Abstract
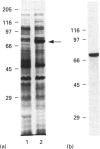
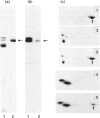
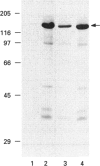
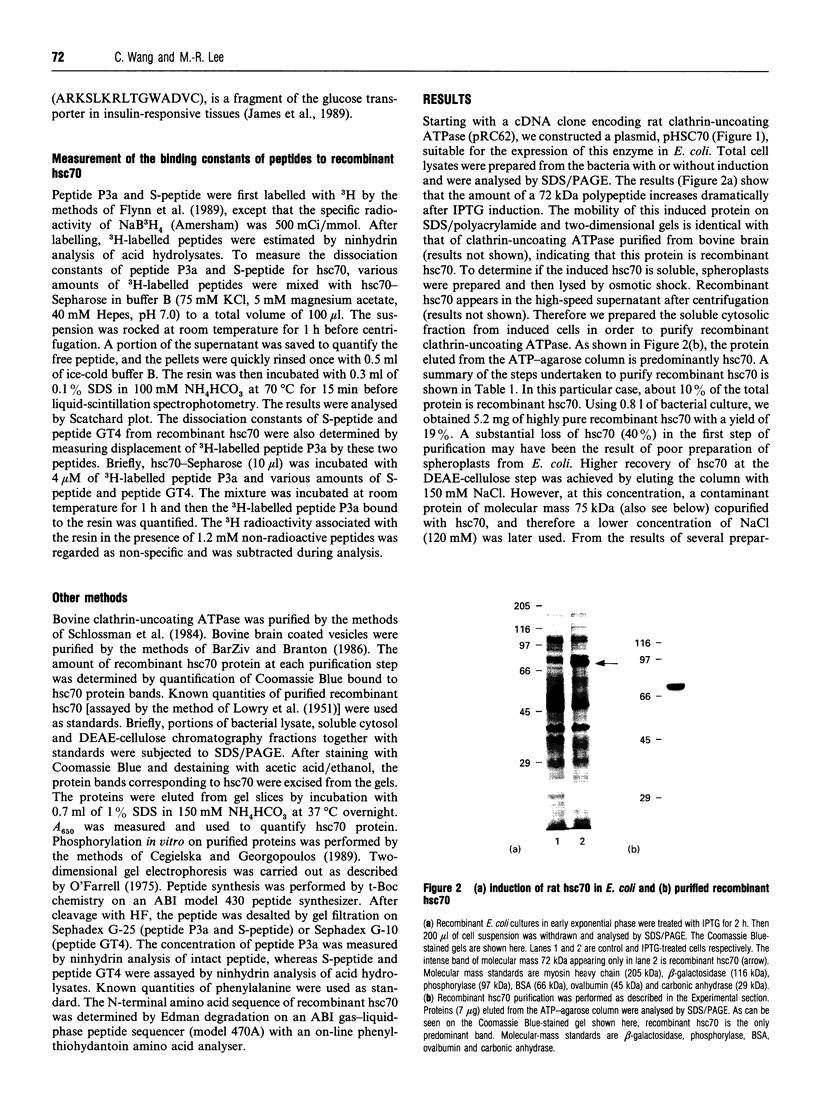
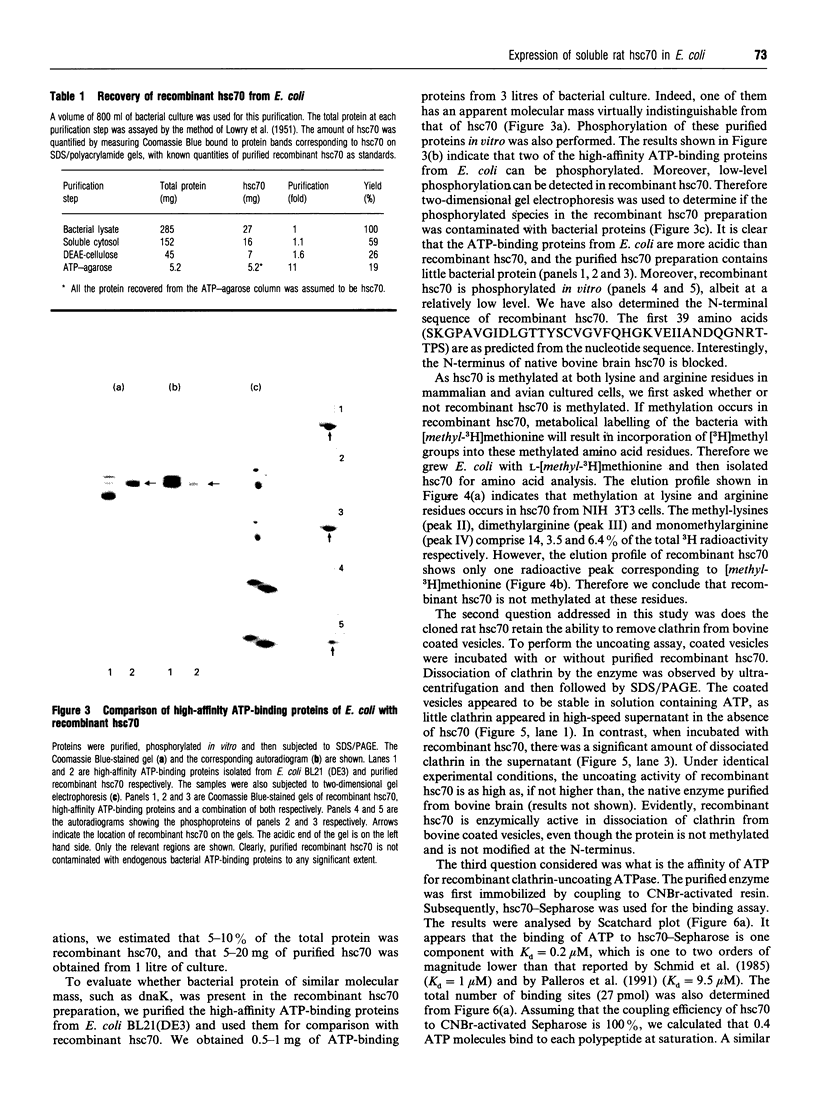
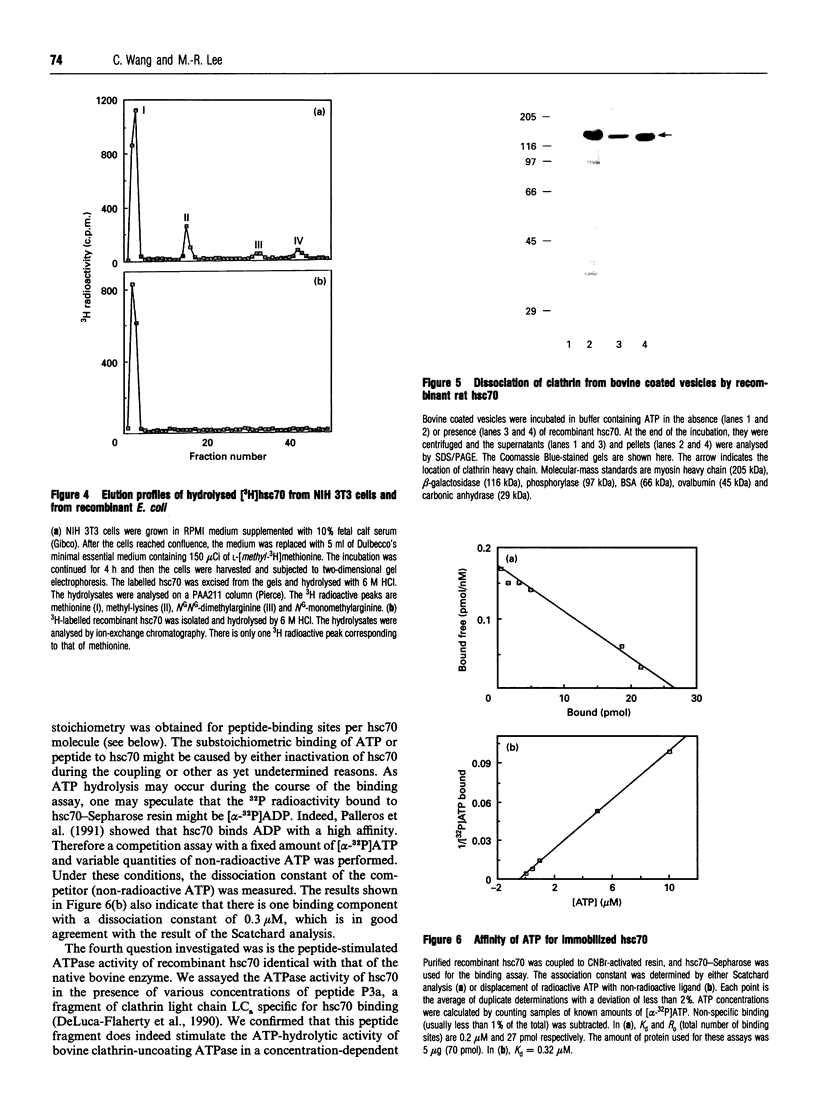
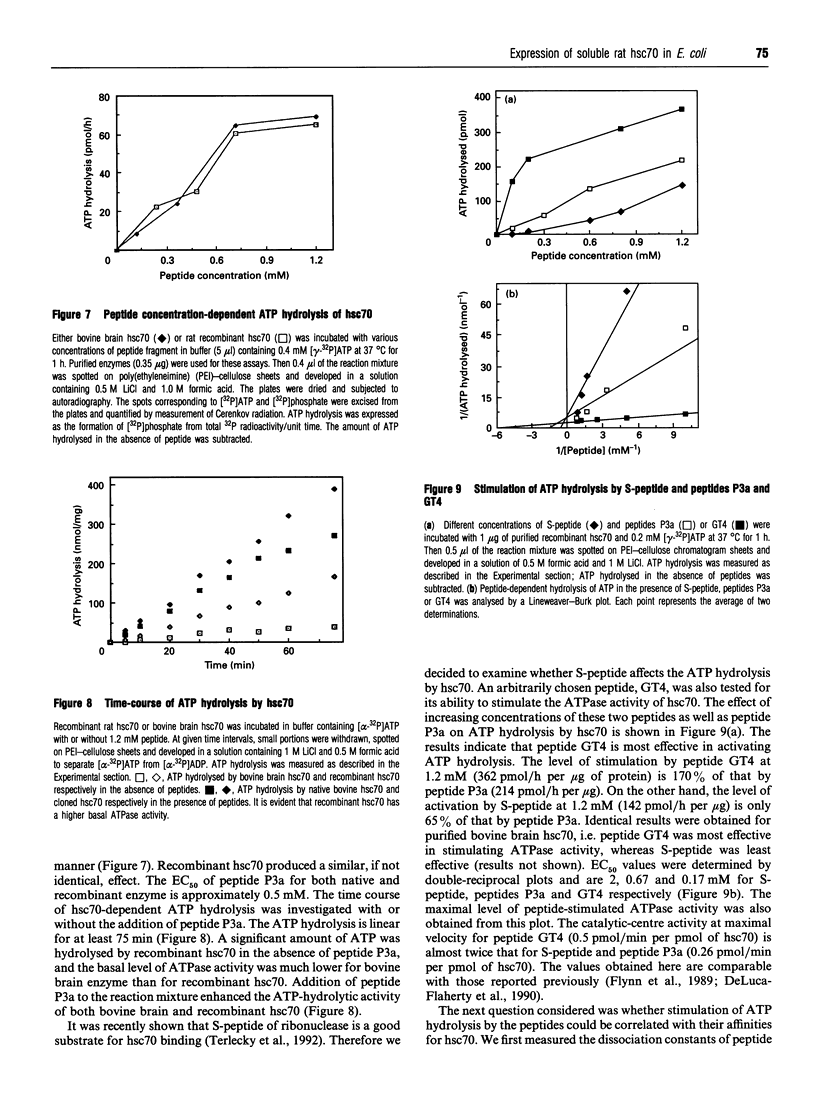
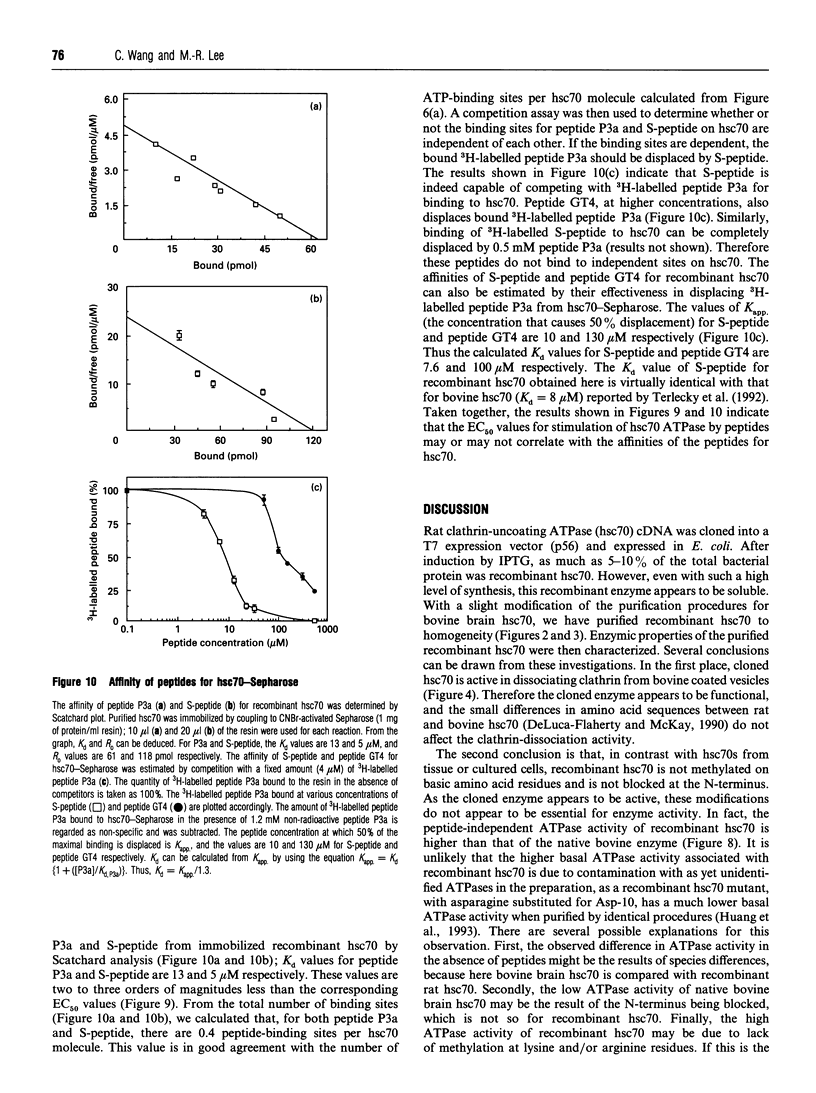
We have cloned the cDNA of rat hsc70 (clathrin-uncoating ATPase) into a T7 expression system and have expressed this enzyme in Escherichia coli. The recombinant clathrin-uncoating ATPase is in the cytosolic fraction of the bacterium and is soluble. It was purified to homogeneity by DEAE-cellulose and ATP-agarose column chromatography. From 1 litre of bacterial culture (0.3-0.4 g of proteins), 5-20 mg of pure recombinant clathrin-uncoating ATPase was routinely obtained. The cloned enzyme is capable of dissociating clathrin from bovine coated vesicle. Furthermore, it is not methylated on basic amino acid residues and is not blocked at the N-terminus, indicating that these modifications on hsc70 are not essential for uncoating of clathrin. Binding of [alpha-32P]ATP by purified recombinant hsc70 was analysed by Scatchard plot. The results indicate that there one high-affinity binding component with a Kd (dissociation constant) of 0.2-0.3 microM. The peptide-stimulated ATPase activities of