Abstract
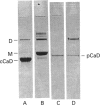
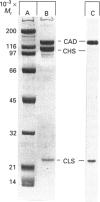
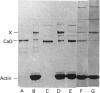
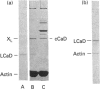
It was reported that chicken gizzard smooth-muscle caldesmon Cys-580 can be disulphide-cross-linked to the C-terminal pen-ultimate residue (Cys-374) of actin, indicating that these residues are close in the protein complex [Graceffa, P. and Jancso, A. (1991) J. Biol. Chem. 266, 20305-20310]. Since the possibility that the cross-link involves a cysteine residue other than actin Cys-374 was not absolutely excluded, more direct evidence was sought for the identify of the cysteine residues involved in the cross-link. We show here that caldesmon could not be disulphide-cross-linked to actin which had Cys-374 removed by carboxypeptidase A digestion, providing direct support for the participation of actin Cys-374 in the cross-link to caldesmon. In order to assign the caldesmon cysteine residue involved in the cross-link, use was made of caldesmon from porcine stomach muscle, which is shown to contain one cysteine residue close to, or at, position 580, in contrast with chicken gizzard caldesmon, which has an additional cysteine residue at position 153. The porcine stomach caldesmon also formed a disulphide-cross-link to actin, further supporting the original conclusion that Cys-580 of the chicken gizzard caldesmon had been cross-linked to actin. Disulphide-cross-linking with similar yield was also observed in native chicken gizzard muscle thin filaments, indicating that the interaction between actin and the C-terminal domain of caldesmon is the same in native and reconstituted thin filaments. The much smaller non-muscle isoform of caldesmon, from rabbit liver, could be similarly cross-linked to actin, consistent with the sequence similarity between the C-terminal domain of muscle and non-muscle caldesmon. The ability to cross-link caldesmon Cys-580 to actin Cys-374 suggests the possibility that the Cys-580 region of caldesmon and the C-terminus of actin form part of the actin-caldesmon binding interface.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam L. P., Gapinski C. J., Hathaway D. R. Phosphorylation sequences in h-caldesmon from phorbol ester-stimulated canine aortas. FEBS Lett. 1992 May 18;302(3):223–226. doi: 10.1016/0014-5793(92)80446-n. [DOI] [PubMed] [Google Scholar]
- Adam L. P., Haeberle J. R., Hathaway D. R. Phosphorylation of caldesmon in arterial smooth muscle. J Biol Chem. 1989 May 5;264(13):7698–7703. [PubMed] [Google Scholar]
- Bartegi A., Fattoum A., Kassab R. Cross-linking of smooth muscle caldesmon to the NH2-terminal region of skeletal F-actin. J Biol Chem. 1990 Feb 5;265(4):2231–2237. [PubMed] [Google Scholar]
- Brauer M., Sykes B. D. 19F nuclear magnetic resonance studies of selectively fluorinated derivatives of G- and F-actin. Biochemistry. 1986 Apr 22;25(8):2187–2191. doi: 10.1021/bi00356a050. [DOI] [PubMed] [Google Scholar]
- Bryan J., Imai M., Lee R., Moore P., Cook R. G., Lin W. G. Cloning and expression of a smooth muscle caldesmon. J Biol Chem. 1989 Aug 15;264(23):13873–13879. [PubMed] [Google Scholar]
- Bryan J., Lee R. Sequence of an avian non-muscle caldesmon. J Muscle Res Cell Motil. 1991 Aug;12(4):372–375. doi: 10.1007/BF01738592. [DOI] [PubMed] [Google Scholar]
- Chantler P. D., Tao T., Stafford W. F., 3rd On the relationship between distance information derived from cross-linking and from resonance energy transfer, with specific reference to sites located on myosin heads. Biophys J. 1991 Jun;59(6):1242–1250. doi: 10.1016/S0006-3495(91)82339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosbie R., Adams S., Chalovich J. M., Reisler E. The interaction of caldesmon with the COOH terminus of actin. J Biol Chem. 1991 Oct 25;266(30):20001–20006. [PMC free article] [PubMed] [Google Scholar]
