Abstract
The glymphatic system (GS) is a whole‐brain perivascular network, consisting of three compartments: the periarterial and perivenous spaces and the interposed brain parenchyma. GS dysfunction has been implicated in neurodegenerative diseases, particularly Alzheimer's disease (AD). So far, comprehensive research on GS in humans has been limited by the absence of easily accessible biomarkers. Recently, promising non‐invasive methods based on magnetic resonance imaging (MRI) along with aquaporin‐4 (AQP4) quantification in the cerebrospinal fluid (CSF) were introduced for an indirect assessment of each of the three GS compartments. We recruited 111 consecutive subjects presenting with symptoms suggestive of degenerative cognitive decline, who underwent 3 T MRI scanning including multi‐shell diffusion‐weighted images. Forty nine out of 111 also underwent CSF examination with quantification of CSF‐AQP4. CSF‐AQP4 levels and MRI measures—including perivascular spaces (PVS) counts and volume fraction (PVSVF), white matter free water fraction (FW‐WM) and mean kurtosis (MK‐WM), diffusion tensor imaging analysis along the perivascular spaces (DTI‐ALPS) (mean, left and right)—were compared among patients with AD (n = 47) and other neurodegenerative diseases (nAD = 24), patients with stable mild cognitive impairment (MCI = 17) and cognitively unimpaired (CU = 23) elderly people. Two runs of analysis were conducted, the first including all patients; the second after dividing both nAD and AD patients into two subgroups based on gray matter atrophy as a proxy of disease stage. Age, sex, years of education, and scanning time were included as confounding factors in the analyses. Considering the whole cohort, patients with AD showed significantly higher levels of CSF‐AQP4 (exp(b) = 2.05, p = .005) and FW‐WM FW‐WM (exp(b) = 1.06, p = .043) than CU. AQP4 levels were also significantly higher in nAD in respect to CU (exp(b) = 2.98, p < .001). CSF‐AQP4 and FW‐WM were significantly higher in both less atrophic AD (exp(b) = 2.20, p = .006; exp(b) = 1.08, p = .019, respectively) and nAD patients (exp(b) = 2.66, p = .002; exp(b) = 1.10, p = .019, respectively) compared to CU subjects. Higher total (exp(b) = 1.59, p = .013) and centrum semiovale PVS counts (exp(b) = 1.89, p = .016), total (exp(b) = 1.50, p = .036) and WM PVSVF (exp(b) = 1.89, p = .005) together with lower MK‐WM (exp(b) = 0.94, p = .006), mean and left ALPS (exp(b) = 0.91, p = .043; exp(b) = 0.88, p = .010 respectively) were observed in more atrophic AD patients in respect to CU. In addition, more atrophic nAD patients exhibited higher levels of AQP4 (exp(b) = 3.39, p = .002) than CU. Our results indicate significant changes in putative MRI biomarkers of GS and CSF‐AQP4 levels in AD and in other neurodegenerative dementias, suggesting a close interaction between glymphatic dysfunction and neurodegeneration, particularly in the case of AD. However, the usefulness of some of these biomarkers as indirect and standalone indices of glymphatic activity may be hindered by their dependence on disease stage and structural brain damage.
Keywords: Alzheimer disease, aquaporin 4, brain perivascular spaces, cerebrospinal fluid, dementia, diffusion magnetic resonance imaging, glymphatic system, neurodegenerative disease
MRI glymphatic biomarkers were analyzed in 111 prospectively recruited subjects with suspected dementia. Forty nine out of 111 subjects also underwent quantification of CSF‐AQP4. Neurodegeneration is associated with diffuse changes of glymphatic biomarkers and AQP4 levels in the CSF, which are partly dependent on disease stage and measures of structural brain measures.
Practitioner Points.
Diffusion MRI and the quantification of AQP4 in the cerebrospinal fluid may serve as indirect biomarkers of glymphatic activity in vivo.
Neurodegeneration is associated with diffuse changes of MRI parameters of glymphatic activity and AQP4 levels in the CSF.
Changes of glymphatic biomarkers are partly depending on disease stage and measures of structural brain damage.
1. INTRODUCTION
The glymphatic system (GS) is a perivascular network which plays a fundamental role in waste removal from the brain. GS has a three‐compartment organization, in which subarachnoid cerebrospinal fluid (CSF) enters the brain's interstitium from the periarterial spaces facilitated by aquaporin‐4 (AQP4), mixes with the interstitial fluid (ISF) and waste solutes—including pathological proteins like amyloid‐β (Aβ) and tau—and is then drained out of the brain by perivenous efflux routes into meningeal and cervical lymphatics (Bohr et al., 2022; Iliff et al., 2012; Rasmussen et al., 2018).
Gold‐standard in‐vivo evaluation tools for GS require intrathecal (Zhang et al., 2021; Zhou et al., 2020) or intravenous (Zhang, Tang, et al., 2023) administration of gadolinium based contrast agents (GBCA) or radioactive tracers (Schubert et al., 2019), whose relative invasiveness has limited comprehensive research in humans. Recently, there has been a growing effort to identify less invasive and more easily accessible biomarkers of GS function.
Promising magnetic resonance imaging (MRI)‐based techniques have been introduced for an indirect evaluation of glymphatic activity. These include enlarged perivascular spaces (PVS) quantification and diffusion‐based techniques.
PVS are fluid‐filled compartments enveloping brain penetrating arterioles and are considered as a major component of the GS. PVS are known to become enlarged in many clinical conditions. Enlarged PVS in the basal ganglia (BG) are primarily associated with aging and hypertension (Bown et al., 2022; Evans et al., 2023), while enlarged PVS in the centrum semiovale (CSO) have been associated with amyloid deposition (Charidimou et al., 2014; Kim et al., 2021; Perosa et al., 2022) and neurodegeneration. Indeed, impaired glymphatic clearance may limit effective removal of pathological proteins from the brain and clog up the system upstream, leading to further reduction of perivascular drainage, PVS enlargement and ultimately neurodegeneration. Once dilated, PVS can be quantified on MRI using visual scoring scales (Paradise et al., 2020, 2021) or, more accurately, with a wide range of segmentation techniques, spanning from classical image processing approaches to deep neural network modeling (Ballerini et al., 2018; Barisano et al., 2022; Pham et al., 2022; Sepehrband et al., 2019).
Diffusion‐weighted (DW)‐MRI enables the evaluation of brain microstructure by probing microscopic diffusivity of water molecules (Bergamino et al., 2021). Most commonly, diffusion‐related parameters are derived using the diffusion tensor (DT) model (i.e., DT‐imaging: DTI) (Pierpaoli et al., 1996). The DTI‐based analysis along the perivascular spaces (ALPS) was developed to evaluate the diffusivity along the PVS of the deep medullary veins, which is regarded as a proxy measure of glymphatic clearance (Taoka et al., 2017). The usefulness of ALPS index as a non‐invasive biomarker of GS function was supported by a recent comparison with data obtained by intrathecal GBCA tracers (Zhang et al., 2021).
DT is a widely acknowledged model that, however, suffers from several limitations (Jones & Cercignani, 2010). To overcome these limitations, a free‐water (FW) correction algorithm was developed to remove the contribution of extracellular FW to DTI‐derived metrics (Pasternak et al., 2009). Elevated FW in the white matter (WM) has been observed in patients with AD, suggesting impaired fluid drainage likely attributable to glymphatic dysfunction (Dumont et al., 2019; Ji et al., 2017; Kamagata et al., 2022; Maier‐Hein et al., 2015), and the FW index has been proposed as a standalone biomarker of AD‐related pathology (Bergamino et al., 2021; Ji et al., 2017; Kamagata et al., 2022; Maillard et al., 2019).
Diffusion‐kurtosis imaging (DKI) is a higher order diffusion model that represents another evolution of the DTI, accounting for non‐Gaussian diffusion of water molecules (Jensen et al., 2005; Jensen & Helpern, 2010). Metrics derived from DKI, particularly mean kurtosis (MK), have been suggested as putative indices of glymphatic activity (Örzsik et al., 2023). In fact, it was recently shown that the expansion of interstitial space associated with sleep results in a reduction of diffusion non‐Gaussianity and hence of diffusion kurtosis.
Besides MRI‐based markers, recent data suggest a potential utility of quantifying CSF‐AQP4 levels for the detection of glymphatic dysregulation (Bergström et al., 2021). AQP4 is a water channel densely expressed on the perivascular side of astrocytes lining PVS. Its polarized localization is essential for the rapid influx of fluid from periarterial spaces into the brain parenchyma. In neurodegeneration due to AD pathology, AQP4 is known to be over‐expressed and mislocalized away from astrocytic endfeet processes (Boespflug et al., 2018; Zeppenfeld et al., 2017), possibly impairing glymphatic clearance of waste solutes and pathological proteins (Buccellato et al., 2022; Lopes et al., 2024). Consistent with this, levels of AQP4 were found to be increased in the CSF of patients affected by neurodegenerative dementia, particularly AD (Arighi et al., 2022; Bergström et al., 2021). Moreover, preliminary data suggest an association between CSF levels of AQP4, tau protein levels (Bergström et al., 2021) and enlarged PVS burden, particularly in the CSO (Sacchi et al., 2023).
Different biomarkers may preferably mirror alterations in one the three compartments of the GS. Although the precise anatomical substrate of enlarged PVS is still under debate, the overall evidence suggests that they represent periarterial spaces (Wardlaw et al., 2020). Given the close spatial and functional relationship between PVS and AQP4, the latter might primarily represent a marker of periarterial pathophysiological mechanisms. Nonetheless, AQP4 is also expressed on brain capillaries and veins (Iliff et al., 2012), and thus might potentially indicate more widespread aspects of GS dysfunction. FW and MK mainly mirror changes in parenchymal microstructure and fluid content, providing information about ISF, while the DTI‐ALPS index is a measure of the diffusivity along the PVS around the deep medullary veins. Together, all these biomarkers may enable an indirect evaluation of the whole GS in vivo.
Against this background, the principal aim of this study was to evaluate the changes of these putative biomarkers of GS function in patients with neurodegenerative diseases, particularly AD, and with stable mild cognitive impairment (MCI) in respect to healthy elderly people. The secondary aims were to evaluate the reciprocal correlations among GS biomarkers and the correlations between these parameters and established markers of AD pathology and neurodegeneration and cognitive scores.
2. MATERIALS AND METHODS
2.1. Study participants
For this study, we prospectively enrolled a cohort of subjects from those referred to the Neurodegenerative Diseases Unit of the Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico in Milan (Italy) in suspicion of dementia, over a 12‐month period. In addition to the assessments needed to formulate (or exclude) a specific diagnosis of dementia, recruited participants were required to undergo an additional 10‐min long acquisition (i.e., multi‐shell DW‐MRI) at the end of their MRI clinical scan, and to give permission to use their clinical data (in anonymous form) for research purposes. Whenever a CSF examination was clinically appropriate, subjects were given the option to agree to the collection of an extra CSF sample for quantification of AQP4 levels.
At baseline, in all subjects the diagnostic work‐up included past medical history; general and neurological examination; neuropsychological assessment; MRI scanning; and, when appropriate, CSF examination.