recombinant hsc70 at 37 degrees C with respect to S-peptide peptides P3a and GT4 at a concentration of 1.2 mM are 142 +/- 6, 214 +/- 8 and 362 +/- 5 pmol/h per micrograms of hsc70 protein respectively. The EC50 values of hsc70 ATPase for S-peptide, peptides P3a and GT4 are 2, 0.67 and 0.17 mM respectively. On the other hand, the dissociation constants of S-peptide, peptides P3a and GT4 for recombinant hsc70 are 7.6, 13 and 100 microM respectively. Thus peptide GT4 is the only peptide examined for which the binding constant is comparable with the EC50 for stimulation ATPase activity, albeit it has the lowest affinity for hsc70.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Zvi D., Branton D. Clathrin-coated vesicles contain two protein kinase activities. Phosphorylation of clathrin beta-light chain by casein kinase II. J Biol Chem. 1986 Jul 25;261(21):9614–9621. [PubMed] [Google Scholar]
- Cegielska A., Georgopoulos C. Functional domains of the Escherichia coli dnaK heat shock protein as revealed by mutational analysis. J Biol Chem. 1989 Dec 15;264(35):21122–21130. [PubMed] [Google Scholar]
- Chappell T. G., Konforti B. B., Schmid S. L., Rothman J. E. The ATPase core of a clathrin uncoating protein. J Biol Chem. 1987 Jan 15;262(2):746–751. [PubMed] [Google Scholar]
