Highlights
-
•
Identification of a novel variant of the BRAT1 gene (c.398A>G;p.His133Arg).
-
•
WES is useful for identifying causative variant in rare neurodevelopmental disorders.
-
•
BRAT1-related disorders have variability in the clinical presentation.
Keywords: BRAT1 gene, Neurodevelopmental disorders, Neurological abnormalities, Whole exome sequencing (WES), Sanger sequencing
Abstract
The BRAT1 gene plays a crucial role in RNA metabolism and brain development, and mutations in this gene have been associated with neurodevelopmental disorders. The variability in the clinical presentation of BRAT1-related disorders is highlighted, emphasizing the importance of considering this condition in the differential diagnosis of neurodevelopmental disorders. This study aimed to identify a causative variant in an Iranian patient affected by developmental delay, speech delay, seizure, and clubfoot through whole exome sequencing (WES) followed by Sanger sequencing. The WES revealed a novel biallelic variant of the BRAT1, c.398A>G (p.His133Arg), in the patient, which segregated within the family. A literature review suggests that the phenotypic variability associated with BRAT1 mutations is likely due to multiple factors, including the location and type of mutation, the specific functions of the protein, and the influence of other genetic and environmental factors. The phenotypic variability of BRAT1-related disorders underscores the importance of considering BRAT1-related disorders in the differential diagnosis of epileptic encephalopathy with rigidity. These findings provide important insights into the role of BRAT1 in neurodevelopmental disorders and highlight the potential clinical implications of identifying and characterizing novel variants in this gene.
1. Introduction
Neurodevelopmental disorders encompass a diverse range of conditions characterized by impaired cognitive, motor, and social functioning. Genetic factors play a significant role in the etiology of these disorders, and the identification of disease-causing genes is crucial for understanding their underlying mechanisms and improving diagnostic accuracy [1]. One such gene of interest is BRAT1, which has been implicated in various neurodevelopmental disorders [2].
BRAT1 (BRCA1-associated protein required for ATM activation-1) is a critical gene involved in DNA repair and the maintenance of genomic stability. Mutations in BRAT1 have been associated with a spectrum of neurodevelopmental disorders, including intellectual disability, epilepsy, speech delay, and motor impairments. Biallelic mutations in this gene have been linked to two phenotypes including, neurodevelopmental disorder with cerebellar atrophy and with or without seizures (NEDCAS #MIM 618056) [20], as well as lethal neonatal rigidity and multifocal seizure syndrome (RMFSL#MIM 614498) [21], [22]. The RMFSL phenotype is the severe form of disease, and the NEDCAS phenotype is the milder form of BRAT1-related disease. The RMFSL phenotype is presented with severe encephalopathy, drug-resistant epilepsy, cerebral atrophy, and early death. In contrast, the NEDCAS phenotype is presented with intellectual disability, cerebellar atrophy, ataxia, nystagmus, and a higher life expectancy. However, the full extent of BRAT1 genotype-phenotype correlations and the underlying disease mechanisms remain to be fully elucidated [2].
The goal of this study is to identify a causative variant through whole exome sequencing (WES) in a patient with neurodevelopmental disorders. Furthermore, we conducted a literature review to compare the clinical features observed in individuals with BRAT1 mutations, which can help to improve our understanding of the relationship between genotype and phenotype in BRAT1-related disorders.