All recruited patients were clinically followed‐up every 6 months for at least 1 year, and reclassified as suffering from a progressive neurodegenerative disease (AD or non‐AD type), or a non‐degenerative condition (stable MCI) if they were discharged with no evidence of neurodegeneration and did not show cognitive deterioration at follow‐up. Cognitively unimpaired (CU) individuals presented with subjective memory complaints and underwent an extensive diagnostic work‐up, which did not provide any evidence for cognitive impairment or an ongoing neurodegenerative condition.
Exclusion criteria for enrolment in the study were: (a) previous history of major psychiatric disorders; (b) MRI evidence of remarkable vascular pathology, cerebral amyloid angiopathy (CAA), or intracranial space‐occupying lesions; (c) coarse movement artifacts on MRI scans. The study involving human participants was reviewed and approved by the Local Ethics Committee (Comitato Etico Area 2 Milano, approval N 859_2021, date 14.09.2021). All participants and their legal guardians (when appropriate) were required to provide written informed consent before entering the study.
2.2. MRI acquisition
MRI data were acquired on a Philips Achieva dStream 3 T scanner (Eindhoven, Netherlands) in a single scanning session. All the subjects underwent the same acquisition protocol, which included:
A volumetric high‐resolution T1‐weighted magnetization prepared gradient recalled echo images (MPRAGE; TE = 3.81 ms; TR = 8.27 ms; flip angle = 8°; matrix = 240 × 240 × 180; slice thickness = 1 mm);
A volumetric fast fluid‐attenuated inversion recovery (FLAIR: TE = 300 ms; TR = 5000 ms; TI = 1700 ms; echo‐train‐length = 182, flip angle = 90°; matrix = 240 × 240 × 180; slice thickness = 1 mm; slice spacing = 1 mm)
A multi‐shell DW‐MRI scan (TE = 85 ms, TR = 8400, b values = 1000/2000 smm−2, number of diffusion directions = 32/32, FoV = 240 × 240 mm2, matrix = 96 × 96, number of slices = 60; slice thickness = 2.5 mm, phase encoding = PA, nine b0 images (PA), three additional b0 images (AP)).
Fifty five subjects were scanned in the morning, while the remaining half (56/111) in the afternoon.
2.3. Regions of interest segmentation
T1‐weighted images (T1w) were pre‐processed and segmented using FreeSurfer 7.11 to obtain binary mask of WM and BG. To reduce the odds of PVS misclassification and partial volume effects, the binary WM mask underwent erosion by one voxel, followed by the subtraction of a dilated mask of the lateral ventricles.
2.4. White matter lesion segmentation
White matter lesions (WML) were segmented applying the lesion growth algorithm (LGA) as implemented in the Lesion Segmentation Tool (LST) toolbox version 3.0.0 (www.statistical-modelling.de/lst.html) from Statistical Parametric Mapping (SPM12, Wellcome Trust Centre for Neuroimaging), setting the initial k value to 0.2. The resulting probability map was then binarized using a threshold of 0.5.
Tissue probability maps of gray matter (GM), WM, and CSF generated in the first step of the LGA were also used for total intracranial volume (TIV) calculation. The WML (WMLVF; WMLVF = WML volume/TIV) and GM (GMVF; GMVF = GM volume/TIV) volume fractions were obtained to correct for interindividual brain size variability.
2.5. PVS rating and segmentation
Enlarged PVS were assessed both visually and quantitatively. First, according to Paradise et al. (2020) the number of enlarged PVS in the slices located 2 mm (BG_vis) and 37 mm (CSO_vis) above the anterior commissure and their sum (ALL_vis) were counted by LS, a neurologist trained in PVS scoring. PVS were defined according to the Standards for Reporting Vascular Changes on Neuroimaging (STRIVE) criteria (Duering et al., 2023; Wardlaw et al., 2013). PVS of any diameter were included if they fulfilled other STRIVE diagnostic criteria.
Then, PVS were mapped from T1w images using an automated quantification method as follows. Native T1w images were denoised using the non‐local mean algorithm available in the scikit‐image library 0.21 (https://scikit-image.org/) (van der Walt et al., 2014), N4 bias corrected (Tustison et al., 2010) and skull‐stripped. Then, Frangi filter (Frangi et al., 1998) was applied to preprocessed T1w images using the scikit‐image library 0.21, and setting default parameters with a scale range of 0.5–5 voxels to maximize vessel inclusion. The resulting vesselness map was then binarized using a raw threshold of 0.05 for WM and 0.1 for BG, determined by consensus among LS, FD, and CC after visual inspection of the Frangi output. To eliminate PVS mis‐segmentation caused by WM hyperintensity, WML were excluded from the PVS mask. The PVS volume fraction (PVSVF; PVSVF = PVS volume/TIV) was measured in the WM and BG and then added to obtain the volume of all PVS (ALL).
2.6. FW and MK calculation
DW images were first corrected for susceptibility induced distortions using the FSL TopUp function (FSL, version 6.0.1) and the additional b0 images, followed by eddy‐current correction (Andersson & Sotiropoulos, 2016). To address motion‐associated signal dropout, slice‐wise outliers were estimated and replaced using Gaussian Process prediction (Andersson & Sotiropoulos, 2016).
Maps of the fractional volume of FW and of the MK were constructed from preprocessed DW images fitting a regularized bitensor model and the DKI, respectively, with the open‐source software package Diffusion Imaging in Python (Dipy) algorithm (dipy.org/) (Garyfallidis et al., 2014). A 3D Gaussian smoothing kernel (FWHM = 1.25 mm) was applied to the DW images before fitting the model.
Images were then co‐registered to their corresponding high resolution T1w image using SPM12.
Mean white matter FW (FW‐WM) and MK (MK‐WM) values were measured within each WM mask, after excluding WML and PVS and reslicing the WM mask to match the resolution of the DW images, thus including the normal appearing WM only.
2.7. DTI ALPS
The DTI‐ALPS index was calculated from DW images using a semi‐automated and highly reliable pipeline developed and validated by Taoka, Ito, Nakamichi, Kamagata, et al. (2022).
Fractional anisotropy (FA) and mean diffusivity (MD) maps as well as diffusivity maps along the directions of the x‐axis (right–left; Dxx), y‐axis (anterior–posterior; Dyy), and z‐axis (inferior–superior; Dzz) were obtained from preprocessed DW images applying the DTI model with Dipy. The FA maps from all participants were first linearly and then nonlinearly registered to the high‐resolution FMRIB58_FA standard space image. The subject with the smallest degree of warping was selected for the regions of interest (ROIs) placement. Using this subject's color‐coded FA map, 5 mm spherical ROIs were placed in the projection and association areas at the level of the lateral ventricle bodies in the left and right hemispheres. ROIs positioning was visually checked in each participant, and manual corrections were performed where needed.
The ALPS index was calculated as reported by Taoka et al. (2017). Left hemisphere ALPS (ALPS_left), right hemisphere ALPS (ALPS_right) and their average (ALPS_mean) were obtained. Average FA and MD of the normal appearing WM were also derived.
2.8. CSF AQP4 and protein determination
Available CSF samples were centrifuged at 1500 × g for 10 min at 4°C. The supernatants were aliquoted in polypropylene tubes and stored at −80°C until use. CSF levels of Aβ42, total tau (T‐tau), and phosphorylated tau at 181 (P‐tau) were assessed using either a ChemiLuminescence Enzyme ImmunoAssay (CLEIA) by a Lumipulse G600II platform (Fujirebio, Ghent, Belgium). The following normality thresholds were used: >640 pg/mL for Aβ42, <61 pg/mL for P‐tau, and < 580 pg/mL for T‐tau (Sacchi et al., 2022).
AQP4 concentration was determined in all available samples of CSF using a specific ELISA kit from Cusabio (www.cusabio.com), as detailed elsewhere (Arighi et al., 2022; Sacchi et al., 2023).
2.9. Statistical analysis
Data were analyzed using R. 4.3.1. Considering that, except for MK‐WM, all variables exhibited significant skewness, non‐parametric testing was favored. The Kruskal‐Wallis tests was employed to assess differences in demographic and clinical characteristics among all groups. Generalized linear models were utilized to investigate differences in GS biomarkers, employing a gamma distribution and a log link function, while incrementally entering additional covariates.
In the basic model (Model 1), age, sex, years of formal education and—for MRI indices only—scanning time (dichotomous) were included. Model 2 incorporated WMLVF and Model 3 WMLVF and GMVF as covariates to account for the influence of WM macrostructural damage and GM atrophy. Model 1 was employed to evaluate differences in all biomarkers; Model 2 and 3 were specifically applied when analyzing MRI parameters (i.e., all except AQP4). Two runs of analysis were conducted, the first one including all patients; the second one after dividing both patients with AD and patients with other neurodegenerative diseases into two subgroups, based on the degree of GM atrophy using the median value of GMVF in each subgroup. Statistical significance was set at an uncorrected p‐value of <.05.
Finally, partial Spearman rank correlation tests, adjusted for age, sex and years of education were conducted to assess reciprocal associations among putative GS biomarkers and their potential relationships with AD CSF biomarkers and Mini‐Mental State Examination (MMSE) scores. The false discovery rate (FDR) was used to correct for multiple correlation tests.
3. RESULTS
3.1. Demographic and clinical characteristics of the study population
A total of 111 subjects were included in the study. Seventy one of them were diagnosed as suffering from a neurodegenerative disease according to the most recent diagnostic criteria available for each condition (Armstrong et al., 2013; Crutch et al., 2017; Dubois et al., 2021; Gorno‐Tempini et al., 2011; McKeith et al., 2017; Rascovsky et al., 2011): Forty seven patients with AD while 11 with FTD, 6 with DLB and 7 with other neurodegenerative disease (non‐AD [nAD] = 24). Based on their clinical follow‐up, 17 subjects were diagnosed with stable MCI while 23 were classified as CU individuals.
Fifty out of 111 participants also underwent lumbar puncture for CSF analysis and 49/50 samples were available for AQP4 measurement (nAD = 12, AD = 29, MCI = 4, CU = 4).
Demographic and clinical characteristics of the study population are summarized in Table 1. Notably, MCI patients were less educated than AD patients (p = .02). No differences in age, sex and disease duration were found between groups.
TABLE 1.
