Highlights
-
•
A total of 105 participants were enrolled; 93% were classified with advanced HIV disease by clusters of differentiation 4 lateral flow assay.
-
•
Clusters of differentiation 4 lateral flow assay showed a high sensitivity (100%) but low specificity (19%) and accuracy (47%).
-
•
There was a high prevalence of fungal infections; mortality was lower than previous hospital reports.
Keywords: HIV, Histoplasmosis, Cryptococcosis, CD4, Antigen testing, Rapid testing
Abstract
Objectives
Despite advancements in HIV diagnosis and treatment, advanced HIV disease (AHD) is still a significant concern worldwide, especially in countries with a high percentage of undiagnosed cases and late-stage diagnoses.
Methods
A prospective pilot study was conducted in Buenos Aires, Argentina to assess the feasibility of implementing a package for people living with HIV, integrating a point-of-care clusters of differentiation (CD4) test, followed by rapid Cryptococcus and Histoplasma antigen (Ag) detection.
Results
A total of 105 people living with HIV were enrolled, during June 2021 to October 2021. The VISITECT CD4 Advanced Disease Lateral Flow Assay (CD4-LFA) (Accubio) classified 98 (93%) patients with AHD. Compared with flow cytometry, the CD4-LFA performed with a high sensitivity (100%) but low specificity (19%) and limited accuracy (47%). In the 98 patients classified with AHD using the CD4-LFA, 16 tested positive for any of the rapid Ag used, including 12 patients positive for the Histoplasma Ag test and four positive for Cryptococcus Ag; all four patients with positive Cryptococcus Ag in sera and were diagnosed with meningitis. In the 30-day follow-up, one death was recorded.
Conclusions
The CD4-LFA correctly classified all patients with CD4 ≤200 cells/µL by flow cytometry, but a high frequency of patients misclassified with AHD was recorded. We also observed a high prevalence of opportunistic fungal infections, as previously observed in the hospital where this pilot study was conducted; however, in contrast with those previous reports, mortality was lower. The study underscores the importance of scaling up comprehensive care strategies and collaborating with governmental and non-governmental partners to enhance access to essential diagnostic tools and treatments for people living with HIV. Further research with larger sample sizes is needed to validate these findings.
Introduction
Despite the accessibility of rapid assays for HIV diagnosis and substantial progress in the efficacy and availability of antiretroviral treatment, advanced HIV disease (AHD) remains a major global public health concern [1]. In Argentina, it is estimated that there are 140,000 people living with HIV (PLHIV), with approximately 13% unaware of their HIV status. Furthermore, in the province of Buenos Aires, 35% of newly diagnosed PLHIV are identified during an advanced stage of the disease [2]. According to the World Health Organization (WHO) definitions, AHD is defined as having a clusters of differentiation (CD) 4 cell counts below 200 cells/µL or with WHO clinical stages III/IV. Due to severe immunosuppression, people with AHD are at the highest risk of developing opportunistic infections (OIs), including cryptococcosis and histoplasmosis, infections highly endemic in the Americas, including Argentina [[3], [4], [5], [6], [7], [8], [9], [10], [11]].
Accurate and affordable CD4 testing is essential for assessing risk of developing OIs among PLHIV. Flow cytometry (FCM) testing is the gold-standard method for CD4 quantification; however, access is limited due to decentralization and the need for complex laboratory capacity; special conditions for specimen collection, transport, and testing; and highly trained laboratory professionals. Recently, Accubio developed the VISITECT CD4 Advanced Disease Lateral Flow Assay (CD4-LFA), a point-of-care test for semi-quantification of CD4 count, and rapid detection of AHD [12].
Cryptococcosis and histoplasmosis are associated with high mortality in PLHIV, especially in people living in low- and middle-income countries. Diagnosis of these infections has greatly improved in the last decade because of highly accurate and rapid assays for antigen detection [8,[13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]]. These assays have increased case detection, reduced diagnosis time, and lowered mortality rates [8,[13], [14], [15], [16], [17], [18], [19], [20]]. Some of these novel tests are based on LFA and enzyme immunoassay (EIA) and have the benefit of being performed using non-invasive specimens such as urine, serum, or fingerstick whole blood. In addition, these rapid tests have presented superior performance compared with conventional culture- and microscopy-based testing [[22], [23], [24],[26], [27], [28], [29], [30], [31]].
