementary materials
REFERENCES
- Abobaker H., Omer N.A., Hu Y., Idriss A.A., Zhao R. In ovo injection of betaine promotes adrenal steroidogenesis in pre-hatched chicken fetuses. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella J.M., Lee E.C., Bailey B.L., Saenz C., Anderson J.M., Craig S.A., Kraemer W.J., Volek J.S., Maresh C.M. Betaine supplementation enhances anabolic endocrine and Akt signaling in response to acute bouts of exercise. Eur. J. Appl. Physiol. 2013;113:793–802. doi: 10.1007/s00421-012-2492-8. [DOI] [PubMed] [Google Scholar]
- Bai X., Wang Y., Wang Z., Cao J., Dong Y., Chen Y. In ovo exposure to monochromatic lights affect posthatch muscle growth and Ssatellite cell proliferation of chicks: role of IGF-1. Growth Factors. 2016;34:107–118. doi: 10.1080/08977194.2016.1199553. [DOI] [PubMed] [Google Scholar]
- Benoit B., Meugnier E., Castelli M., Chanon S., Vieille-Marchiset A., Durand C., Bendridi N., Pesenti S., Monternier P.A., Durieux A.C., Freyssenet D., Rieusset J., Lefai E., Vidal H., Ruzzin J. Fibroblast growth factor 19 regulates skeletal muscle mass and ameliorates muscle wasting in mice. Nat. Med. 2017;23:990–996. doi: 10.1038/nm.4363. [DOI] [PubMed] [Google Scholar]
- Biniszkiewicz D., Gribnau J., Ramsahoye B., Gaudet F., Eggan K., Humpherys D., Mastrangelo M.A., Jun Z., Walter J., Jaenisch R. Dnmt1 overexpression causes genomic hypermethylation, loss of imprinting, and embryonic lethality. Mol Cell Biol. 2002;22:2124–2135. doi: 10.1128/MCB.22.7.2124-2135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloise F.F., Cordeiro A., Ortiga-Carvalho T.M. Role of thyroid hormone in skeletal muscle physiology. J. Endocrinol. 2018;236:R57–R68. doi: 10.1530/JOE-16-0611. [DOI] [PubMed] [Google Scholar]
- Chargé S.B., Rudnicki M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Chen W., Lv Y.T., Zhang H.X., Ruan D., Wang S., Lin Y.C. Developmental specificity in skeletal muscle of late-term avian embryos and its potential manipulation. Poult. Sci. 2013;92:2754–2764. doi: 10.3382/ps.2013-03099. [DOI] [PubMed] [Google Scholar]
- Chen R., Zhuang S., Chen Y.P., Cheng Y.F., Wen C., Zhou Y.M. Betaine improves the growth performance and muscle growth of partridge shank broiler chickens via altering myogenic gene expression and insulin-like growth factor-1 signaling pathway. Poult. Sci. 2018;97:4297–4305. doi: 10.3382/ps/pey303. [DOI] [PubMed] [Google Scholar]
- Chen R., Wen C., Cheng Y., Chen Y., Zhuang S., Zhou Y. Effects of dietary supplementation with betaine on muscle growth, muscle amino acid contents and meat quality in Cherry Valley ducks. J. Anim. Physiol. Anim. Nutr. (Berl.) 2019;103:1050–1059. doi: 10.1111/jpn.13083. [DOI] [PubMed] [Google Scholar]
- Chen M.M., Zhao Y.P., Zhao Y., Deng S.L., Yu K. Regulation of myostatin on the growth and development of skeletal muscle. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.785712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Shi Y., Li J., Shao C., Ma S., Shen C., Zhao R. Dietary bile acids improve breast muscle growth in chickens through FXR/IGF2 pathway. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2023.103346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang J.Y. Bile acids: regulation of synthesis. J. Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duo T., Liu X., Mo D., Bian Y., Cai S., Wang M., Li R., Zhu Q., Tong X., Liang Z., Jiang W., Chen S., Chen Y., He Z. Single-base editing in IGF2 improves meat production and intramuscular fat deposition in Liang Guang Small Spotted pigs. J. Anim. Sci. Biotechnol. 2023;14:141–159. doi: 10.1186/s40104-023-00930-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Li Y., Jiang W., Hu Y., Jia Y., Zhao R. GR-mediated transcriptional regulation of m (6)A metabolic genes contributes to diet-induced fatty liver in hens. J. Anim. Sci. Biotechnol. 2021;12:117–127. doi: 10.1186/s40104-021-00642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera W.R., Ochala J. Skeletal muscle: a brief review of structure and function. Calcif. Tissue Int. 2015;96:183–195. doi: 10.1007/s00223-014-9915-y. [DOI] [PubMed] [Google Scholar]