Demographics and clinical characteristics of the study population.
| nAD | AD | MCI | CU | p‐Value (fdr) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 24 | n = 47 | n = 17 | n = 23 | nADvsADvs MCIvsCU | nADvs AD | nADvs MCI | ADvs MCI | nADvs CU | Advs CU | MCIvs CU | |
| Sex (M/F) | 9/15 | 22/25 | 8/9 | 14/9 | .36 | ||||||
| Age (y) | 75 (70.75, 77.25) | 71 (67.5,76) | 74 (69,76) | 70 (60, 75.5) | .12 | ||||||
| Education (y) | 13 (7.75, 14.25) | 13 (12,18) | 8 (8,13) | 13 (8, 18) | .042 | .031 | |||||
| Disease duration (y) | 3.02 (2.32, 5.6) | 2.51 (1.69, 4.69) | 2.73 (1.70, 3.48) | 2.80 (2.30, 4.22) | .49 | ||||||
| MMSE (N) | 24 (18.75, 26) | 23 (20.5,26) | 27 (25, 28) | 29 (28, 30) | <.001 | .012 | .001 | <.001 | <.001 | .001 | |
| Aβ42 (pg/mL) | 829 (700, 891) | 496 (435, 591) | 1218 (1158.5, 1330) | 854 (762, 945) | <.001 | <.001 | .038 | .001 | .002 | ||
| T‐tau (pg/mL) | 518 (192, 851) | 669 (407, 922) | 252 (205, 261) | 501 (276, 750) | 0.056 | ||||||
| P‐tau (pg/mL) | 84 (33, 119) | 122 (68, 154) | 34 (30, 35) | 88 (34, 140) | .049 | ||||||
| T‐tau/Aβ42 | 0.78 (0.24, 1.11) | 1.29 (0.78, 1.93) | 0.19 (0.16, 0.21) | 0.53 (0.38, 0.75) | <.001 | .017 | .038 | .002 | .031 | ||
| P‐tau/Αβ42 | 0.12 (0.03, 0.17) | 0.22 (0.15, 0.32) | 0.02 (0.02, 0.03) | 0.09 (0.05, 0.14) | <.001 | .007 | .046 | .002 | .046 | ||
| WMLVF | 0.004 (0.002, 0.008) | 0.003 (0.002, 0.007) | 0.003 (0.001, 0.008) | 0.002 (0.0006, 0.004) | 0.1 | ||||||
| GMVF | 0.371 (0.344, 0.425) | 0.387 (0.354, 0.425) | 0.418 (0.379, 0.454) | 0.421 (0.398, 0.457) | .013 | .03 | .03 | ||||
Note: Data are presented as median (Q1, Q3). N represents the number of patients. p values reported in bold denote statistical significance at p < .05 (Kruskal‐Wallis test, FDR corrected).
Abbreviations: Aβ42, amyloid β 42; AD, Alzheimer's disease patients; CU, cognitively unimpaired subjects; GMVF, gray matter volume fraction; MCI, mild cognitive impairment patients; MMSE, Mini‐Mental State Examination; nAD, non Alzheimer's disease degenerative patients; P‐tau, phosphorylated tau; T‐tau, total tau; WMLVF, white matter lesion volume fraction.
3.2. Group comparisons in the whole cohort
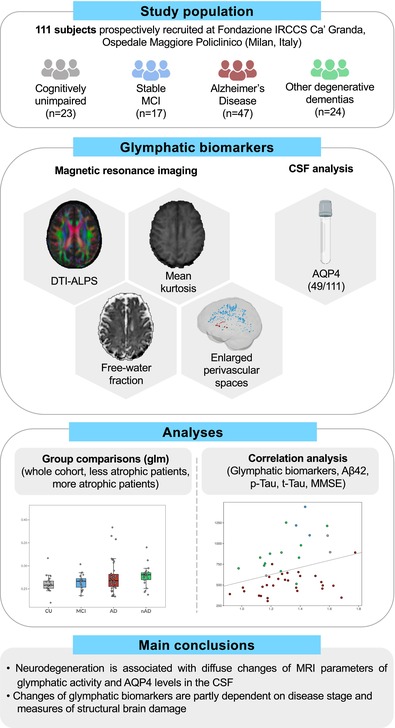
AQP4 levels were significantly higher in AD (exp(b) = 2.05, [Confidence Interval (CI) = 1.25–3.21], p = .005) and nAD patients (exp(b) = 2.98, CI = [1.76–4.85], p < .001) compared to CU individuals. Patients with AD showed a higher FW‐WM (exp(b) = 1.06, CI = [1.00–1.11], p = .043) than CU individuals (Figure 1).
FIGURE 1.
Group comparisons in the whole cohort. Boxplots of the differences in AQP4 levels and FW‐WM among cognitively unimpaired (CU) individuals, patients with MCI, patients with AD and patients with degenerative diseases different from AD (nAD). The p values correspond to the generalized linear model analysis (Model 1). Statistical significance was set at p < .05.
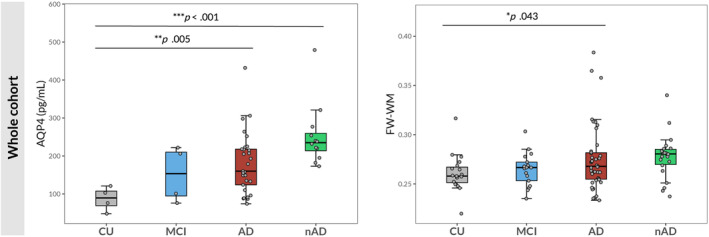
In Models 2 and 3, AD patients still showed significantly higher FW‐WM than CU subjects (exp(b) = 1.06, CI = [1.01–1.12], p = .028 in Model 2; exp(b) = 1.06, CI = [1.01–1.12], p = .031 in Model 3) (Figure 4a).
FIGURE 4.
Heatmaps summarizing significant differences in GS biomarkers among groups. a for MRI biomarkers only. (a) comparisons in the whole cohort; (b) comparisons including AD and nAD patients with lower GM atrophy; (c) comparisons including AD and nAD patients with higher GM atrophy.
3.3. Group comparisons including only degenerative patients with less GM atrophy
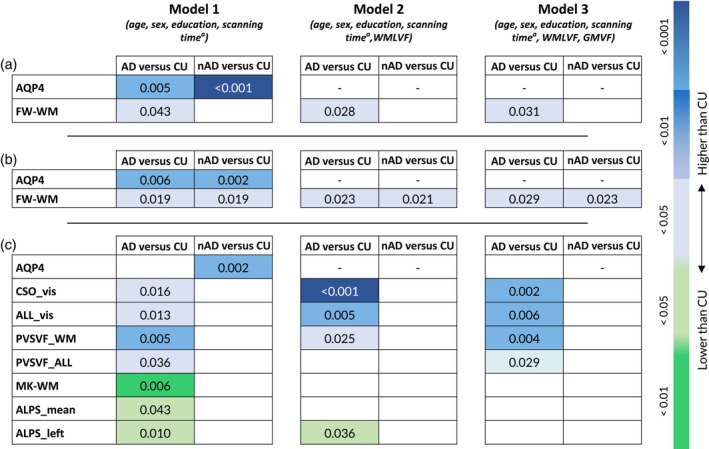
When considering AD (n = 23) and nAD (=12) patients with less GM atrophy, AQP4 and FW‐WM were significantly higher in the AD (exp(b) = 2.20, CI = [1.29–3.59], p = .006; exp(b) = 1.08, CI = [1.01–1.15], p = .019, respectively) and in the nAD groups (exp(b) = 2.66, CI = [1.50–4.62], p = .002; exp(b) = 1.10, CI = [1.02–1.19], p = .019, respectively) compared to CU subjects (Figure 2). In Models 2 and 3, FW‐WM was still higher in both AD (exp(b) = 1.08, CI = [1.01–1.15], p = .023, Model 2; exp(b) = 1.08, CI = [1.01–1.15], p = .029, Model 3) and nAD groups (exp(b) = 1.10, CI = [1.02–1.19], p = .021, Model 2; exp(b) = 1.10, CI = [1.02–1.19], p = .023, Model 3) compared to CU individuals (Figure 4b).
FIGURE 2.
Group including only less atrophic degenerative patients. Boxplots of the differences in AQP4 levels and FW‐WM among cognitively unimpaired (CU) individuals, patients with MCI, patients with AD with lower gray matter atrophy and patients with degenerative diseases different from AD and lower gray matter atrophy (nAD). The p values correspond to the generalized linear model analysis (Model 1). Statistical significance was set at p < .05.
3.4. Group comparisons including only degenerative patients with higher GM atrophy
When focusing on AD (n = 24) and nAD (n = 12) patients with more remarkable GM atrophy, the former group showed higher CSO_vis (exp(b) = 1.89, CI = [1.15–3.10], p = .016), ALL_vis (exp(b) = 1.59, CI = [1.12–2.24], p = .013), PVSVF_WM (exp(b) = 1.89, CI = [1.23–2.90], = 0.005) and PVSVF_ALL (exp(b) = 1.50, CI = [1.04–2.16], p = .036) together with lower MK‐WM (exp(b) = 0.94, CI = [0.90–0.98], p = .006), ALPS_mean (exp(b) = 0.91, CI = [0.84–0.99], p = .043) and ALPS_left (exp(b) = 0.88, CI = [0.81–0.97], p = .010). Moreover, nAD patients showed higher levels of AQP4 (exp(b) = 3.39, CI = [1.76–6.49], p = .002) compared to CU individuals, while, in the same comparison, AD patients showed a trend toward statistical significance only (p = .052) (Figure 3).
FIGURE 3.
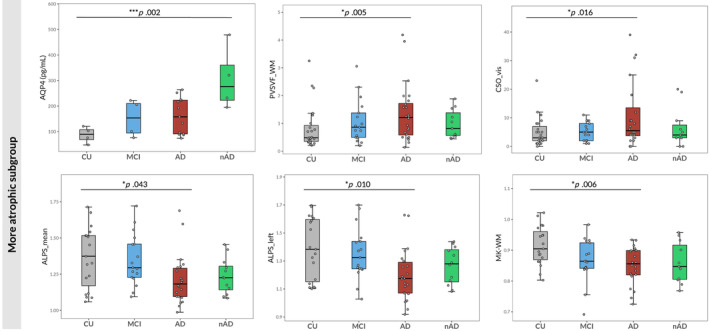
Group comparisons including only more atrophic degenerative patients. Boxplots of the differences in AQP4 levels, PVSVF_WM, visual scores of PVS in the CSO, ALPS_mean, ALPS_left and MK‐WM among cognitively unimpaired (CU) individuals, patients with MCI, patients with AD with higher GM atrophy and patients with degenerative diseases different from AD with higher GM atrophy (nAD). The p values correspond to the generalized linear model analysis (Model 1). Statistical significance was set at p < .05.
In Model 2, CSO_vis (exp(b) = 2.42, CI = [1.47–3.97], p < .001), ALL_vis (exp(b) = 1.72, CI = [1.21–2.46], p = .005) and PVSVF_WM (exp(b) = 1.64, CI = [1.08–2.48], p = .025) remained significantly higher, whereas ALPS_left significantly lower (exp(b) = 0.90, CI = [0.82–0.99], p = .036) in AD patients compared to CU subjects. Conversely, differences in MK‐WM, PVSVF_ALL and ALPS_mean did no longer reach statistical significance.
In Model 3, results obtained in Model 2 were partially preserved. AD patients still showed higher CSO_vis (exp(b) = 2.54, CI = [1.39–4.58], p = .002), ALL_vis (exp(b) = 1.83, CI = [1.19–2.79], p = .006), PVSVF_WM (exp(b) = 2.07, CI = [1.27–3.32], p = .004) and PVSVF_ALL (exp(b) = 1.59, CI[1.05–2.41], p = .029) than CU. However, the observed difference in ALPS_left was no longer significant (Figure 4c).