The aim of this study was to implement a comprehensive package of care for PLHIV, incorporating Point-of-Care CD4 screening for AHD, followed by rapid antigen testing for Cryptococcus and Histoplasma in people classified as having AHD using the CD4-LFA.
Materials and methods
Participants and study design
A prospective pilot study was conducted from June 2021 to October 2021 at Hospital Juan A. Fernández in Buenos Aires, Argentina. This hospital is a major public and academic institution affiliated with the University of Buenos Aires, providing free health care and education. The infectious diseases clinic serves over 4000 patients with HIV, offering antiretroviral treatment, clinical care, and approximately 36,200 scheduled appointments annually. The unit ensures quick access to specialized care, facilitates early hospital discharges, and supports patients until clinical stability. This study was conducted in the outpatient setting, consecutively enrolling patients with new HIV diagnoses, patients with known HIV status, and those returning to the HIV program.
We excluded individuals with undetectable viral load during the 3 months before enrollment, as well as those diagnosed with cryptococcosis or histoplasmosis within the previous year. In addition, we excluded individuals who had been on antifungal treatment in the last 14 months.
There were no rejections for participating in the pilot study. All enrolled individuals underwent a first testing using the VISITECT CD4 Advanced Disease Lateral Flow Assay (Accubio); this CD4-LFA testing was done using the same blood specimen used for FCM. Those patients with <200 CD4 cells/µL according to the CD4-LFA were further evaluated for the presence of Cryptococcus antigen (Ag) in sera using the IMMY's Cryptococcus Ag LFA (CrAg-LFA), and Histoplasma antigen in urine using the MiraVista Ag detection LFA (HistoAg-LFA), and the IMMY Clarus Histoplasma GM Ag detection EIA (HistoAg-EIA). Tests were performed following the manufacturer's instructions.
In addition, the following standard of care testing was performed: CD4 cells count by FCM (FACScalibur, Becton-Dickinson), HIV viral load (m2000 RealTime System, Abbott), histopathology, blood culture (BD, BACTEC Myco/F Lytic). Other laboratory testing, including special stains, cultures, and GeneXpert for tuberculosis were performed based on patient symptomatology and clinical suspicion of medical staff.
Patients with positive results by the Cryptococcus or the Histoplasma Ag assays, regardless of symptomatology, were clinically evaluated by medical staff before starting specific treatment. In patients with positive CrAg in sera, lumbar puncture was performed to rule out Cryptococcus meningitis, following WHO recommendations. Cerebrospinal fluid (CSF) was collected and tested by India ink, CrAg-LFA, and culture [32].
Statistical analysis
Based on data showing that AHD prevalence in the Provincia de Buenos Aires, Argentina is 35% [2] and using 2 × 2 tables, we calculated the analytical performance of the VISITECT CD4-LFA, including sensitivity, specificity, accuracy, positive predictive value, and negative predictive value, and calculated 95% confidence intervals [2]. For CD4-LFA validation, we used the FCM as a referent test. We calculated the absolute and relative frequencies of variables collected. All analyses were performed using STATA 11.0 or Microsoft Excel.
Results
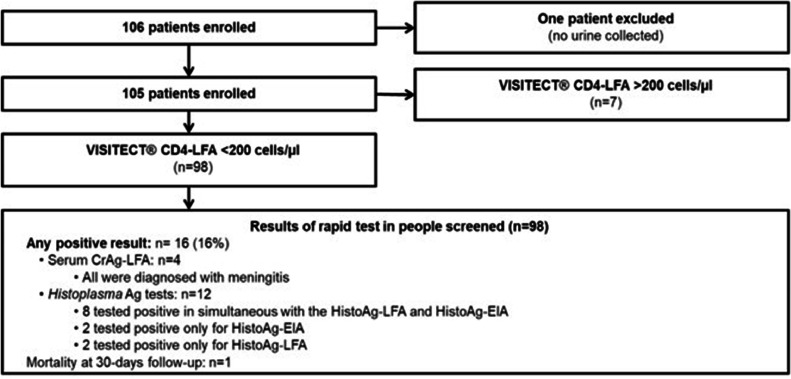
A total of 106 patients were enrolled; one patient was excluded because the patient did not have a urine specimen (Figure 1). The mean age was 41 years (SD 11.2); 61% (n = 64) were male. By FCM, the median CD4 count was 140 cells/µL (interquartile range [IQR]: 53-254). A total of 71% of patients were presented with symptoms compatible with OIs.