- Fuentes E.N., Valdés J.A., Molina A., Björnsson B.T. Regulation of skeletal muscle growth in fish by the growth hormone−insulin-like growth factor system. Gen. Comp. Endocrinol. 2013;192:136–148. doi: 10.1016/j.ygcen.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Ge X.K., Wang A.A., Ying Z.X., Zhang L.G., Su W.P., Cheng K., Feng C.C., Zhou Y.M., Zhang L.L., Wang T. Effects of diets with different energy and bile acids levels on growth performance and lipid metabolism in broilers. Poult. Sci. 2019;98:887–895. doi: 10.3382/ps/pey434. [DOI] [PubMed] [Google Scholar]
- Guo A., Li K., Tian H.C., Fan Z., Chen Q.N., Yang Y.F., Yu J., Wu Y.X., Xiao Q. FGF19 protects skeletal muscle against obesity-induced muscle atrophy, metabolic derangement and abnormal irisin levels via the AMPK/SIRT-1/PGC-α pathway. J. Cell. Mol. Med. 2021;25:3585–3600. doi: 10.1111/jcmm.16448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O., Piestun Y., Rozenboim I., Yablonka-Reuveni Z. In ovo exposure to monochromatic green light promotes skeletal muscle cell proliferation and affects myofiber growth in posthatch chicks. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1062–R1070. doi: 10.1152/ajpregu.00378.2005. [DOI] [PubMed] [Google Scholar]
- Hu Y., Sun Q., Li X., Wang M., Cai D., Li X., Zhao R. In ovo injection of betaine affects hepatic cholesterol metabolism through epigenetic gene regulation in newly hatched chicks. PloS One. 2015;10 doi: 10.1371/journal.pone.0122643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Feng Y., Ding Z., Lv L., Sui Y., Sun Q., Abobaker H., Cai D., Zhao R. Maternal betaine supplementation decreases hepatic cholesterol deposition in chicken offspring with epigenetic modulation of SREBP2 and CYP7A1 genes. Poult. Sci. 2020;99:3111–3120. doi: 10.1016/j.psj.2019.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Price F., Rudnicki M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idriss A.A., Hu Y., Sun Q., Jia L., Jia Y., Omer N.A., Abobaker H., Zhao R. Prenatal betaine exposure modulates hypothalamic expression of cholesterol metabolic genes in cockerels through modifications of DNA methylation. Poult. Sci. 2017;96:1715–1724. doi: 10.3382/ps/pew437. [DOI] [PubMed] [Google Scholar]
- Jia Y., Song H., Gao G., Cai D., Yang X., Zhao R. Maternal betaine supplementation during gestation enhances expression of mtDNA-encoded genes through D-Loop DNA hypomethylation in the skeletal muscle of newborn piglets. J. Agric. Food Chem. 2015;63:10152–10160. doi: 10.1021/acs.jafc.5b04418. [DOI] [PubMed] [Google Scholar]
- Jin C., Zhuo Y., Wang J., Zhao Y., Xuan Y., Mou D., Liu H., Zhou P., Fang Z., Che L., Xu S., Feng B., Li J., Jiang X., Lin Y., Wu D. Methyl donors dietary supplementation to gestating sows diet improves the growth rate of offspring and is associating with changes in expression and DNA methylation of insulin-like growth factor-1 gene. J. Anim. Physiol. Anim. Nutr. (Berl) 2018;102:1340–1350. doi: 10.1111/jpn.12933. [DOI] [PubMed] [Google Scholar]
- Kovacheva V.P., Mellott T.J., Davison J.M., Wagner N., Lopez-Coviella I., Schnitzler A.C., Blusztajn J.K. Gestational choline deficiency causes global and Igf2 gene DNA hypermethylation by up-regulation of Dnmt1 expression. J. Biol. Chem. 2007;282:31777–31788. doi: 10.1074/jbc.M705539200. [DOI] [PubMed] [Google Scholar]
- Lever M., Slow S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin. Biochem. 2010;9:732–744. doi: 10.1016/j.clinbiochem.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Li Y., Yuan L., Yang X., Ni Y., Xia D., Barth S., Grossmann R., Zhao R. Effect of early feed restriction on myofibre types and expression of growth-related genes in the gastrocnemius muscle of crossbred broiler chickens. Br. J. Nutr. 2007;98:310–319. doi: 10.1017/S0007114507699383. [DOI] [PubMed] [Google Scholar]
- Liu H.H., Wang J.W., Zhang R.P., Chen X., Yu H.Y., Jin H.B., Li L., Han C.C., Xu F., Kang B., He H., Xu H.Y. In ovo feeding of IGF-1 to ducks influences neonatal skeletal muscle hypertrophy and muscle mass growth upon satellite cell activation. J. Cell. Physiol. 2012;227:1465–1475. doi: 10.1002/jcp.22862. [DOI] [PubMed] [Google Scholar]