3.5. Correlation analysis in the whole population
Considering the entire study population, CSO_vis exhibited positive association with increased FW‐WM (p fdr <.001). MK‐WM was positively correlated with ALPS_right and ALPS_mean (rho = 0.29, p fdr = .019; rho = 0.27, p fdr = .046, respectively). GS MRI parameters were not associated with CSF biomarkers of AD nor MMSE in the whole population.
3.6. Correlation analysis in the AD subgroup
When focusing on patients with AD, higher PVSVF_WM, PVSVF_ALL, and CSO_vis were associated with higher FW‐WM (rho = 0.46, p fdr = .018; rho = 0.44, p fdr = .028; rho = 0.52, p fdr = .006 respectively). PVSVF_WM and PVSVF_ALL showed a significant negative correlation with ALPS_right (rho = −0.48, p fdr = .015; rho = −0.37, p fdr = .027 respectively).
After correction for multiple correlations, lower ALPS_right, ALPS_left, and ALPS_mean showed a trend toward association with lower Aβ42 levels (rho = 0.57, p fdr = .095; rho = 0.52, p fdr = .095; rho = 0.53, p fdr = .095 respectively). GS MRI parameters were not associated with MMSE in the AD subgroup.
Uncorrected correlation between GS biomarkers and FA, MD, WMLVF, GMVF, and age are reported in Supplementary Table 1.
4. DISCUSSION
In this study, we combined the use of non‐invasive MRI metrics and measurement of CSF‐AQP4 levels to attempt a comprehensive characterization of the GS function in patients suffering from neurodegenerative dementia, with a specific focus on AD.
According to our a priori hypothesis, we observed significant alterations of all the examined biomarkers in patients with AD and, to a lesser extent, in those with other forms of degenerative dementia when compared to CU individuals. In particular, patients with degenerative diseases exhibited higher CSF‐AQP4 levels and increased FW‐WM compared to CU subjects. Moreover, AD patients compared to CU subjects showed a greater burden of enlarged PVS in the WM, reduced MK and diffusivity along perivascular spaces obtained by ALPS index, though these latter findings were statistically significant only when focusing the analysis on AD patients with higher GM atrophy.
Overall, these findings suggest an alteration of the GS in association with neurodegeneration and AD pathology. However, variations among different patient subgroups and the absence of correlation with cognitive scores require further discussion.
Previous investigations have consistently demonstrated an association between worsening AD pathology and over‐expression and loss of perivascular localization of AQP4 (Boespflug et al., 2018; Zeppenfeld et al., 2017). In line with this, ourselves and other groups have previously described higher levels of AQP4 in the CSF of patients with AD and other forms of degenerative dementia, when compared to healthy controls (Arighi et al., 2022). Here, we replicated the same finding in an entirely new cohort of patients, which indicates robustness of this observation and its potential pathophysiological value. However, AQP4 levels were not correlated with tau, contrary to previous observations from our group (Arighi et al., 2022; Sacchi et al., 2023), and were unrelated to the other biomarkers of glymphatic activity, hindering speculation regarding the pathophysiological origin of AQP4 over‐expression.
Further investigation would help to elucidate whether AQP4 may be considered either a proxy measure of glymphatic dysfunction or rather a marker of reactive astrocytosis in the context of neurodegeneration.
The significantly higher FW in the normal appearing WM of patients affected by AD and other neurodegenerative diseases is consistent with previous data (Bergamino et al., 2021; Dumont et al., 2019; Ji et al., 2017; Kamagata et al., 2022). Indeed, two studies analyzing ADNI data (Dumont et al., 2019; Kamagata et al., 2022), along with a third monocentric study from Singapore (Ji et al., 2017), consistently reported higher FW in the normal appearing WM of AD patients compared to controls. Conversely, we did not observe a significant difference in FW‐WM between MCI patients and CU subjects, aligning with findings by Kamagata et al. (2022), but contrasting with those by Dumont et al. (2019). In our study, increase of FW content was particularly pronounced within the subgroup of neurodegenerative patients exhibiting a lower degree of GM atrophy. Moreover, it was only marginally affected when accounting for WMLVF, in contrast to what has been observed by Kamagata et al. (2022) and GMVF.
Taken altogether, these observations indicate that FW might reflect early interstitial water accumulation, which is at least partially independent from WM damage and GM atrophy. This phenomenon might therefore signify expansion and stasis of ISF (Chad et al., 2018), possibly due to glymphatic dysfunction. However, several non‐mutually exclusive mechanisms, including edema, modulation of BBB permeability, and neuroinflammation (Pasternak et al., 2012) are known to occur and may result in an increase in extracellular water fraction. The observed association between increase FW‐WM and a higher burden of enlarged PVS in the WM may support an interpretation of this metric as a marker of impaired interstitial bulk flow.
Differences in the other biomarkers were only detected in AD patients with a higher degree of GM atrophy compared to CU individuals. This result may prompt some general considerations. First, regarding brain atrophy as a surrogate of disease severity, AQP4, and FW might capture early alterations in glymphatic function, possibly linked to an astrocytic response to damage (Iliff et al., 2014; Ren et al., 2013). In contrast, the remaining biomarkers might reflect later pathophysiological aspects of glymphatic decompensation. This for example, has been suggested for ALPS‐index (Ma et al., 2021). Second, although glymphatic failure is thought to be a common mechanism in neurodegenerative diseases, the distinctive pattern of alterations that we observed in the AD subgroup may imply a specific pathophysiological role of GS dysfunction in AD brains. This is particularly evident in the case of ALPS index and PVS, whose deviations from normal levels were more pronounced in AD patients compared to those with other neurodegenerative dementias. Additional considerations pertaining to individual biomarkers are discussed in the following sections.
When comparing patients with AD and more pronounced GM atrophy against CU subjects, the former showed PVS enlargements more significantly distributed in the WM but not in the BG. Post‐mortem studies have reported direct correlations between PVS in the WM, cortical Aβ deposition, CAA severity, and APOE ε4 polymorphism (Perosa et al., 2022; Roher et al., 2003; van Veluw et al., 2016). Nonetheless, some studies failed to identify an association between PVS in the WM and amyloid load, as assessed by either positron emission tomography (Banerjee et al., 2017; Wang et al., 2021) or CSF‐Aβ levels (Maier‐Hein et al., 2015; Murdock et al., 2024). A possible explanation for this inconsistency is that enlarged PVS in the WM are associated with AD but not Aβ biomarkers because they are manifestations amyloid‐independent processes of AD, for example a tau protein‐related process. In fact, ourselves and other groups have previously described a significant association between tau levels in the CSF (Chad et al., 2018; Maier‐Hein et al., 2015) or tau deposition (Wang et al., 2021) and enlarged PVS, particularly in the CSO (Moses et al., 2022). Indeed, differences in PVS burden in the WM of AD patients were enhanced by the inclusion of WMLVF and GMVF as confounding factors. This suggests that enlarged PVS burden in AD exceeds what is associated with vascular pathology and could be linked to accelerated neurodegeneration secondary to GS dysfunction.
DKI derived metrics were recently identified as a potential biomarker of glymphatic clearance (Örzsik et al., 2023). According to available data, we hypothesized a reduction in MK of the normal appearing WM associated with ISF expansion secondary to GS dysfunction. We observed the expected changes when comparing patients with higher levels of atrophy against CU individuals. This was not the case when comparing the whole cohort of patients or the less atrophic subgroup to CU subjects. Considering GM atrophy as a proxy of disease stage, these findings may be partially explained by the nonlinear relationship between amyloid burden and changes in WM diffusivity (Benitez et al., 2022; Collij et al., 2021) across disease progression. We argue that the less remarkable changes in WM diffusivity seen at early AD stages may reflect a mixture of compensating and inflammatory mechanisms, which are followed by more widespread changes in diffusivity driven by neurodegeneration. Moreover, MK values were strongly dependent on GMVF, aging and measures of WM integrity (Supplementary Table 1). This suggests that MK reduction may primarily reflect myelin sheath damage and decreased axonal density. In contrast to other techniques (e.g., FW), MK is likely unable to single out the increase in interstitial volume fraction due to GS dysfunction and ISF stasis in the context of neurodegeneration.
Overall, previous studies reported a significant decrease in ALPS index of AD patients, and less consistently MCI (Steward et al., 2021; Zhong et al., 2023), as compared to controls (Kamagata et al., 2022; Steward et al., 2021; Taoka et al., 2017; Zhang, Wang, et al., 2023; Zhong et al., 2023). Our findings are partially consistent with these data. We observed a significant reduction of ALPS index in AD patients with more GM atrophy, but not in the less atrophic subgroup. Μοreover, we did not detect any significant difference between patients with MCI and CU, in contrast to results by Steward et al. (2021) and Zhong et al. (2023) but as already described by Kamagata et al. (2022). The inconsistent findings in relation to MCI patients may be due to the lack of consensus about the clinical diagnostic criteria and their operationalization in different studies, leading to prominent heterogeneity of the MCI groups across different studies. Indeed, a recent study by Huang (2024) found significantly lower ALPS index values in amyloid‐positive, but not amyloid negative MCI patients compared to controls. Considering the defining features of our MCI group, this result may help explain our finding. Conversely, the absence of significant differences in ALPS‐indices when incorporating less atrophic patients need to be further addressed. Most of our AD patients could be classified as mild. By contrast, previous studies have mostly included AD patients at more advanced disease stages (Steward et al., 2021; Taoka et al., 2017; Zhang, Wang, et al., 2023; Zhong et al., 2023), which could partially account for some of the inconsistencies. Interestingly, in a recent study by Kamagata et al., a significant reduction of mean ALPS index was detectable even in patients with rather mild AD who were selected from the ADNI database. Given the comparable sample size and the similar statistics, such a discrepancy might be attributable to their incorporation of APOE ε4 as a confounding factor. Unfortunately, we do not have APOE ε4 data available for our cohort of patients to further support this speculation.
Moreover, as already pointed out by Kamagata et al., the differences in ALPS indices were significantly reduced after the inclusion of WMLVF as covariate, and disappeared after the additional incorporation of GMVF in the models, indicating the influence of WML and GM atrophy on measures of glymphatic dysfunction in AD brains (Hsu et al., 2023; Naganawa et al., 2019; Siow et al., 2022; Song et al., 2022; Taoka, Ito, Nakamichi, Nakane, et al., 2022). However, based on their own findings, Hsu et al. have recently proposed that the decline in the ALPS index may precede and partially mediate GMVF alterations in individuals with AD (Hsu et al., 2023). Furthermore, Huang et al. observed that the abnormality of ALPS index prominently increased before the threshold of CSF Aβ42 positivity and then plateaued (Huang, 2024). Nevertheless, in the absence of any conclusive evidence from longitudinal data or animal models (Harrison et al., 2020; Iliff et al., 2012; Peng et al., 2016), an alternative interpretation is that changes in ALPS index might represent a late epiphenomenon of structural brain damage.