Figure 1.
Flow chart of patients enrolled and rapid testing performed during the implementation of package of care strategy in people living with HIV in Argentina.
CD4-LFA, VISITECT CD4 Advanced Disease Lateral Flow Assay; CrAg, Cryptococcus Ag.
Performance of VISITECT CD4-LFA: According to the CD4-LFA, 98 of 105 (93%) enrolled patients were classified with AHD. FCM CD4 count was available for 100 of the 105 patients included. FCM classified 63 (63%) patients with <200 cells/µL and 37 (37%) with >200 CD4 cells/µL. Table 1a shows the sensitivity, specificity, accuracy, positive predictive value, and negative predictive value with their 95% confidence interval ranges of the CD4-LFA compared with the gold standard, FCM. This assay performed with a sensitivity of 100% but a specificity of 19%. A total of 21 of the 30 patients classified by the CD4-LFA as <200 cells/µL had between 200 and 350 CD4 cells/µL according to the FCM. The remaining nine had over 350 cells/µL. Conversely, the FCM range of the seven specimens classified by the CD4-LFA as >200 cells/µL was 369 to 990 cells/µL.
Table 1.
Analytical performance of the VISITECT CD4 Advanced Disease LFA. Comparison against the flow cytometry results obtained by the FACScalibur, Becton-Dickinson. Performance calculated using a prevalence of 35% of advanced HIV disease.
| FACScalibur, Becton-Dickinson |
|||
|---|---|---|---|
| Table 1a | ≤200 CD4 | >200 CD4 | |
| VISITECT CD4-Advanced disease LFA | ≤200 CD4 | 63 | 30 |
| >200 CD4 | 0 | 7 | |
| Sensitivity | 100% (95%-CI: 94-100) | ||
| Specificity | 19% (95%-CI: 8-35) | ||
| Accuracy | 47% (95%-CI: 37-57) | ||
| Positive predictive value | 40% (95%-CI: 36-44) | ||
| Negative predictive value | 100% (95%-CI: 59%-100%) | ||
| FACScalibur, Becton-Dickinson |
|||
| Table 1b | ≤350 CD4 | >350 CD4 | |
| VISITECT CD4-Advanced disease LFA | ≤200 CD4 | 84 | 9 |
| >200 CD4 | 0 | 7 | |
| Sensitivity | 100% (95%-CI: 96-100) | ||
| Specificity | 44% (95%-CI: 20-70) | ||
| Accuracy | 63% (95%-CI: 53-73) | ||
| Positive predictive value | 49% (95%-CI: 38-60) | ||
| Negative predictive value | 100% (95%-CI: 59-100) | ||
CD, cluster of differentiation; CI, confidence interval; LFA, lateral flow assay.
Advance HIV prevalence: 35% [2].
Aiming to improve the analytical performance of the CD4-LFA, we recalculated the performance of the assay using the following cutoff of the FCM: 250 cells/µL, 300 cells/µL, and 350 cells/µL. We saw that by increasing the threshold of the CD4 cell count calculated by FCM, the analytical performance of the CD4-LFA improved, especially the specificity and accuracy. We observed that using a cutoff of 350 cells/µL by FCM, the CD4-LFA presented a sensitivity of 100% and a specificity of 44% (Table 1b).
OI screening
Among the 98 patients classified with AHD by the CD4-LFA, 16 (16%) had life-threatening infections; of those, 12 were diagnosed with histoplasmosis and four with Cryptococcus meningitis. All cases were symptomatic.
