Abstract
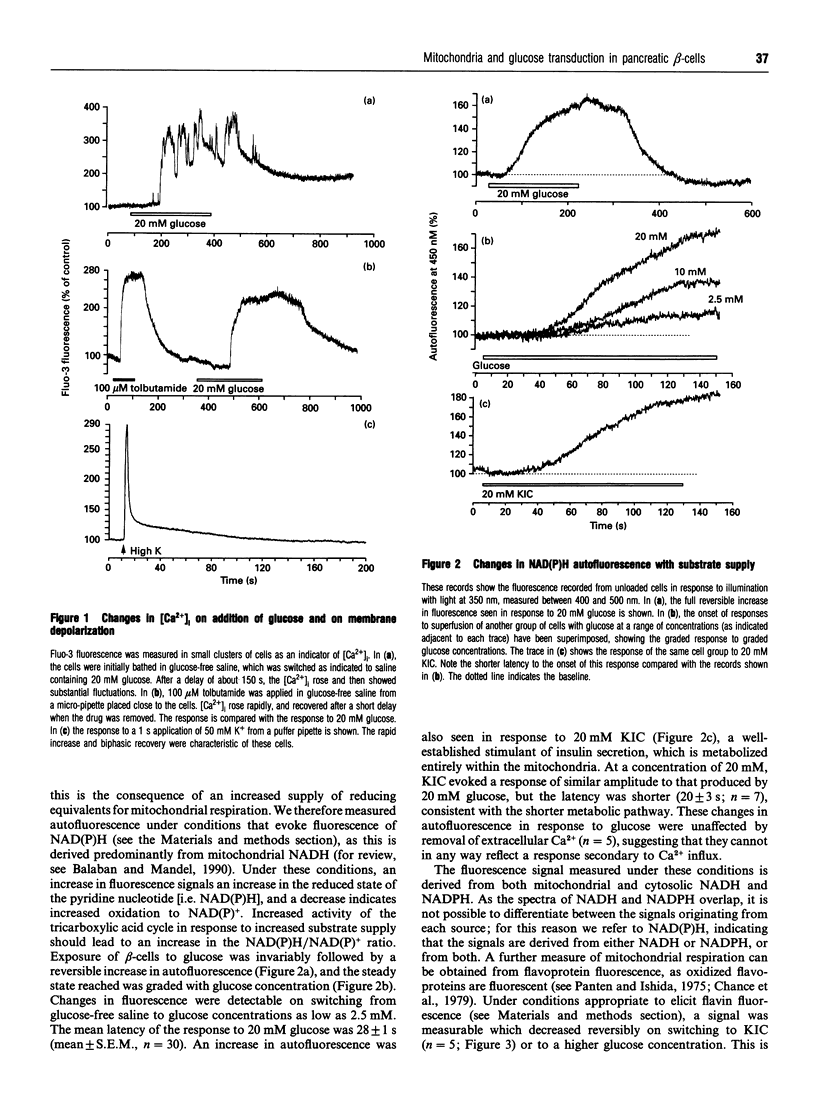
Microfluorimetric and patch-clamp techniques have been combined to determine the relationship between changes in mitochondrial metabolism, the activity of KATP channels and changes in intracellular free calcium concentration ([Ca2+]i) in isolated pancreatic beta-cells in response to glucose, ketoisocaproic acid (KIC) and the electron donor couple tetramethyl p-phenylenediamine (TMPD) and ascorbate. Exposure of cells to 20 mM glucose raised NAD(P)H autofluorescence after a delay of 28 +/- 1 s (mean +/- S.E.M., n = 30). The mitochondrial inner membrane potential, delta psi m (monitored using rhodamine 123 fluorescence), hyperpolarized with a latency of 49 +/- 6 s (n = 17), and the [Ca2+]i rose after 129 +/- 13 s (n = 5). The amplitudes of the metabolic changes were graded appropriately with glucose concentration over the range 2.5-20 mM. All variables responded to KIC with shorter latencies: NAD(P)H autofluorescence rose after a delay of 20 +/- 3 s (n = 5) and rhodamine 123 changed after 21 +/- 3 s (n = 6). The electron donor couple, TMPD with ascorbate, rapidly hyperpolarized delta psi m and raised [Ca2+]i. When [Ca2+]i was raised by sustained exposure to 20 mM glucose, TMPD had no further effect. TMPD also decreased whole-cell KATP currents and depolarized the cell membrane, measured with the perforated patch configuration. These data are consistent with a central role for mitochondrial oxidative phosphorylation in coupling changes in glucose concentration with the secretion of insulin.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft F. M., Ashcroft S. J., Harrison D. E. Effects of 2-ketoisocaproate on insulin release and single potassium channel activity in dispersed rat pancreatic beta-cells. J Physiol. 1987 Apr;385:517–529. doi: 10.1113/jphysiol.1987.sp016505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Ashcroft S. J., Harrison D. E. Properties of single potassium channels modulated by glucose in rat pancreatic beta-cells. J Physiol. 1988 Jun;400:501–527. doi: 10.1113/jphysiol.1988.sp017134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Balaban R. S. Regulation of oxidative phosphorylation in the mammalian cell. Am J Physiol. 1990 Mar;258(3 Pt 1):C377–C389. doi: 10.1152/ajpcell.1990.258.3.C377. [DOI] [PubMed] [Google Scholar]
- Best L., Trebilcock R., Tomlinson S. 2-Ketoisocaproate transport in insulin-secreting cells. Biosci Rep. 1992 Feb;12(1):69–76. doi: 10.1007/BF01125829. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R. Responses of type I cells dissociated from the rabbit carotid body to hypoxia. J Physiol. 1990 Sep;428:39–59. doi: 10.1113/jphysiol.1990.sp018199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunting J. R. Influx and efflux kinetics of cationic dye binding to respiring mitochondria. Biophys Chem. 1992 Feb;42(2):163–175. doi: 10.1016/0301-4622(92)85006-p. [DOI] [PubMed] [Google Scholar]