The strong association between ALPS index, WM damage and GM atrophy may also explain its association with patient cognitive scores, particularly with the MMSE of more atrophic patients (Hsu et al., 2023; Taoka et al., 2017; Zhang, Wang, et al., 2023; Zhong et al., 2023). We did not detect any association in the whole cohort. This is contrary to findings in most prior studies but aligns with recent research by Matsushita et al. (2023). In both cases, disagreement may be attributable to patient stratification (predominantly very mild to mild AD) and the relatively narrow range of MMSE scores.
As previously described (Kamagata et al., 2022), we also observed a trend toward association between ALPS index in the left hemisphere and CSF‐Αβ42 in the AD subgroup. It is notable that the observed trend was specific to the left ALPS. This could be attributed to anatomical differences between the two hemispheres and the inclusion of patients affected by progressive aphasia, which primarily impacts the left hemisphere. Nonetheless, the relationship between ALPS index and amyloid deposition in the cerebral cortex remains unclear (Hsu et al., 2023; Matsushita et al., 2023) and requires further investigations.
In summary, according to our study, ALPS index may behave like a proxy measure of late GS failure associated with neurodegeneration, rather than an early biomarker of glymphatic dysfunction. On the other hand, the distinct changes observed in ALPS within the AD subgroup and its correlation with Aβ suggest a specific association with accumulation of AD pathology.
5. LIMITATIONS
The major limitation of this study is the lack of direct pathophysiological support to our measures. At the time of writing, only the DTI‐ALPS index had been directly correlated with measures obtained with GBCA CSF tracers (Zhang et al., 2021). However, concerns still remain about its true capacity to represent glymphatic clearance, primarily based on anatomical considerations (Ringstad, 2024). Since the exact histopathologic processes underlying the observed alterations is not fully understood, our results need to be interpreted with caution.
Second, we only evaluated cross‐sectional data from a single memory clinic. Future studies should focus on the longitudinal changes of these biomarkers and their interactions with amyloid and tau protein deposition and measure of cognitive dysfunction.
Third, the subgroups of MCI and CU individuals with available AQP4 dosing were rather small, because we prioritized homogeneity of the sample. However, statistical significances did not change when (1) joining MCI and CU groups AND (2) adding all CU patients with available AQP4 measurements from our lab (8 additional subjects) (Supplementary Figure 1).
Fourth, our PVS segmentation relied solely on T1w images due to the unavailability of T2 volumetric scans, in contrast to what has been suggested by Sepehrband et al. to enhance PVS contrast (Sepehrband et al., 2019). We also acknowledge the limitations of the PVS segmentation method. To deal with this limitation and strengthen our findings, we associated visual rating scores to volumetric PVS quantification.
Another limitation is that AQP4 genetic and APOE ε4 allelic variations were not included as confounding factors due to unavailability. AQP4 mutations are known to modulate glymphatic activity and associate with Aβ clearance (Rainey‐Smith et al., 2018; Sapkota et al., 2022), while APOE allelic mutations may impact the number of PVS (Paradise et al., 2021; Shams et al., 2017). Furthermore, despite the exclusion of subjects with significant vascular brain damage, we did not control for cardiovascular risk factors and sleep disturbances, which are both known to influence glymphatic activity.
Lastly, we used GM atrophy to identify patients at different stages of the disease. Though evidence for this choice is sound (Alzheimer's disease Neuroimaging Initiative1, 2011; Jack et al., 2013; Planche et al., 2022), cognitive scores or severity scale could have been used instead. However, CDR was not available for all the participants, while the MMSE sensitivity is lower in the early stages of the disease due to cognitive reserve (Pettigrew et al., 2023; Zhu et al., 2021) and ceiling effect (Franco‐Marina et al., 2010; Scollard et al., 2023). Moreover, it could not be representative of the disease stage in patients affected by subtypes of progressive aphasia, who were present, though rare, in both the nAD and the AD groups in our study.
6. CONCLUSIONS
Our results revealed that AQP4 and FW‐WM are significantly increased in patients with neurodegenerative dementia compared to CU subjects at the early stages of the disease as assessed by lower levels of GM atrophy. Additionally, we confirmed substantial GS alterations in more advanced AD patients, characterized by an increased burden of enlarged PVS in the WM, lower MK and lower DTI‐ALPS. DTI‐ALPS may also serve as a marker of disease progression in AD.
While the bulk of these findings align with existing literature reporting significant alterations of putative GS biomarkers in neurodegenerative diseases, particularly AD, we hereby describe a heavy dependency of some of these indices, particularly MK‐WM and DTI‐ALPS, on measures of structural brain damage. This may partly hinder their usefulness as standalone and indirect markers of glymphatic activity until validated by comparative studies against established gold standard.
Notwithstanding this limitation, our results suggest a close interaction between glymphatic function and neurodegeneration, particularly in the case of AD. This interaction could potentially serve as a target for dementia prevention, by managing physiological factors like sleep hygiene (Himali et al., 2023) and cardiovascular risk (Xie et al., 2024), which are known to influence GS activity. In addition, in patients who are already suffering from cognitive decline, this might represent a potential target for therapeutic interventions, whether pharmacological (Mohaupt et al., 2023) or physical (Murdock et al., 2024), capable of modulating glymphatic clearance.
AUTHOR CONTRIBUTIONS
LS and AA designed the study, analyzed, and interpreted the data. LS drafted the manuscript. AA, TC, AMP, LG, FD, CC, and MP contributed to the analysis and interpretation of the data. MA, MS, and CF performed CSF analyses. MB, MC, BO, VP, FD, and CC helped in the analysis of MRI data. FT and GC acquired MRI data. AA, MB, LL, and DG drafted and revised the manuscript for intellectual content. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study.
Supporting information
SUPPLEMENTARY FIGURE 1. Comparisons of AQP4 levels among groups. (Left) Boxplots of the differences in AQP4 levels among cognitively unimpaired (CU) individuals + patients with MCI, patients with AD and patients with degenerative diseases different from AD (nAD). (Right) Boxplots of the differences in AQP4 levels among CU including eight more subjects, MCI, AD, and nAD. The p values correspond to the generalized linear model analysis (Model 1). Statistical significance was set at p < .05.
SUPPLEMENTARY TABLE 1. Correlation analysis between biomarkers of glymphatic function, age and measures of white matter and gray matter damage.
ACKNOWLEDGMENTS
This work was supported by grants from the Italian Ministry of Health (Ricerca Corrente). MS. is supported by the Italian Ministry of Health, grant GR‐2019‐12369100.
Sacchi, L. , D'Agata, F. , Campisi, C. , Arcaro, M. , Carandini, T. , Örzsik, B. , Dal Maschio, V. P. , Fenoglio, C. , Pietroboni, A. M. , Ghezzi, L. , Serpente, M. , Pintus, M. , Conte, G. , Triulzi, F. , Lopiano, L. , Galimberti, D. , Cercignani, M. , Bozzali, M. , & Arighi, A. (2024). A “glympse” into neurodegeneration: Diffusion MRI and cerebrospinal fluid aquaporin‐4 for the assessment of glymphatic system in Alzheimer's disease and other dementias. Human Brain Mapping, 45(12), e26805. 10.1002/hbm.26805
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Alzheimer's disease Neuroimaging Initiative1 , Zhang, N. , Song, X. , Zhang, Y. , Chen, W. , D'Arcy, R. C. N. , Darvesh, S. , Fisk, J. D. , & Rockwood, K. (2011). An MRI brain atrophy and lesion index to assess the progression of structural changes in Alzheimer's disease, mild cognitive impairment, and Normal aging: A follow‐up study. Journal of Alzheimer's Disease, 26, 359–367. [DOI] [PubMed] [Google Scholar]
- Andersson, J. L. R. , & Sotiropoulos, S. N. (2016). An integrated approach to correction for off‐resonance effects and subject movement in diffusion MR imaging. NeuroImage, 125, 1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arighi, A. , Arcaro, M. , Fumagalli, G. G. , Carandini, T. , Pietroboni, A. M. , Sacchi, L. , Fenoglio, C. , Serpente, M. , Sorrentino, F. , Isgrò, G. , Turkheimer, F. , Scarpini, E. , & Galimberti, D. (2022). Aquaporin‐4 cerebrospinal fluid levels are higher in neurodegenerative dementia: Looking at glymphatic system dysregulation. Alzheimer's Research & Therapy, 14, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, M. J. , Litvan, I. , Lang, A. E. , Bak, T. H. , Bhatia, K. P. , Borroni, B. , Boxer, A. L. , Dickson, D. W. , Grossman, M. , Hallett, M. , Josephs, K. A. , Kertesz, A. , Lee, S. E. , Miller, B. L. , Reich, S. G. , Riley, D. E. , Tolosa, E. , Tröster, A. I. , Vidailhet, M. , & Weiner, W. J. (2013). Criteria for the diagnosis of corticobasal degeneration. Neurology, 80, 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballerini, L. , Lovreglio, R. , Valdés Hernández, M. C. , Ramirez, J. , MacIntosh, B. J. , Black, S. E. , & Wardlaw, J. M. (2018). Perivascular spaces segmentation in brain MRI using optimal 3D filtering. Scientific Reports, 8, 2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee, G. , Kim, H. J. , Fox, Z. , Jäger, H. R. , Wilson, D. , Charidimou, A. , Na, H. K. , Na, D. L. , Seo, S. W. , & Werring, D. J. (2017). MRI‐visible perivascular space location is associated with Alzheimer's disease independently of amyloid burden. Brain, 140, 1107–1116. [DOI] [PubMed] [Google Scholar]