Among the 12 patients with positive Histoplasma antigen testing, eight tested positive by HistoAg-LFA and HistoAg-EIA, two tested positive only for HistoAg-EIA, and two tested positive for HistoAg-LFA only. The median CD4 count of these patients was 53 cells/µL (IQR 40-125). By the HistoAg-EIA, the antigen concentration ranged from 1.4 ng/ml to >25 ng/mL, the upper limit value of the test detection. Histoplasmosis was proven by culture in six (50%) of these 12 patients with a positive Ag test: three isolated from skin lesions, two from lymph nodes, and one from a biopsy of the thyroid (Table 2). Eight patients started treatment with liposomal amphotericin B and three with oral itraconazole; one did not receive treatment because the patient left the hospital against medical advice. At 30 days of follow-up, one patient died; this patient was also diagnosed with tuberculosis (Table 2, patient 6).
Table 2.
Performance of the assays used in the diagnosis of 18 patients with positive results for Cryptococcus and Histoplasma Ag assays.
| # | CD4 count | Result of Ag testing |
Case definition based on rapid test | ||||
|---|---|---|---|---|---|---|---|
| Histoplasma Ag lateral flow assay | Histoplasma Ag enzyme immunoassay (concentration) | Serum CrAg (titer) | Cerebrospinal fluid CrAg (titer) | Culture | |||
| 1 | 53 | Positive | Positive (>25 ng/ml) | Negative | Negative | Histoplasma capsulatum | Proven histoplasmosisa |
| 2 | 94 | Positive | Positive (>25 ng/ml) | Negative | Negative | Histoplasma capsulatum | Proven histoplasmosis |
| 3 | 52 | Positive | Positive (>25 ng/ml) | Negative | Negative | Histoplasma capsulatum | Proven histoplasmosis |
| 4 | 42 | Positive | Positive (2.6 ng/ml) | Negative | Negative | Histoplasma capsulatum | Proven histoplasmosis |
| 5 | 283 | Negative | Positive (1.4 ng/ml) | Negative | Negative | Histoplasma capsulatum | Proven histoplasmosis |
| 6 | 12 | Positive | Positive (>25 ng/ml) | Negative | Negative | Histoplasma capsulatum | Proven histoplasmosis / tuberculosisb |
| 7 | 156 | Negative | Positive (2.7 ng/ml) | Negative | Negative | Negative | Probable histoplasmosis |
| 8 | 54 | Positive | Positive (5.3 ng/ml) | Negative | Negative | Negative | Probable histoplasmosis |
| 9 | 30 | Positive | Positive (12.0 ng/ml) | Negative | Negative | Negative | Probable histoplasmosis |
| 10 | 46 | Positive | Positive (1.5 ng/ml) | Negative | Negative | Negative | Probable histoplasmosis |
| 11 | 194 | Positive | Negative | Negative | Negative | Negative | Probable histoplasmosis |
| 12 | 39 | Positive | Negative | Negative | Negative | Negative | Probable histoplasmosis |
| 13 | 53 | Negative | Negative | P (>1:2560) | P (>1:2560) | Cryptococcus neoformans | Cryptococcal meningitis |
| 14 | 36 | Negative | Negative | P (>1:2560) | P (1:80) | Cryptococcus neoformans | Cryptococcal meningitis |
| 15 | 8 | Negative | Negative | P (>1:2560) | P (1:20) | Cryptococcus neoformans | Cryptococcal meningitis |
| 16 | 41 | Negative | Negative | P (1:320) | P (>1:2560) | Cryptococcus neoformans | Cryptococcal meningitis |
Ag, antigen; CrAg, Cryptococcus Ag.
Acid-fast bacilli in urine smear.
Died during the 30-day follow-up.
The four patients with positive serum CrAg-LFA were symptomatic. The patients had a median CD4 cell count of 33 cells/µL (IQR 29-43). All of them had meningitis, with positive antigen and culture of CSF. Antigen titration in serum and CSF is detailed in Table 2. Three of four patients had a CSF positive India ink test; the negative CSF India ink test corresponded with the lower antigen titer in CSF (1:20). Patients were treated according to the national recommendations. All four patients were alive at the 30-day follow-up (Table 2).
The turn-around time for the results from the CD4-LFA, CrAg-LFA, and HistoAg-LFA was a maximum of 2 hours. The HistoAg-EIA was performed once a week, with results available within 1-7 days. Upon reporting a positive result for Cryptococcus or Histoplasma Ag, the infectious disease unit analyzed the case and implemented the specific antifungal treatment on the same day the result was reported.