- CHANCE B., COHEN P., JOBSIS F., SCHOENER B. Intracellular oxidation-reduction states in vivo. Science. 1962 Aug 17;137(3529):499–508. doi: 10.1126/science.137.3529.499. [DOI] [PubMed] [Google Scholar]
- Chance B., Schoener B., Oshino R., Itshak F., Nakase Y. Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples. NADH and flavoprotein fluorescence signals. J Biol Chem. 1979 Jun 10;254(11):4764–4771. [PubMed] [Google Scholar]
- Chapman J. B. Fluorometric studies of oxidative metabolism in isolated papillary muscle of the rabbit. J Gen Physiol. 1972 Feb;59(2):135–154. doi: 10.1085/jgp.59.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A. M., Harris D. A. Defects in regulation of mitochondrial ATP synthase in cardiomyocytes from spontaneously hypertensive rats. Am J Physiol. 1990 Oct;259(4 Pt 2):H1264–H1269. doi: 10.1152/ajpheart.1990.259.4.H1264. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. Ca2+ as a second messenger within mitochondria of the heart and other tissues. Annu Rev Physiol. 1990;52:451–466. doi: 10.1146/annurev.ph.52.030190.002315. [DOI] [PubMed] [Google Scholar]
- Duchen M. R., Biscoe T. J. Mitochondrial function in type I cells isolated from rabbit arterial chemoreceptors. J Physiol. 1992 May;450:13–31. doi: 10.1113/jphysiol.1992.sp019114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen M. R., Biscoe T. J. Relative mitochondrial membrane potential and [Ca2+]i in type I cells isolated from the rabbit carotid body. J Physiol. 1992 May;450:33–61. doi: 10.1113/jphysiol.1992.sp019115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen M. R. Ca(2+)-dependent changes in the mitochondrial energetics in single dissociated mouse sensory neurons. Biochem J. 1992 Apr 1;283(Pt 1):41–50. doi: 10.1042/bj2830041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton P. L., Wilson D. F., Lee C. P. Oxidation-reduction potentials of cytochromes in mitochondria. Biochemistry. 1970 Dec 22;9(26):5077–5082. doi: 10.1021/bi00828a006. [DOI] [PubMed] [Google Scholar]
- Emaus R. K., Grunwald R., Lemasters J. J. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochim Biophys Acta. 1986 Jul 23;850(3):436–448. doi: 10.1016/0005-2728(86)90112-x. [DOI] [PubMed] [Google Scholar]
- Hellerström C. Effects of carbohydrates on the oxygen consumption of isolated pancreatic islets of mice. Endocrinology. 1967 Jul;81(1):105–112. doi: 10.1210/endo-81-1-105. [DOI] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Evidence for mediated transport of glucose in mammalian pancreatic -cells. Biochim Biophys Acta. 1971 Jul 6;241(1):147–154. doi: 10.1016/0005-2736(71)90312-9. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. D-glucose inhibits potassium efflux from pancreatic islet cells. Nature. 1978 Jan 19;271(5642):271–273. doi: 10.1038/271271a0. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Malaisse W. J. Dynamics of O2 consumption in rat pancreatic islets. Diabetologia. 1980 May;18(5):395–405. doi: 10.1007/BF00276821. [DOI] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Chen L. B. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci U S A. 1980 Feb;77(2):990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöbsis F. F., Duffield J. C. Oxidative and glycolytic recovery metabolism in muscle. J Gen Physiol. 1967 Mar;50(4):1009–1047. doi: 10.1085/jgp.50.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiranadi B., Bangham J. A., Smith P. A. Inhibition of electrical activity in mouse pancreatic beta-cells by the ATP/ADP translocase inhibitor, bongkrekic acid. FEBS Lett. 1991 May 20;283(1):93–96. doi: 10.1016/0014-5793(91)80561-g. [DOI] [PubMed] [Google Scholar]
- Lernmark A. The preparation of, and studies on, free cell suspensions from mouse pancreatic islets. Diabetologia. 1974 Oct;10(5):431–438. doi: 10.1007/BF01221634. [DOI] [PubMed] [Google Scholar]
- Panten U., Christians J., von Kriegstein E., Poser W., Hasselblatt A. Effect of carbohydrates upon fluorescence of reduced pyridine nucleotides from perifused isolated pancreatic islets. Diabetologia. 1973 Dec;9(6):477–482. doi: 10.1007/BF00461692. [DOI] [PubMed] [Google Scholar]
- Panten U., Ishida H. Fluorescence of oxidized flavoproteins from perifused isolated pancreatic islets. Diabetologia. 1975 Dec;11(6):569–573. doi: 10.1007/BF01222108. [DOI] [PubMed] [Google Scholar]
- Pralong W. F., Bartley C., Wollheim C. B. Single islet beta-cell stimulation by nutrients: relationship between pyridine nucleotides, cytosolic Ca2+ and secretion. EMBO J. 1990 Jan;9(1):53–60. doi: 10.1002/j.1460-2075.1990.tb08079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. A., Ashcroft F. M., Rorsman P. Simultaneous recordings of glucose dependent electrical activity and ATP-regulated K(+)-currents in isolated mouse pancreatic beta-cells. FEBS Lett. 1990 Feb 12;261(1):187–190. doi: 10.1016/0014-5793(90)80667-8. [DOI] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- Valdeolmillos M., Nadal A., Contreras D., Soria B. The relationship between glucose-induced K+ATP channel closure and the rise in [Ca2+]i in single mouse pancreatic beta-cells. J Physiol. 1992 Sep;455:173–186. doi: 10.1113/jphysiol.1992.sp019295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON J. R. GLYCOLYTIC CONTROL MECHANISMS. I. INHIBITION OF GLYCOLYSIS BY ACETATE AND PYRUVATE IN THE ISOLATED, PERFUSED RAT HEART. J Biol Chem. 1965 Jun;240:2308–2321. [PubMed] [Google Scholar]
- Zoratti M., Pietrobon D., Azzone G. F. On the relationship between rate of ATP synthesis and H+ electrochemical gradient in rat-liver mitochondria. Eur J Biochem. 1982 Sep 1;126(3):443–451. doi: 10.1111/j.1432-1033.1982.tb06800.x. [DOI] [PubMed] [Google Scholar]