- Barisano, G. , Lynch, K. M. , Sibilia, F. , Lan, H. , Shih, N. C. , Sepehrband, F. , & Choupan, J. (2022). Imaging perivascular space structure and function using brain MRI. NeuroImage, 257, 119329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez, A. , Jensen, J. H. , Thorn, K. , Dhiman, S. , Fountain‐Zaragoza, S. , Rieter, W. J. , Spampinato, M. V. , Hamlett, E. D. , Nietert, P. J. , Falangola, M. F. , & Helpern, J. A. (2022). Greater diffusion restriction in White matter in preclinical Alzheimer disease. Annals of Neurology, 91, 864–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamino, M. , Walsh, R. R. , & Stokes, A. M. (2021). Free‐water diffusion tensor imaging improves the accuracy and sensitivity of white matter analysis in Alzheimer's disease. Scientific Reports, 11, 6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström, S. , Remnestål, J. , Yousef, J. , Olofsson, J. , Markaki, I. , Carvalho, S. , Corvol, J. C. , Kultima, K. , Kilander, L. , Löwenmark, M. , Ingelsson, M. , Blennow, K. , Zetterberg, H. , Nellgård, B. , Brosseron, F. , Heneka, M. T. , Bosch, B. , Sanchez‐Valle, R. , Månberg, A. , … Nilsson, P. (2021). Multi‐cohort profiling reveals elevated CSF levels of brain‐enriched proteins in Alzheimer's disease. Annals of Clinical Translational Neurology, 8, 1456–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boespflug, E. L. , Simon, M. J. , Leonard, E. , Grafe, M. , Woltjer, R. , Silbert, L. C. , Kaye, J. A. , & Iliff, J. J. (2018). Targeted assessment of enlargement of the perivascular space in Alzheimer's disease and vascular dementia subtypes implicates Astroglial involvement specific to Alzheimer's disease. Journal of Alzheimer's Disease, 66, 1587–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr, T. , Hjorth, P. G. , Holst, S. C. , Hrabětová, S. , Kiviniemi, V. , Lilius, T. , Lundgaard, I. , Mardal, K. A. , Martens, E. A. , Mori, Y. , Nägerl, U. V. , Nicholson, C. , Tannenbaum, A. , Thomas, J. H. , Tithof, J. , Benveniste, H. , Iliff, J. J. , Kelley, D. H. , & Nedergaard, M. (2022). The glymphatic system: Current understanding and modeling. iScience, 25, 104987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bown, C. W. , Carare, R. O. , Schrag, M. S. , & Jefferson, A. L. (2022). Physiology and clinical relevance of enlarged perivascular spaces in the aging brain. Neurology, 98, 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccellato, F. R. , D'Anca, M. , Serpente, M. , Arighi, A. , & Galimberti, D. (2022). The role of glymphatic system in Alzheimer's and Parkinson's disease pathogenesis. Biomedicine, 10, 2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad, J. A. , Pasternak, O. , Salat, D. H. , & Chen, J. J. (2018). Re‐examining age‐related differences in white matter microstructure with free‐water corrected diffusion tensor imaging. Neurobiology of Aging, 71, 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charidimou, A. , Jaunmuktane, Z. , Baron, J. C. , Burnell, M. , Varlet, P. , Peeters, A. , Xuereb, J. , Jäger, R. , Brandner, S. , & Werring, D. J. (2014). White matter perivascular spaces: An MRI marker in pathology‐proven cerebral amyloid angiopathy? Neurology, 82, 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collij, L. E. , Ingala, S. , Top, H. , Wottschel, V. , Stickney, K. E. , Tomassen, J. , Konijnenberg, E. , ten Kate, M. , Sudre, C. , Lopes Alves, I. , Yaqub, M. M. , Wink, A. M. , van ‘t Ent, D. , Scheltens, P. , van Berckel, B. N. M. , Visser, P. J. , Barkhof, F. , & Braber, A. D. (2021). White matter microstructure disruption in early stage amyloid pathology. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring, 13, e12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutch, S. J. , Schott, J. M. , Rabinovici, G. D. , Murray, M. , Snowden, J. S. , van der Flier, W. M. , Dickerson, B. C. , Vandenberghe, R. , Ahmed, S. , Bak, T. H. , Boeve, B. F. , Butler, C. , Cappa, S. F. , Ceccaldi, M. , de Souza, L. C. , Dubois, B. , Felician, O. , Galasko, D. , Graff‐Radford, J. , … Alzheimer's Association ISTAART Atypical Alzheimer's Disease and Associated Syndromes Professional Interest Area . (2017). Consensus classification of posterior cortical atrophy. Alzheimer's & Dementia, 13, 870–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois, B. , Villain, N. , Frisoni, G. B. , Rabinovici, G. D. , Sabbagh, M. , Cappa, S. , Bejanin, A. , Bombois, S. , Epelbaum, S. , Teichmann, M. , Habert, M. O. , Nordberg, A. , Blennow, K. , Galasko, D. , Stern, Y. , Rowe, C. C. , Salloway, S. , Schneider, L. S. , Cummings, J. L. , & Feldman, H. H. (2021). Clinical diagnosis of Alzheimer's disease: Recommendations of the international working group. The Lancet Neurology, 20, 484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duering, M. , Biessels, G. J. , Brodtmann, A. , Chen, C. , Cordonnier, C. , de Leeuw, F. E. , Debette, S. , Frayne, R. , Jouvent, E. , Rost, N. S. , ter Telgte, A. , al‐Shahi Salman, R. , Backes, W. H. , Bae, H. J. , Brown, R. , Chabriat, H. , de Luca, A. , deCarli, C. , Dewenter, A. , … Wardlaw, J. M. (2023). Neuroimaging standards for research into small vessel disease—Advances since 2013. The Lancet Neurology, 22, 602–618. [DOI] [PubMed] [Google Scholar]
- Dumont, M. , Roy, M. , Jodoin, P. M. , Morency, F. C. , Houde, J. C. , Xie, Z. , Bauer, C. , Samad, T. A. , van Dijk, K. R. A. , Goodman, J. A. , Descoteaux, M. , & Alzheimer's Disease Neuroimaging Initiative . (2019). Free water in White matter differentiates MCI and AD from control subjects. Frontiers in Aging Neuroscience, 11, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, T. E. , Knol, M. J. , Schwingenschuh, P. , Wittfeld, K. , Hilal, S. , Ikram, M. A. , Dubost, F. , van Wijnen, K. , Katschnig, P. , Yilmaz, P. , de Bruijne, M. , Habes, M. , Chen, C. , Langer, S. , Völzke, H. , Ikram, M. K. , Grabe, H. J. , Schmidt, R. , Adams, H. H. H. , & Vernooij, M. W. (2023). Determinants of perivascular spaces in the general population: A pooled cohort analysis of individual participant data. Neurology, 100, e107–e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco‐Marina, F. , García‐González, J. J. , Wagner‐Echeagaray, F. , Gallo, J. , Ugalde, O. , Sánchez‐García, S. , Espinel‐Bermúdez, C. , Juárez‐Cedillo, T. , Rodríguez, M. Á. V. , & García‐Peña, C. (2010). The mini‐mental state examination revisited: Ceiling and floor effects after score adjustment for educational level in an aging Mexican population. International Psychogeriatrics, 22, 72–81. [DOI] [PubMed] [Google Scholar]
- Frangi, A. F. , Niessen, W. J. , Vincken, K. L. , & Viergever, M. A. (1998). Multiscale vessel enhancement filtering. In Wells W. M., Colchester A., & Delp S. (Eds.), Medical image computing and computer‐assisted intervention—MICCAI'98 (Vol. 1496, pp. 130–137). Springer. [Google Scholar]
- Garyfallidis, E. , Brett, M. , Amirbekian, B. , Rokem, A. , van der Walt, S. , Descoteaux, M. , Nimmo‐Smith, I. , & Dipy Contributors . (2014). Dipy, a library for the analysis of diffusion MRI data. Frontiers in Neuroinformatics, 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno‐Tempini, M. L. , Hillis, A. E. , Weintraub, S. , Kertesz, A. , Mendez, M. , Cappa, S. F. , Ogar, J. M. , Rohrer, J. D. , Black, S. , Boeve, B. F. , Manes, F. , Dronkers, N. F. , Vandenberghe, R. , Rascovsky, K. , Patterson, K. , Miller, B. L. , Knopman, D. S. , Hodges, J. R. , Mesulam, M. M. , & Grossman, M. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76, 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, I. F. , Ismail, O. , Machhada, A. , Colgan, N. , Ohene, Y. , Nahavandi, P. , Ahmed, Z. , Fisher, A. , Meftah, S. , Murray, T. K. , Ottersen, O. P. , Nagelhus, E. A. , O'Neill, M. J. , Wells, J. A. , & Lythgoe, M. F. (2020). Impaired glymphatic function and clearance of tau in an Alzheimer's disease model. Brain, 143, 2576–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himali, J. J. , Baril, A. A. , Cavuoto, M. G. , Yiallourou, S. , Wiedner, C. D. , Himali, D. , DeCarli, C. , Redline, S. , Beiser, A. S. , Seshadri, S. , & Pase, M. P. (2023). Association between slow‐wave sleep loss and incident dementia. JAMA Neurology, 80, 1326–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, J. , Wei, Y. C. , Toh, C. H. , Hsiao, I. T. , Lin, K. J. , Yen, T. C. , Liao, M. F. , & Ro, L. S. (2023). magnetic resonance images implicate that Glymphatic alterations mediate cognitive dysfunction in alzheimer disease . Annals of Neurology, 93, 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S. , Zhang, Y. R. , Guo, Y. , Du, J. , Ren, P. , Wu, B. S. , Feng, J. F. , Alzheimer's Disease Neuroimaging Initiative , Cheng, W. , & Yu, J. T. (2024). Glymphatic system dysfunction predicts amyloid deposition, neurodegeneration, and clinical progression in Alzheimer's disease. Alzheimer's & Dementia, 20, 3251–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff, J. J. , Chen, M. J. , Plog, B. A. , Zeppenfeld, D. M. , Soltero, M. , Yang, L. , Singh, I. , Deane, R. , & Nedergaard, M. (2014). Impairment of Glymphatic pathway function promotes tau pathology after traumatic brain injury. The Journal of Neuroscience, 34, 16180–16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff, J. J. , Wang, M. , Liao, Y. , Plogg, B. A. , Peng, W. , Gundersen, G. A. , Benveniste, H. , Vates, G. E. , Deane, R. , Goldman, S. A. , Nagelhus, E. A. , & Nedergaard, M. (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, Including Amyloid β. Science Translational Medicine, 4, 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack, C. R. , Knopman, D. S. , Jagust, W. J. , Petersen, R. C. , Weiner, M. W. , Aisen, P. S. , Shaw, L. M. , Vemuri, P. , Wiste, H. J. , Weigand, S. D. , & Lesnick, T. G. (2013). Tracking pathophysiological processes in Alzheimer's disease: An updated hypothetical model of dynamic biomarkers. The Lancet Neurology, 12, 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, J. H. , & Helpern, J. A. (2010). MRI quantification of non‐Gaussian water diffusion by kurtosis analysis. NMR in Biomedicine, 23, 698–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, J. H. , Helpern, J. A. , Ramani, A. , Lu, H. , & Kaczynski, K. (2005). Diffusional kurtosis imaging: The quantification of non‐gaussian water diffusion by means of magnetic resonance imaging. Magnetic Resonance in Medicine, 53, 1432–1440. [DOI] [PubMed] [Google Scholar]