Discussion
In this pilot study, a sequential rapid diagnostic package of care in PLHIV was successfully implemented. This package of care included rapid CD4 screening, followed by rapid antigen detection for Cryptococcus and Histoplasma. One of five people screened for OI tested positive for these two life-threatening fungal OIs.
The CD4-LFA succeeded in correctly identifying all PLHIV with <200 cells/µL; however, 30 of 37 patients with >200 cells/µL were misclassified as patients with AHD. However, it is important to highlight that 70% of those patients had CD4 counts between 200 and 350 cells/µL, meaning that these persons were at risk of developing OIs, as demonstrated in other studies [8,[15], [16], [17]]. We diagnosed a case of proved histoplasmosis in a patient with a CD4 count of 283 cells/µL. In this study, VISITECT CD4 Advanced Disease LFA identified patients with CD4 <350 cells/µL, with a sensitivity of 100% but a specificity of 44%, meaning that around one in two patients screened would have between 350 and 369 CD4 cells/µL according to our results. Future research could assess whether the automatic reading of VISITECT CD4 Advanced Disease LFA could improve interpretation and performance of this assay. Recent advancements were seen with the CrAg-LFA, where the use of a smartphone's application “TIRASPOT” improved the capacity of testing interpretation [33].
We observed that one of 10 patients screened was positive for HistoAg and all of them had symptoms compatible with disseminated histoplasmosis. This finding is consistent with data from previous studies where histoplasmosis prevalence ranges from 2% to 59% (median 20%) [5,21]. Previous studies performed in the Hospital Fernández showed that prevalence differs depending on the diagnostic assay used, as this study has also shown [34,35]. In that study, conventional laboratory assays such as microscopy and culture were used to diagnose eight histoplasmosis cases (0.6 cases per month), whereas the antigen test was positive in 12 cases, meaning 2.4 cases per month [35]. Subsequently, another study with 123 PLHIV showed similar results: 12 cases of histoplasmosis were diagnosed (incidence 10%; 1.2 cases per month); all had a positive Ag test but only eight had a positive antibody test [34]. This reinforces the idea that antigen detection is much more sensitive than conventional diagnostic tools and, thus, may be beneficial in any settings where PLHIV receive care.
Hospital Fernandez has routinely implemented Cryptococcus antigen testing since 1992. During the early implementation of CrAg testing, a total of 10 cases were detected in 123 PLHIV evaluated (8% prevalence). Six (60%) of these patients had meningitis, and two died during hospitalization [35]. In addition, we routinely titrate the CrAg because it is well-known that CrAg titers in sera higher than 1:160 are associated with meningitis [36]. This finding could be an alternative for ruling out the suspicion of meningitis in patients where testing of CSF is not possible but further studies are needed.
The mortality in this study was lower than our historical data. In a previous study, 33% of patients with Cryptococcus meningitis died; here, all patients survived at the 30-day follow-up [35]. For histoplasmosis, our previous data showed 20% mortality; here, we observed one death [34]. Due to the small sample size and the pilot design of this study, we are not able to draw conclusions regarding whether the testing package improved patient outcomes. However, an acceptable and quick assessment of OIs risk can be done with the VISITECT CD4 test, allowing the screening patients within the same day and facilitating the rapid initiation of effective treatment for life-threatening fungal diseases. Studies are ongoing to confirm these findings. Our group is working with government and non-governmental partners in the implementation of this package of care strategy on a large scale.
Conclusion
The implementation of a comprehensive care package for PLHIV, which includes point-of-care CD4 testing and rapid turn-around diagnosis of OIs, has the potential to reduce PLHIV mortality rates. It is well-documented that rapid diagnosis and targeted treatment have a positive impact on patient survival. There are ongoing studies with larger sample sizes aiming to confirm these findings, with the goal of scaling up the implementation of comprehensive care strategies. Collaboration with governmental and non-governmental partners is essential to improve access to essential diagnostic tools and treatments for PLHIV.
Declarations of competing interest
The authors declare no conflicts of interest. Diego H. Caceres has been an IMMY employee since December 2021.