- Chappell T. G., Welch W. J., Schlossman D. M., Palter K. B., Schlesinger M. J., Rothman J. E. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell. 1986 Apr 11;45(1):3–13. doi: 10.1016/0092-8674(86)90532-5. [DOI] [PubMed] [Google Scholar]
- DeLuca-Flaherty C., McKay D. B. Nucleotide sequence of the cDNA of a bovine 70 kilodalton heat shock cognate protein. Nucleic Acids Res. 1990 Sep 25;18(18):5569–5569. doi: 10.1093/nar/18.18.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca-Flaherty C., McKay D. B., Parham P., Hill B. L. Uncoating protein (hsc70) binds a conformationally labile domain of clathrin light chain LCa to stimulate ATP hydrolysis. Cell. 1990 Sep 7;62(5):875–887. doi: 10.1016/0092-8674(90)90263-e. [DOI] [PubMed] [Google Scholar]
- Ellis R. J., van der Vies S. M. Molecular chaperones. Annu Rev Biochem. 1991;60:321–347. doi: 10.1146/annurev.bi.60.070191.001541. [DOI] [PubMed] [Google Scholar]
- Flaherty K. M., DeLuca-Flaherty C., McKay D. B. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990 Aug 16;346(6285):623–628. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- Flynn G. C., Chappell T. G., Rothman J. E. Peptide binding and release by proteins implicated as catalysts of protein assembly. Science. 1989 Jul 28;245(4916):385–390. doi: 10.1126/science.2756425. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Dissociation of clathrin from coated vesicles by the uncoating ATPase. J Biol Chem. 1990 Apr 25;265(12):6682–6687. [PubMed] [Google Scholar]