- Drabikowski W., Lehrer S., Nagy B., Gergely J. Loss of Cu2+-binding to actin upon removal of the C-terminal phenylalanine by carboxypeptidase A. Arch Biochem Biophys. 1977 May;181(1):359–361. doi: 10.1016/0003-9861(77)90515-x. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Field V. L., Bowen W. J. The C-terminal residue of actin and its role in reactions of actin and myosin. Arch Biochem Biophys. 1968 Sep 20;127(1):59–64. doi: 10.1016/0003-9861(68)90201-4. [DOI] [PubMed] [Google Scholar]
- Fürst D. O., Cross R. A., De Mey J., Small J. V. Caldesmon is an elongated, flexible molecule localized in the actomyosin domains of smooth muscle. EMBO J. 1986 Feb;5(2):251–257. doi: 10.1002/j.1460-2075.1986.tb04206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graceffa P., Jancsó A. Disulfide cross-linking of caldesmon to actin. J Biol Chem. 1991 Oct 25;266(30):20305–20310. [PubMed] [Google Scholar]
- Hayashi K., Fujio Y., Kato I., Sobue K. Structural and functional relationships between h- and l-caldesmons. J Biol Chem. 1991 Jan 5;266(1):355–361. [PubMed] [Google Scholar]
- Hayashi K., Kanda K., Kimizuka F., Kato I., Sobue K. Primary structure and functional expression of h-caldesmon complementary DNA. Biochem Biophys Res Commun. 1989 Oct 16;164(1):503–511. doi: 10.1016/0006-291x(89)91748-8. [DOI] [PubMed] [Google Scholar]
- Horiuchi K. Y., Chacko S. Caldesmon inhibits the cooperative turning-on of the smooth muscle heavy meromyosin by tropomyosin-actin. Biochemistry. 1989 Nov 14;28(23):9111–9116. doi: 10.1021/bi00449a023. [DOI] [PubMed] [Google Scholar]
- Humphrey M. B., Herrera-Sosa H., Gonzalez G., Lee R., Bryan J. Cloning of cDNAs encoding human caldesmons. Gene. 1992 Mar 15;112(2):197–204. doi: 10.1016/0378-1119(92)90376-z. [DOI] [PubMed] [Google Scholar]
- Jacobson G. R., Schaffer M. H., Stark G. R., Vanaman T. C. Specific chemical cleavage in high yield at the amino peptide bonds of cysteine and cystine residues. J Biol Chem. 1973 Oct 10;248(19):6583–6591. [PubMed] [Google Scholar]
- Johannes F. J., Gallwitz D. Site-directed mutagenesis of the yeast actin gene: a test for actin function in vivo. EMBO J. 1991 Dec;10(12):3951–3958. doi: 10.1002/j.1460-2075.1991.tb04965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama E. Assignment of the positions of chymotryptic fragments and cysteinyl groups in the primary structure of caldesmon in relation to a conformational change. J Biochem. 1989 Dec;106(6):988–993. doi: 10.1093/oxfordjournals.jbchem.a122987. [DOI] [PubMed] [Google Scholar]
- Kołakowski J., Makuch R., Dabrowska R. Lys-373 of actin is involved in binding to caldesmon. FEBS Lett. 1992 Aug 31;309(1):65–67. doi: 10.1016/0014-5793(92)80740-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lash J. A., Sellers J. R., Hathaway D. R. The effects of caldesmon on smooth muscle heavy actomeromyosin ATPase activity and binding of heavy meromyosin to actin. J Biol Chem. 1986 Dec 5;261(34):16155–16160. [PubMed] [Google Scholar]
- Levine B. A., Moir A. J., Audemard E., Mornet D., Patchell V. B., Perry S. V. Structural study of gizzard caldesmon and its interaction with actin. Binding involves residues of actin also recognised by myosin subfragment 1. Eur J Biochem. 1990 Nov 13;193(3):687–696. doi: 10.1111/j.1432-1033.1990.tb19388.x. [DOI] [PubMed] [Google Scholar]
- Liu D. F., Wang D., Stracher A. The accessibility of the thiol groups on G- and F-actin of rabbit muscle. Biochem J. 1990 Mar 1;266(2):453–459. doi: 10.1042/bj2660453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch W. P., Riseman V. M., Bretscher A. Smooth muscle caldesmon is an extended flexible monomeric protein in solution that can readily undergo reversible intra- and intermolecular sulfhydryl cross-linking. A mechanism for caldesmon's F-actin bundling activity. J Biol Chem. 1987 May 25;262(15):7429–7437. [PubMed] [Google Scholar]