Acknowledgments
Author Contributions
All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. Kits used for testing were donated by IMMY, CDC, GAFFI, and Accubio.
Acknowledgments
The authors thank IMMY, GAFFI, CDC, and Accubio (former OMEGA diagnostics) for kit donations. The authors also want to thank Jeremy A. W. Gold for critical review and editing of the manuscript.
Institutional review board statement
This study was approved by the ethical committee of the Hospital Juan A. Fernández, Buenos Aires, Argentina, approval #3370, on November 9, 2020.
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Contributor Information
Mariana Andreani, Email: mariandreani1@gmail.com.
Claudia E. Frola, Email: frola.claudia.e@gmail.com.
Diego H. Cáceres, Email: diegocaceres84@gmail.com.
Claudia Bozzano, Email: xclaudiabozzano@gmail.com.
Liliana Diaz, Email: lildiaz98@gmail.com.
Maria E. Cattani, Email: mariaeugeniacatt@gmail.com.
Juan L. Rodriguez-Tudela, Email: jlrodrigueztudela@gaffi.com.
Maria J. Rolón, Email: mjrolon@gmail.com.
Liliana Guelfand, Email: liguelfand@gmail.com.
References
- 1.UNAIDS. Full report — Danger: UNAIDS Global Aids Update 2022. 2022. https://www.unaids.org/en/resources/documents/2022/in-danger-global-aids-update; [accessed 27 July 2022].
- 2.Ministerio de Salud de la Nación Argentina. Dirección de Respuesta al VIH ITS. Hepatitis virales y tuberculosis. Boletín N 40 Respuesta al VIH y las ITS en la Argentina, 2022. 2023. https://bancos.salud.gob.ar/sites/default/files/2023-11/boletin-ndeg-40-respuesta-al-vih-y-las-its-en-la-argentina.pdf; [accessed 01 December 2023].
- 3.World Health Organization (WHO). Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. 2017. https://www.who.int/hiv/pub/guidelines/advanced-HIV-disease/en/; [accessed 01 July 2017]. [PubMed]
- 4.Centers for Disease Control and Pevention (U.S.) 2021. AIDS and opportunistic infections 2021. [Google Scholar]; https://www.cdc.gov/hiv/basics/livingwithhiv/opportunisticinfections.html; [accessed 31 December 2021].
- 5.Cáceres DH, Gómez BL, Tobón ÁM, Restrepo Á, Chiller T, Lindsley MD, et al. Tackling histoplasmosis infection in people living with HIV from Latin America: from diagnostic strategy to public health solutions. J Fungi (Basel) 2023;9:558. doi: 10.3390/jof9050558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caceres DH, Arauz AB, Flores C, Santiago E, Montoya S, Saenz C, et al. Implementation of rapid diagnostics assays for detection of histoplasmosis and cryptococcosis in Central American people living with HIV. Mycoses. 2021;64:1396–1401. doi: 10.1111/myc.13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forno D, Samayoa B, Medina N, Arathoon E, Mejia CR, Gordillo R, et al. Diagnosis of fungal opportunistic infections in people living with HIV from Guatemala and El Salvador. Mycoses. 2021;64:1563–1570. doi: 10.1111/myc.13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medina N, Alastruey-Izquierdo A, Bonilla O, Gamboa O, Mercado D, Pérez JC, et al. A rapid screening program for histoplasmosis, tuberculosis, and cryptococcosis reduces mortality in HIV patients from Guatemala. J Fungi (Basel) 2021;7:268. doi: 10.3390/jof7040268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez F, Caceres DH, Ford N, Ravasi G, Gomez BL, Pasqualotto AC, et al. Summary of guidelines for managing histoplasmosis among people living with HIV. J Fungi (Basel) 2021;7:134. doi: 10.3390/jof7020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimaro GD, Guinness L, Shiri T, Kivuyo S, Chanda D, Bottomley C, et al. Cryptococcal meningitis screening and community-based early adherence support in people with advanced human immunodeficiency virus infection starting antiretroviral therapy in Tanzania and Zambia: a cost-effectiveness analysis. Clin Infect Dis. 2020;70:1652–1657. doi: 10.1093/cid/ciz453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Limper AH, Adenis A, Le T, Harrison TS. Fungal infections in HIV/AIDS. Lancet Infect Dis. 2017;17:e334–e343. doi: 10.1016/S1473-3099(17)30303-1. [DOI] [PubMed] [Google Scholar]