- Hightower L. E. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991 Jul 26;66(2):191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Huang S. P., Tsai M. Y., Tzou Y. M., Wu W. G., Wang C. Aspartyl residue 10 is essential for ATPase activity of rat hsc70. J Biol Chem. 1993 Jan 25;268(3):2063–2068. [PubMed] [Google Scholar]
- Hwang J. L., Shyy S. H., Chen A. Y., Juan C. C., Whang-Peng J. Studies of topoisomerase-specific antitumor drugs in human lymphocytes using rabbit antisera against recombinant human topoisomerase II polypeptide. Cancer Res. 1989 Feb 15;49(4):958–962. [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Malley K., Mauron A., Barchas J. D., Kedes L. Constitutively expressed rat mRNA encoding a 70-kilodalton heat-shock-like protein. Mol Cell Biol. 1985 Dec;5(12):3476–3483. doi: 10.1128/mcb.5.12.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palleros D. R., Welch W. J., Fink A. L. Interaction of hsp70 with unfolded proteins: effects of temperature and nucleotides on the kinetics of binding. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5719–5723. doi: 10.1073/pnas.88.13.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossman D. M., Schmid S. L., Braell W. A., Rothman J. E. An enzyme that removes clathrin coats: purification of an uncoating ATPase. J Cell Biol. 1984 Aug;99(2):723–733. doi: 10.1083/jcb.99.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S. L., Braell W. A., Rothman J. E. ATP catalyzes the sequestration of clathrin during enzymatic uncoating. J Biol Chem. 1985 Aug 25;260(18):10057–10062. [PubMed] [Google Scholar]
- Schmid S. L., Braell W. A., Schlossman D. M., Rothman J. E. A role for clathrin light chains in the recognition of clathrin cages by 'uncoating ATPase'. Nature. 1984 Sep 20;311(5983):228–231. doi: 10.1038/311228a0. [DOI] [PubMed] [Google Scholar]
- Shi Y., Thomas J. O. The transport of proteins into the nucleus requires the 70-kilodalton heat shock protein or its cytosolic cognate. Mol Cell Biol. 1992 May;12(5):2186–2192. doi: 10.1128/mcb.12.5.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Terlecky S. R., Chiang H. L., Olson T. S., Dice J. F. Protein and peptide binding and stimulation of in vitro lysosomal proteolysis by the 73-kDa heat shock cognate protein. J Biol Chem. 1992 May 5;267(13):9202–9209. [PubMed] [Google Scholar]
- Ungewickell E. The 70-kd mammalian heat shock proteins are structurally and functionally related to the uncoating protein that releases clathrin triskelia from coated vesicles. EMBO J. 1985 Dec 