- Ji, F. , Pasternak, O. , Liu, S. , Loke, Y. M. , Choo, B. L. , Hilal, S. , Xu, X. , Ikram, M. K. , Venketasubramanian, N. , Chen, C. L. H. , & Zhou, J. (2017). Distinct white matter microstructural abnormalities and extracellular water increases relate to cognitive impairment in Alzheimer's disease with and without cerebrovascular disease. Alzheimer's Research & Therapy, 9, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D. K. , & Cercignani, M. (2010). Twenty‐five pitfalls in the analysis of diffusion MRI data. NMR in Biomedicine, 23, 803–820. [DOI] [PubMed] [Google Scholar]
- Kamagata, K. , Andica, C. , Takabayashi, K. , Saito, Y. , Taoka, T. , Nozaki, H. , Kikuta, J. , Fujita, S. , Hagiwara, A. , Kamiya, K. , Wada, A. , Akashi, T. , Sano, K. , Nishizawa, M. , Hori, M. , Naganawa, S. , Aoki, S. , & Alzheimer's Disease Neuroimaging Initiative . (2022). Association of MRI indices of glymphatic system with amyloid deposition and cognition in mild cognitive impairment and Alzheimer disease. Neurology, 99, e2648–e2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. J. , Cho, H. , Park, M. , Kim, J. W. , Ahn, S. J. , Lyoo, C. H. , Suh, S. H. , & Ryu, Y. H. (2021). MRI‐visible perivascular spaces in the centrum Semiovale are associated with brain amyloid deposition in patients with Alzheimer disease–related cognitive impairment. AJNR. American Journal of Neuroradiology, 42, 1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes, D. M. , Wells, J. A. , Ma, D. , Wallis, L. , Park, D. , Llewellyn, S. K. , Ahmed, Z. , Lythgoe, M. F. , & Harrison, I. F. (2024). Glymphatic inhibition exacerbates tau propagation in an Alzheimer's disease model. Alzheimer's Research & Therapy, 16, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, X. , Li, S. , Li, C. , Wang, R. , Chen, M. , Chen, H. , & Su, W. (2021). Diffusion tensor imaging along the perivascular space index in different stages of Parkinson's disease. Frontiers in Aging Neuroscience, 13, 773951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier‐Hein, K. H. , Westin, C. F. , Shenton, M. E. , Weiner, M. W. , Raj, A. , Thomann, P. , Kikinis, R. , Stieltjes, B. , & Pasternak, O. (2015). Widespread white matter degeneration preceding the onset of dementia. Alzheimer's & Dementia, 11, 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard, P. , Fletcher, E. , Singh, B. , Martinez, O. , Johnson, D. K. , Olichney, J. M. , Farias, S. T. , & DeCarli, C. (2019). Cerebral white matter free water: A sensitive biomarker of cognition and function. Neurology, 92, e2221–e2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita, S. , Tatekawa, H. , Ueda, D. , Takita, H. , Horiuchi, D. , Tsukamoto, T. , Shimono, T. , & Miki, Y. (2023). The Association of Metabolic Brain mri, amyloid pet, and clinical factors: A study of Alzheimer's disease and Normal controls from the open access series of imaging studies dataset. Magnetic Resonance Imaging, 59, 1341–1348. 10.1002/jmri.28892 [DOI] [PubMed] [Google Scholar]
- McKeith, I. G. , Boeve, B. F. , Dickson, D. W. , Halliday, G. , Taylor, J. P. , Weintraub, D. , Aarsland, D. , Galvin, J. , Attems, J. , Ballard, C. G. , Bayston, A. , Beach, T. G. , Blanc, F. , Bohnen, N. , Bonanni, L. , Bras, J. , Brundin, P. , Burn, D. , Chen‐Plotkin, A. , … Kosaka, K. (2017). Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology, 89, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohaupt, P. , Vialaret, J. , Hirtz, C. , & Lehmann, S. (2023). Readthrough isoform of aquaporin‐4 (AQP4) as a therapeutic target for Alzheimer's disease and other proteinopathies. Alzheimer's Research & Therapy, 15, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses, J. , Sinclair, B. , Schwartz, D. L. , Silbert, L. C. , O'Brien, T. J. , Law, M. , & Vivash, L. (2022). Perivascular spaces as a marker of disease severity and neurodegeneration in patients with behavioral variant frontotemporal dementia. Frontiers in Neuroscience, 16, 1003522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock, M. H. , Yang, C. Y. , Sun, N. , Pao, P. C. , Blanco‐Duque, C. , Kahn, M. C. , Kim, T. H. , Lavoie, N. S. , Victor, M. B. , Islam, M. R. , Galiana, F. , Leary, N. , Wang, S. , Bubnys, A. , Ma, E. , Akay, L. A. , Sneve, M. , Qian, Y. , Lai, C. , … Tsai, L. H. (2024). Multisensory gamma stimulation promotes glymphatic clearance of amyloid. Nature, 627, 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganawa, S. , Nakane, T. , Kawai, H. , & Taoka, T. (2019). Age dependence of gadolinium leakage from the cortical veins into the cerebrospinal fluid assessed with whole brain 3D‐real inversion recovery MR imaging. Magnetic Resonance in Medical Sciences, 18, 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Örzsik, B. , Palombo, M. , Asllani, I. , Dijk, D. J. , Harrison, N. A. , & Cercignani, M. (2023). Higher order diffusion imaging as a putative index of human sleep‐related microstructural changes and glymphatic clearance. NeuroImage, 274, 120124. [DOI] [PubMed] [Google Scholar]
- Paradise, M. , Crawford, J. D. , Lam, B. C. P. , Wen, W. , Kochan, N. A. , Makkar, S. , Dawes, L. , Trollor, J. , Draper, B. , Brodaty, H. , & Sachdev, P. S. (2021). Association of dilated perivascular spaces with cognitive decline and incident dementia. Neurology, 96, e1501–e1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradise, M. B. , Beaudoin, M. S. , Dawes, L. , Crawford, J. D. , Wen, W. , Brodaty, H. , & Sachdev, P. S. (2020). Development and validation of a rating scale for perivascular spaces on 3T MRI. Journal of the Neurological Sciences, 409, 116621. [DOI] [PubMed] [Google Scholar]
- Pasternak, O. , Shenton, M. E. , & Westin, C.‐F. (2012). Estimation of extracellular volume from regularized multi‐shell diffusion MRI. In Ayache N., Delingette H., Golland P., & Mori K. (Eds.), Medical image computing and computer‐assisted intervention—MICCAI 2012 (Vol. 7511, pp. 305–312). Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak, O. , Sochen, N. , Gur, Y. , Intrator, N. , & Assaf, Y. (2009). Free water elimination and mapping from diffusion MRI. Magnetic Resonance in Medicine, 62, 717–730. [DOI] [PubMed] [Google Scholar]
- Peng, W. , Achariyar, T. M. , Li, B. , Liao, Y. , Mestre, H. , Hitomi, E. , Regan, S. , Kasper, T. , Peng, S. , Ding, F. , Benveniste, H. , Nedergaard, M. , & Deane, R. (2016). Suppression of glymphatic fluid transport in a mouse model of Alzheimer's disease. Neurobiology of Disease, 93, 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perosa, V. , Oltmer, J. , Munting, L. P. , Freeze, W. M. , Auger, C. A. , Scherlek, A. A. , van der Kouwe, A. J. , Iglesias, J. E. , Atzeni, A. , Bacskai, B. J. , Viswanathan, A. , Frosch, M. P. , Greenberg, S. M. , & van Veluw, S. J. (2022). Perivascular space dilation is associated with vascular amyloid‐β accumulation in the overlying cortex. Acta Neuropathologica, 143, 331–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew, C. , Nazarovs, J. , Soldan, A. , Singh, V. , Wang, J. , Hohman, T. , Dumitrescu, L. , Libby, J. , Kunkle, B. , Gross, A. L. , Johnson, S. , Lu, Q. , Engelman, C. , Masters, C. L. , Maruff, P. , Laws, S. M. , Morris, J. C. , Hassenstab, J. , Cruchaga, C. , … Albert, M. (2023). Alzheimer's disease genetic risk and cognitive reserve in relationship to long‐term cognitive trajectories among cognitively normal individuals. Alzheimer's Research & Therapy, 15, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham, W. , Lynch, M. , Spitz, G. , O'Brien, T. , Vivash, L. , Sinclair, B. , & Law, M. (2022). A critical guide to the automated quantification of perivascular spaces in magnetic resonance imaging. Frontiers in Neuroscience, 16, 1021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli, C. , Jezzard, P. , Basser, P. J. , Barnett, A. , & Di Chiro, G. (1996). Diffusion tensor MR imaging of the human brain. Radiology, 201, 637–648. [DOI] [PubMed] [Google Scholar]
- Planche, V. , Manjon, J. V. , Mansencal, B. , Lanuza, E. , Tourdias, T. , Catheline, G. , & Coupé, P. (2022). Structural progression of Alzheimer's disease over decades: The MRI staging scheme. Brain Communications, 4, fcac109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey‐Smith, S. R. , Mazzucchelli, G. N. , Villemagne, V. L. , Brown, B. M. , Porter, T. , Weinborn, M. , Bucks, R. S. , Milicic, L. , Sohrabi, H. R. , Taddei, K. , Ames, D. , Maruff, P. , Masters, C. L. , Rowe, C. C. , Salvado, O. , Martins, R. N. , Laws, S. M. , & for the AIBL Research Group . (2018). Genetic variation in Aquaporin‐4 moderates the relationship between sleep and brain Aβ‐amyloid burden. Translational Psychiatry, 8, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky, K. , Hodges, J. R. , Knopman, D. , Mendez, M. F. , Kramer, J. H. , Neuhaus, J. , van Swieten, J. C. , Seelaar, H. , Dopper, E. G. P. , Onyike, C. U. , Hillis, A. E. , Josephs, K. A. , Boeve, B. F. , Kertesz, A. , Seeley, W. W. , Rankin, K. P. , Johnson, J. K. , Gorno‐Tempini, M. L. , Rosen, H. , … Miller, B. L. (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain, 134, 2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen, M. K. , Mestre, H. , & Nedergaard, M. (2018). The glymphatic pathway in neurological disorders. The Lancet Neurology, 17, 1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, Z. , Iliff, J. J. , Yang, L. , Yang, J. , Chen, X. , Chen, M. J. , Giese, R. N. , Wang, B. , Shi, X. , & Nedergaard, M. (2013). ‘Hit & run’ model of closed‐skull traumatic brain injury (TBI) reveals complex patterns of post‐traumatic AQP4 dysregulation. Journal of Cerebral Blood Flow and Metabolism, 33, 834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstad, G. (2024). Glymphatic imaging: A critical look at the DTI‐ALPS index. Neuroradiology, 66, 157–160. [DOI] [PubMed] [Google Scholar]
- Roher, A. E. , Kuo, Y. M. , Esh, C. , Knebel, C. , Weiss, N. , Kalback, W. , Luehrs, D. C. , Childress, J. L. , Beach, T. G. , Weller, R. O. , & Kokjohn, T. A. (2003). Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer's disease. Molecular Medicine, 9, 112–122. [PMC free article] [PubMed] [Google Scholar]