- 12.Kestens L, Mandy F. Thirty-five years of CD4 T-cell counting in HIV infection: from flow cytometry in the lab to point-of-care testing in the field. Cytometry B Clin Cytom. 2017;92:437–444. doi: 10.1002/cyto.b.21400. [DOI] [PubMed] [Google Scholar]
- 13.Pasqualotto AC, Queiroz-Telles F, Chebabo A, Leitao TMJS, Falci DR, Xavier MO, et al. The “Histoplasmosis Porto Alegre manifesto”-Addressing disseminated histoplasmosis in AIDS. PLoS Negl Trop Dis. 2023;17 doi: 10.1371/journal.pntd.0010960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falci DR, Monteiro AA, Braz Caurio CF, Magalhães TCO, Xavier MO, Basso RP, et al. Histoplasmosis, an underdiagnosed disease affecting people living with HIV/AIDS in Brazil: results of a multicenter prospective cohort study using both classical mycology tests and Histoplasma urine antigen detection. Open Forum Infect Dis. 2019;6 doi: 10.1093/ofid/ofz073. ofz073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medina N, Rodriguez-Tudela JL, Aguirre L, Salazar LR, Gamboa O, Bonilla O, et al. Incidence of histoplasmosis in a cohort of people with HIV: from estimations to reality. Microorganisms. 2021;9:2596. doi: 10.3390/microorganisms9122596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samayoa B, Aguirre L, Bonilla O, Medina N, Lau-Bonilla D, Mercado D, et al. The diagnostic laboratory Hub: a new health care system reveals the incidence and mortality of tuberculosis, histoplasmosis, and cryptococcosis of PWH in Guatemala. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofz534. ofz534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medina N, Alastruey-Izquierdo A, Mercado D, Bonilla O, Pérez JC, Aguirre L, et al. Comparative performance of the laboratory assays used by a Diagnostic Laboratory Hub for opportunistic infections in people living with HIV. AIDS. 2020;34:1625–1632. doi: 10.1097/QAD.0000000000002631. [DOI] [PubMed] [Google Scholar]
- 18.Samayoa B, Roy M, Cleveland AA, Medina N, Lau-Bonilla D, Scheel CM, et al. High mortality and Coinfection in a prospective cohort of human immunodeficiency virus/acquired immune deficiency syndrome patients with histoplasmosis in Guatemala. Am J Trop Med Hyg. 2017;97:42–48. doi: 10.4269/ajtmh.16-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nacher M, Leitao TS, Gómez BL, Couppié P, Adenis A, Damasceno L, et al. The fight against HIV-associated disseminated histoplasmosis in the Americas: unfolding the different stories of four centers. J Fungi (Basel) 2019;5:51. doi: 10.3390/jof5020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caceres DH, Zuluaga A, Arango-Bustamante K, de Bedout C, Tobón ÁM, Restrepo Á, et al. Implementation of a training course increased the diagnosis of histoplasmosis in Colombia. Am J Trop Med Hyg. 2015;93:662–667. doi: 10.4269/ajtmh.15-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreani M, Frola CE, Caceres DH, Canteros CE, Rolón MJ, Chiller T, et al. Validation of a lateral flow assay for rapid diagnosis of histoplasmosis in advanced HIV disease, Buenos Aires, Argentina. Appl Microbiol. 2022;2:950–955. doi: 10.3390/applmicrobiol2040072. [DOI] [Google Scholar]
- 22.Cáceres DH, Gómez BL, Tobón ÁM, Minderman M, Bridges N, Chiller T, et al. Validation and concordance analysis of a new lateral flow assay for detection of Histoplasma antigen in urine. J Fungi (Basel) 2021;7:799. doi: 10.3390/jof7100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cáceres DH, Samayoa BE, Medina NG, Tobón AM, Guzmán BJ, Mercado D, et al. Multicenter validation of commercial antigenuria reagents to diagnose progressive disseminated histoplasmosis in people living with HIV/AIDS in two Latin American countries. J Clin Microbiol. 