- Makuch R., Kołakowski J., Dabrowska R. The importance of C-terminal amino acid residues of actin to the inhibition of actomyosin ATPase activity by caldesmon and troponin I. FEBS Lett. 1992 Feb 10;297(3):237–240. doi: 10.1016/0014-5793(92)80546-s. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Lehman W. Caldesmon is a Ca2+-regulatory component of native smooth-muscle thin filaments. Biochem J. 1985 Nov 1;231(3):517–522. doi: 10.1042/bj2310517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S. B., Redwood C. S. Inhibition of actin-tropomyosin activation of myosin MgATPase activity by the smooth muscle regulatory protein caldesmon. J Biol Chem. 1992 Aug 25;267(24):16796–16800. [PubMed] [Google Scholar]
- Marston S. B., Redwood C. S. The molecular anatomy of caldesmon. Biochem J. 1991 Oct 1;279(Pt 1):1–16. doi: 10.1042/bj2790001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossakowska M., Moraczewska J., Khaitlina S., Strzelecka-Golaszewska H. Proteolytic removal of three C-terminal residues of actin alters the monomer-monomer interactions. Biochem J. 1993 Feb 1;289(Pt 3):897–902. doi: 10.1042/bj2890897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novy R. E., Lin J. L., Lin J. J. Characterization of cDNA clones encoding a human fibroblast caldesmon isoform and analysis of caldesmon expression in normal and transformed cells. J Biol Chem. 1991 Sep 5;266(25):16917–16924. [PubMed] [Google Scholar]
- O'Donoghue S. I., Miki M., dos Remedios C. G. Removing the two C-terminal residues of actin affects the filament structure. Arch Biochem Biophys. 1992 Feb 14;293(1):110–116. doi: 10.1016/0003-9861(92)90372-4. [DOI] [PubMed] [Google Scholar]
- Porzio M. A., Pearson A. M. Improved resolution of myofibrillar proteins with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1977 Jan 25;490(1):27–34. doi: 10.1016/0005-2795(77)90102-7. [DOI] [PubMed] [Google Scholar]
- Riseman V. M., Lynch W. P., Nefsky B., Bretscher A. The calmodulin and F-actin binding sites of smooth muscle caldesmon lie in the carboxyl-terminal domain whereas the molecular weight heterogeneity lies in the middle of the molecule. J Biol Chem. 1989 Feb 15;264(5):2869–2875. [PubMed] [Google Scholar]
- Sobue K., Sellers J. R. Caldesmon, a novel regulatory protein in smooth muscle and nonmuscle actomyosin systems. J Biol Chem. 1991 Jul 5;266(19):12115–12118. [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stafford W. F., Jancso A., Graceffa P. Caldesmon from rabbit liver: molecular weight and length by analytical ultracentrifugation. Arch Biochem Biophys. 1990 Aug 15;281(1):66–69. doi: 10.1016/0003-9861(90)90413-s. [DOI] [PubMed] [Google Scholar]
- Sutherland C., Walsh M. P. Phosphorylation of caldesmon prevents its interaction with smooth muscle myosin. J Biol Chem. 1989 Jan 5;264(1):578–583. [PubMed] [Google Scholar]
- Thomas D. D., Seidel J. C., Gergely J. Rotational dynamics of spin-labeled F-actin in the sub-millisecond time range. J Mol Biol. 1979 Aug 15;132(3):257–273. doi: 10.1016/0022-2836(79)90259-6. [DOI] [PubMed] [Google Scholar]
- Velaz L., Ingraham R. H., Chalovich J. M. Dissociation of the effect of caldesmon on the ATPase activity and on the binding of smooth heavy meromyosin to actin by partial digestion of caldesmon. J Biol Chem. 1990 Feb 15;265(5):2929–2934. [PubMed] [Google Scholar]
- Vorotnikov A. V., Gusev N. B. Some properties of duck gizzard caldesmon. Biochem J. 1991 Jan 1;273(Pt 1):161–163. doi: 10.1042/bj2730161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. L., Chalovich J. M., Graceffa P., Lu R. C., Mabuchi K., Stafford W. F. A long helix from the central region of smooth muscle caldesmon. J Biol Chem. 1991 Jul 25;266(21):13958–13963. [PMC free article] [PubMed] [Google Scholar]
- Wang C. L., Wang L. W., Xu S. A., Lu R. C., Saavedra-Alanis V., Bryan J. Localization of the calmodulin- and the actin-binding sites of caldesmon. J Biol Chem. 1991 May 15;266(14):9166–9172. [PubMed] [Google Scholar]
- Yamashiro S., Yamakita Y., Hosoya H., Matsumura F. Phosphorylation of non-muscle caldesmon by p34cdc2 kinase during mitosis. Nature. 1991 Jan 10;349(6305):169–172. doi: 10.1038/349169a0. [DOI] [PubMed] [Google Scholar]