- Sacchi, L. , Arcaro, M. , Carandini, T. , Pietroboni, A. M. , Fumagalli, G. G. , Fenoglio, C. , Serpente, M. , Sorrentino, F. , Visconte, C. , Pintus, M. , Conte, G. , Contarino, V. E. , Scarpini, E. , Triulzi, F. , Galimberti, D. , & Arighi, A. (2023). Association between enlarged perivascular spaces and cerebrospinal fluid aquaporin‐4 and tau levels: Report from a memory clinic. Frontiers in Aging Neuroscience, 15, 1191714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi, L. , Carandini, T. , Fumagalli, G. G. , Pietroboni, A. M. , Contarino, V. E. , Siggillino, S. , Arcaro, M. , Fenoglio, C. , Zito, F. , Marotta, G. , Castellani, M. , Triulzi, F. , Galimberti, D. , Scarpini, E. , & Arighi, A. (2022). Unravelling the association between amyloid‐PET and cerebrospinal fluid biomarkers in the Alzheimer's disease Spectrum: Who really deserves an a+? Journal of Alzheimer's Disease, 85, 1009–1020. [DOI] [PubMed] [Google Scholar]
- Sapkota, D. , Florian, C. , Doherty, B. M. , White, K. M. , Reardon, K. M. , Ge, X. , Garbow, J. R. , Yuede, C. M. , Cirrito, J. R. , & Dougherty, J. D. (2022). Aqp4 stop codon readthrough facilitates amyloid‐β clearance from the brain. Brain, 145, 2982–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert, J. J. , Veronese, M. , Marchitelli, L. , Bodini, B. , Tonietto, M. , Stankoff, B. , Brooks, D. J. , Bertoldo, A. , Edison, P. , & Turkheimer, F. E. (2019). Dynamic11 C‐PiB PET shows cerebrospinal fluid flow alterations in Alzheimer disease and multiple sclerosis. Journal of Nuclear Medicine, 60, 1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scollard, P. , Choi, S. E. , Lee, M. L. , Mukherjee, S. , Trittschuh, E. H. , Sanders, R. E. , Gibbons, L. E. , Joshi, P. , Devine, S. , Au, R. , Dams‐O'Connor, K. , Saykin, A. J. , Seshadri, S. , Beiser, A. , Aparicio, H. J. , Salinas, J. , Gonzales, M. M. , Pase, M. P. , Ghosh, S. , … Crane, P. K. (2023). Ceiling effects and differential measurement precision across calibrated cognitive scores in the Framingham study. Neuropsychology, 37, 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehrband, F. , Barisano, G. , Sheikh‐Bahaei, N. , Cabeen, R. P. , Choupan, J. , Law, M. , & Toga, A. W. (2019). Image processing approaches to enhance perivascular space visibility and quantification using MRI. Scientific Reports, 9, 12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams, S. , Martola, J. , Charidimou, A. , Larvie, M. , Granberg, T. , Shams, M. , Kristoffersen‐Wiberg, M. , & Wahlund, L. O. (2017). Topography and determinants of magnetic resonance imaging (MRI)‐visible perivascular spaces in a large memory clinic cohort. Journal of the American Heart Association, 6, e006279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siow, T. Y. , Toh, C. H. , Hsu, J. L. , Liu, G. H. , Lee, S. H. , Chen, N. H. , Fu, C. J. , Castillo, M. , & Fang, J. T. (2022). Association of Sleep, neuropsychological performance, and gray matter volume with Glymphatic function in community‐dwelling older adults. Neurology, 98, e829–e838. [DOI] [PubMed] [Google Scholar]
- Song, H. , Ruan, Z. , Gao, L. , Lv, D. , Sun, D. , Li, Z. , Zhang, R. , Zhou, X. , Xu, H. , & Zhang, J. (2022). Structural network efficiency mediates the association between glymphatic function and cognition in mild VCI: A DTI‐ALPS study. Frontiers in Aging Neuroscience, 14, 974114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward, C. E. , Venkatraman, V. K. , Lui, E. , Malpas, C. B. , Ellis, K. A. , Cyarto, E. V. , Vivash, L. , O'Brien, T. J. , Velakoulis, D. , Ames, D. , Masters, C. L. , Lautenschlager, N. T. , Bammer, R. , & Desmond, P. M. (2021). Assessment of the DTI‐ALPS parameter along the perivascular space in older adults at risk of dementia. Journal of Neuroimaging, 31, 569–578. [DOI] [PubMed] [Google Scholar]
- Taoka, T. , Ito, R. , Nakamichi, R. , Kamagata, K. , Sakai, M. , Kawai, H. , Nakane, T. , Abe, T. , Ichikawa, K. , Kikuta, J. , Aoki, S. , & Naganawa, S. (2022). Reproducibility of diffusion tensor image analysis along the perivascular space (DTI‐ALPS) for evaluating interstitial fluid diffusivity and glymphatic function: CHanges in Alps index on multiple conditiON acquIsition eXperiment (CHAMONIX) study. Japanese Journal of Radiology, 40, 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoka, T. , Ito, R. , Nakamichi, R. , Nakane, T. , Sakai, M. , Ichikawa, K. , Kawai, H. , & Naganawa, S. (2022). Diffusion‐weighted image analysis along the perivascular space (DWI–ALPS) for evaluating interstitial fluid status: Age dependence in normal subjects. Japanese Journal of Radiology, 40, 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoka, T. , Masutani, Y. , Kawai, H. , Nakane, T. , Matsuoka, K. , Yasuno, F. , Kishimoto, T. , & Naganawa, S. (2017). Evaluation of glymphatic system activity with the diffusion MR technique: Diffusion tensor image analysis along the perivascular space (DTI‐ALPS) in Alzheimer's disease cases. Japanese Journal of Radiology, 35, 172–178. [DOI] [PubMed] [Google Scholar]
- Tustison, N. J. , Avants, B. B. , Cook, P. A. , Zheng, Y. , Egan, A. , Yushkevich, P. A. , & Gee, J. C. (2010). N4ITK: Improved N3 bias correction. IEEE Transactions on Medical Imaging, 29, 1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Walt, S. , Schönberger, J. L. , Nunez‐Iglesias, J. , Boulogne, F. , Warner, J. D. , Yager, N. , Gouillart, E. , Yu, T. , & scikit‐image contributors . (2014). Scikit‐image: Image processing in python. PeerJ, 2, e453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veluw, S. J. , Biessels, G. J. , Bouvy, W. H. , Spliet, W. G. M. , Zwanenburg, J. J. M. , Luijten, P. R. , Macklin, E. A. , Rozemuller, A. J. M. , Gurol, M. E. , Greenberg, S. M. , Viswanathan, A. , & Martinez‐Ramirez, S. (2016). Cerebral amyloid angiopathy severity is linked to dilation of juxtacortical perivascular spaces. Journal of Cerebral Blood Flow and Metabolism, 36, 576–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M.‐L. , Yu, M.‐M. , Wei, X.‐E. , Li, W.‐B. , & Li, Y.‐H. (2021). Association of enlarged perivascular spaces with Aβ and tau deposition in cognitively normal older population. Neurobiology of Aging, 100, 32–38. [DOI] [PubMed] [Google Scholar]
- Wardlaw, J. M. , Benveniste, H. , Nedergaard, M. , Zlokovic, B. V. , Mestre, H. , Lee, H. , Doubal, F. N. , Brown, R. , Ramirez, J. , MacIntosh, B. J. , Tannenbaum, A. , Ballerini, L. , Rungta, R. L. , Boido, D. , Sweeney, M. , Montagne, A. , Charpak, S. , Joutel, A. , Smith, K. J. , … colleagues from the Fondation Leducq Transatlantic Network of Excellence on the Role of the Perivascular Space in Cerebral Small Vessel Disease . (2020). Perivascular spaces in the brain: Anatomy, physiology and pathology. Nature Reviews. Neurology, 16, 137–153. [DOI] [PubMed] [Google Scholar]
- Wardlaw, J. M. , Smith, E. E. , Biessels, G. J. , Cordonnier, C. , Fazekas, F. , Frayne, R. , Lindley, R. I. , O'Brien, J. T. , Barkhof, F. , Benavente, O. R. , Black, S. E. , Brayne, C. , Breteler, M. , Chabriat, H. , Decarli, C. , de Leeuw, F. E. , Doubal, F. , Duering, M. , Fox, N. C. , … STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1) . (2013). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. The Lancet Neurology, 12, 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, L. , Zhang, Y. , Hong, H. , Xu, S. , Cui, L. , Wang, S. , Li, J. , Liu, L. , Lin, M. , Luo, X. , Li, K. , Zeng, Q. , Zhang, M. , Zhang, R. , & Huang, P. (2024). Higher intracranial arterial pulsatility is associated with presumed imaging markers of the glymphatic system: An explorative study. NeuroImage, 288, 120524. [DOI] [PubMed] [Google Scholar]
- Zeppenfeld, D. M. , Simon, M. , Haswell, J. D. , D'Abreo, D. , Murchison, C. , Quinn, J. F. , Grafe, M. R. , Woltjer, R. L. , Kaye, J. , & Iliff, J. J. (2017). Association of perivascular localization of aquaporin‐4 with cognition and Alzheimer disease in aging brains. JAMA Neurology, 74, 91–99. [DOI] [PubMed] [Google Scholar]
- Zhang, M. , Tang, J. , Xia, D. , Xue, Y. , Ren, X. , Huang, Q. , Shi, L. , Tang, W. , & Fu, J. (2023). Evaluation of glymphatic‐meningeal lymphatic system with intravenous gadolinium‐based contrast‐enhancement in cerebral small‐vessel disease. European Radiology, 33, 6096–6106. [DOI] [PubMed] [Google Scholar]
- Zhang, W. , Zhou, Y. , Wang, J. , Gong, X. , Chen, Z. , Zhang, X. , Cai, J. , Chen, S. , Fang, L. , Sun, J. , & Lou, M. (2021). Glymphatic clearance function in patients with cerebral small vessel disease. NeuroImage, 238, 118257. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Wang, Y. , Jiao, B. , Wang, Z. , Shi, J. , Zhang, Y. , Bai, X. , Li, Z. , Li, S. , Bai, R. , & Sui, B. (2023). Glymphatic system impairment in Alzheimer's disease: Associations with perivascular space volume and cognitive function. European Radiology, 34, 1314–1323. 10.1007/s00330-023-10122-3 [DOI] [PubMed] [Google Scholar]
- Zhong, J. , Zhang, X. , Xu, H. , Zheng, X. , Wang, L. , Jiang, J. , & Li, Y. (2023). Unlocking the enigma: Unraveling multiple cognitive dysfunction linked to glymphatic impairment in early Alzheimer's disease. Frontiers in Neuroscience, 17, 1222857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Cai, J. , Zhang, W. , Gong, X. , Yan, S. , Zhang, K. , Luo, Z. , Sun, J. , Jiang, Q. , & Lou, M. (2020). Impairment of the Glymphatic pathway and putative meningeal lymphatic vessels in the aging human. Annals of Neurology, 87, 357–369. [DOI] [PubMed] [Google Scholar]
- Zhu, W. , Li, X. , Li, X. , Wang, H. , Li, M. , Gao, Z. , Wu, X. , Tian, Y. , Zhou, S. , Wang, K. , & Yu, Y. (2021). The protective impact of education on brain structure and function in Alzheimer's disease. BMC Neurology, 21, 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY FIGURE 1. Comparisons of AQP4 levels among groups. (Left) Boxplots of the differences in AQP4 levels among cognitively unimpaired (CU) individuals + patients with MCI, patients with AD and patients with degenerative diseases different from AD (nAD). (Right) Boxplots of the differences in AQP4 levels among CU including eight more subjects, MCI, AD, and nAD. The p values correspond to the generalized linear model analysis (Model 1). Statistical significance was set at p < .05.
SUPPLEMENTARY TABLE 1. Correlation analysis between biomarkers of glymphatic function, age and measures of white matter and gray matter damage.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