16;4(13A):3385–3391. doi: 10.1002/j.1460-2075.1985.tb04094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Brennan W. A., Jr Rat skeletal muscle, liver and brain have different fetal and adult forms of the glucose transporter. Biochim Biophys Acta. 1988 Dec 8;946(1):11–18. doi: 10.1016/0005-2736(88)90451-8. [DOI] [PubMed] [Google Scholar]
- Wang C., Gomer R. H., Lazarides E. Heat shock proteins are methylated in avian and mammalian cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3531–3535. doi: 10.1073/pnas.78.6.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Lazarides E. Arsenite-induced changes in methylation of the 70,000 dalton heat shock proteins in chicken embryo fibroblasts. Biochem Biophys Res Commun. 1984 Mar 15;119(2):735–743. doi: 10.1016/s0006-291x(84)80312-5. [DOI] [PubMed] [Google Scholar]
- Wang C., Lazarides E., O'Connor C. M., Clarke S. Methylation of chicken fibroblast heat shock proteins at lysyl and arginyl residues. J Biol Chem. 1982 Jul 25;257(14):8356–8362. [PubMed] [Google Scholar]
- Wang C., Lin J. M., Lazarides E. Methylations of 70,000-Da heat shock proteins in 3T3 cells: alterations by arsenite treatment, by different stages of growth and by virus transformation. Arch Biochem Biophys. 1992 Aug 15;297(1):169–175. doi: 10.1016/0003-9861(92)90656-h. [DOI] [PubMed] [Google Scholar]