2018;56 doi: 10.1128/JCM.01959-17. e01959–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cáceres DH, Zuluaga A, Tabares ÁM, Chiller T, González Á, Gómez BL. Evaluation of a Cryptococcal antigen lateral flow assay in serum and cerebrospinal fluid for rapid diagnosis of cryptococcosis in Colombia. Rev Inst Med Trop Sao Paulo. 2017;59:e76. doi: 10.1590/S1678-9946201759076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caceres DH, Scheel CM, Tobón AM, Ahlquist Cleveland A, Restrepo A, Brandt ME, et al. Validation of an enzyme-linked immunosorbent assay that detects Histoplasma capsulatum antigenuria in Colombian patients with AIDS for diagnosis and follow-up during therapy. Clin Vaccine Immunol. 2014;21:1364–1368. doi: 10.1128/CVI.00101-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blan BDS, Poester VR, Basso RP, Benelli JL, Sanchotene KO, Caceres DH, et al. Optimization of a commercial Histoplasma galactomannan EIA test in a population from an endemic area of histoplasmosis in southern Brazil. Mycoses. 2023;66:304–307. doi: 10.1111/myc.13554. [DOI] [PubMed] [Google Scholar]
- 27.Kanyama C, Chagomerana MB, Chawinga C, Ngoma J, Shumba I, Kumwenda W, et al. Implementation of tuberculosis and cryptococcal meningitis rapid diagnostic tests amongst patients with advanced HIV at Kamuzu Central Hospital, Malawi, 2016–2017. BMC Infect Dis. 2022;22:224. doi: 10.1186/s12879-022-07224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huerga H, Mathabire Rucker SC, Bastard M, Mpunga J, Amoros Quiles I, Kabaghe C, et al. Urine lipoarabinomannan testing for all HIV patients hospitalized in medical wards identifies a large proportion of patients with tuberculosis at risk of death. Open Forum Infect Dis. 2021;8 doi: 10.1093/ofid/ofaa639. ofaa639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nathavitharana RR, Lederer P, Chaplin M, Bjerrum S, Steingart KR, Shah M. Impact of diagnostic strategies for tuberculosis using lateral flow urine lipoarabinomannan assay in people living with HIV. Cochrane Database Syst Rev. 2021;8 doi: 10.1002/14651858.CD014641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricks S, Denkinger CM, Schumacher SG, Hallett TB, Arinaminpathy N. The potential impact of urine-LAM diagnostics on tuberculosis incidence and mortality: a modelling analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basso RP, Poester VR, Benelli JL, Stevens DA, MO Xavier. Disseminated histoplasmosis in persons with HIV/AIDS, Southern Brazil, 2010–2019. Emerg Infect Dis. 2022;28:721–724. doi: 10.3201/eid2803.212150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization (WHO) 2018. Guidelines for the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. Supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. [PubMed] [Google Scholar]; https://www.who.int/hiv/pub/guidelines/cryptococcal-disease/en/; [accessed 31 December 2018].
- 33.Bermejo-Peláez D, Medina N, Álamo E, Soto-Debran JC, Bonilla O, Luengo-Oroz M, et al. Digital platform for automatic qualitative and quantitative reading of a cryptococcal antigen point-of-care assay leveraging smartphones and artificial intelligence. J Fungi (Basel) 2023;9:217. doi: 10.3390/jof9020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frola C, Bermejo V, Spadaccini L, Guelfand L, Pérez H. Impact of disseminated histoplasmosis in HIV positive patients. Actual Sida Infectol. 2013;21:37–41. [Google Scholar]
- 35.Frola C. Universidad Nacional del Nordeste.; 2019. Utilidad de técnicas de diagnóstico rápido en histoplasmosis y criptococosis en pacientes con infección avanzada por HIV. [Google Scholar]
- 36.Wake RM, Britz E, Sriruttan C, Rukasha I, Omar T, Spencer DC, et al. High cryptococcal antigen titers in blood are predictive of subclinical cryptococcal meningitis among human immunodeficiency virus-infected patients. Clin Infect Dis. 2018;66:686–692. doi: 10.1093/cid/cix872. [DOI] [PMC free article] [PubMed] [Google Scholar]