- Li X., Sun Q., Li X., Cai D., Sui S., Jia Y., Song H., Zhao R. Dietary betaine supplementation to gestational sows enhances hippocampal IGF2 expression in newborn piglets with modified DNA methylation of the differentially methylated regions. Eur. J. Nutr. 2015;54:1201–1210. doi: 10.1007/s00394-014-0799-4. [DOI] [PubMed] [Google Scholar]
- Mahmoud A.M., Ali M.M. Methyl donor micronutrients that modify DNA methylation and cancer outcome. Nutrients. 2019;11:608–637. doi: 10.3390/nu11030608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Xu S., Zhao Y., Wang H., Feng J. Dietary betaine addition promotes hepatic cholesterol synthesis, bile acid conversion, and export in rats. Nutrients. 2020;12:1399–1414. doi: 10.3390/nu12051399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancin L., Wu G.D., Paoli A. Gut microbiota-bile acid-skeletal muscle axis. Trends Microbiol. 2023;31:254–269. doi: 10.1016/j.tim.2022.10.003. [DOI] [PubMed] [Google Scholar]
- Ma S., Wang Y., Chen L., Wang W., Zhuang X., Liu Y., Zhao R. Parental betaine supplementation promotes gosling growth with epigenetic modulation of IGF gene family in the liver. J. Anim. Sci. 2024;102:skae065. doi: 10.1093/jas/skae065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyyarikkandy M.S., Schlesinger M., Ren Y., Gao M., Liefeld A., Reed S., Amalaradjou M.A. In ovo probiotic supplementation promotes muscle growth and development in broiler embryos. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perino A., Demagny H., Velazquez-Villegas L., Schoonjans K. Molecular physiology of bile acid signaling in health, disease, and aging. Physiol. Rev. 2021;101:683–731. doi: 10.1152/physrev.00049.2019. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Yu J., Li Y., Yang F., Yu H., Xue M., Zhang F., Jiang X., Ji X., Bao Z. Depletion of gut microbiota induces skeletal muscle atrophy by FXR-FGF15/19 signalling. Ann. Med. 2021;53:508–522. doi: 10.1080/07853890.2021.1900593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Yu J., Ji X., Yu H., Xue M., Zhang F., Li Y., Bao Z. Ileal FXR-FGF15/19 signaling activation improves skeletal muscle loss in aged mice. Mech Ageing Dev. 2022;202 doi: 10.1016/j.mad.2022.111630. [DOI] [PubMed] [Google Scholar]
- Sato M., Sato K., Furuse M. Change in hepatic and plasma bile acid contents and its regulatory gene expression in the chicken embryo. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2008;150:344–347. doi: 10.1016/j.cbpb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;169:361–371. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- Senesi P., Luzi L., Montesano A., Mazzocchi N., Terruzzi I. Betaine supplement enhances skeletal muscle differentiation in murine myoblasts via IGF-1 signaling activation. J. Transl. Med. 2013;11:174–185. doi: 10.1186/1479-5876-11-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D.J., Wang L. Bile acid-activated receptors: A Review on FXR and Other Nuclear Receptors. Handb Exp Pharmacol. 2019;256:51–72. doi: 10.1007/164_2019_236. [DOI] [PubMed] [Google Scholar]
- van der Wagt I., de Jong I.C., Mitchell M.A., Molenaar R., van den Brand H. A review on yolk sac utilization in poultry. Poult. Sci. 2020;99:2162–2175. doi: 10.1016/j.psj.2019.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velloso C.P. Regulation of muscle mass by growth hormone and IGF-I. Br. J. Pharmacol. 2008;154:557–568. doi: 10.1038/bjp.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Zhao N., Yang Y., Hu Y., Dong H., Zhao R. Mitotically stable modification of DNA methylation in IGF2/H19 imprinting control region is associated with activated hepatic IGF2 expression in offspring rats from betaine-supplemented dams. J. Agric. Food. Chem. 2018;66:2704–2713. doi: 10.1021/acs.jafc.7b05418. [DOI] [PubMed] [Google Scholar]
- Zammit P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017;72:19–32. doi: 10.1016/j.semcdb.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Zeisel S.H., Mar M.-H., Howe J.C., Holden J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003;133:1302–1307. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.