2. Clinical presentation
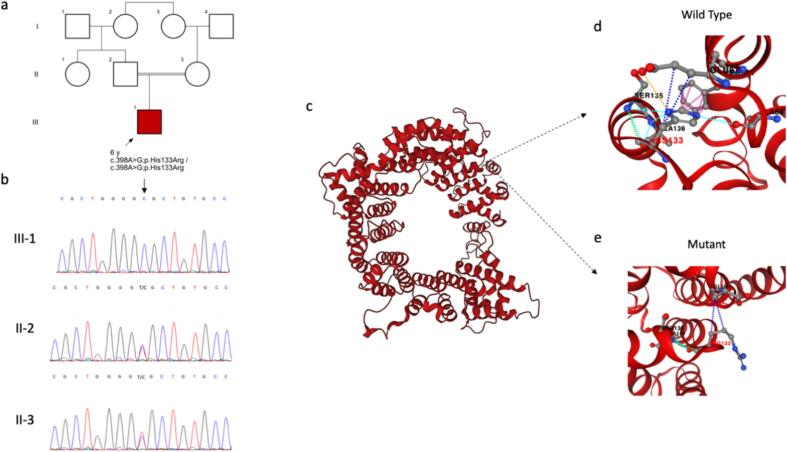
The proband was a 6-year-old male patient who was the only child of consanguineous parents (first cousins, Fig. 1). He had been diagnosed with a range of developmental and neurological abnormalities. Specifically, the patient presented with congenital clubfoot (also known as talipes equinovarus), which underwent surgical correction. The patient subsequently experienced delayed psychomotor development, including a delay in acquiring age-appropriate motor skills as well as the onset of independent walking. The proband had delayed psychomotor development, achieved head control at five months, independent sitting at nine months, started walking at 19 months and started to run by three years of age. At present, at the age of six, the proband exhibits proficient walking and running skills. Nonetheless, difficulties arise when attempting to hop on one foot, and an unsteady gait is evident, suggesting potential challenges with balance and coordination during movement. Additionally, the patient exhibited delayed development of speech and language skills, impaired cognitive functioning, and intellectual abilities below average for his age. The proband began verbalizing at three and a half years old, initially using a few words. His first sentences emerged at the age of five. Despite undergoing years of speech therapy, at six years old, he continues to encounter difficulties in speaking and can only say simple sentences. Moreover, he has a history of experiencing seizures, with the initial occurring at two and a half years old and occurring three times during one month. The seizures were not related to fever and were all atonic, once preceded by a brief myoclonic seizure, and each lasted about ten seconds. Following this event, he has been consistently medicated with sodium valproate, effectively preventing further seizures. There were no abnormal findings in the electroencephalography (EEG). Magnetic resonance imaging (MRI) revealed cerebellar atrophy, which could be responsible for motor coordination and balance disturbances seen in the proband, and microcephaly, which may suggest reduced brain growth and development. No dysmorphic features were detected in the proband. Given the multifaceted nature of the patient's presentation, a multidisciplinary approach involving neurology, genetics, orthopedics, and developmental specialists was employed to ensure accurate diagnosis and appropriate management.
Fig. 1.
A) The pedigree of the proband. b) Electropherogram of nucleotide change c.398A>G in the BRAT1 gene in the patient and his parents. c) Protein model of BRCA1-associated protein required for ATM activation-1 protein based on the AlphaFold structure prediction (ID MOU2). d) His133 in the wild type protein and its interactions based on the prediction of PremPS computational method. d) Arg133 in the mutated protein and its interactions based on the prediction of PremPS computational method. Navy blue dotted line: hydrophobic interaction, Arctic blue dotted line: polar interaction, green dotted line: Van der Waals interaction, yellow dotted line: ionic interaction, purple dotted line: aromatic interaction.
3. Methods
3.1. DNA extraction and Whole-Exome sequencing (WES)
To extract genomic DNA from the proband and their parents' peripheral blood, we employed the salting out technique [3]. The concentration and quality of genomic DNA were assessed using NanoDrop 1000 (Thermo Fisher Scientific, Inc., Wilmington, DE, USA). WES was performed on the Illumina Novaseq6000 platform, generating 101-bp paired-end reads, using the proband's genomic DNA. SureSelectXT2 V7 kits were utilized to enrich the exonic and surrounding exon–intron boundary regions.
The raw data obtained from the sequencing process amounted to approximately 100 gigabases. Subsequently, it was aligned to the human reference genome (GRCh37/hg19) using the Burrows-Wheeler Aligner (BWA) [4]. Sequence alignment map (SAM) tools were employed for further analysis of the resulting binary alignment map (BAM) files [5], and Picard (https://broadinstitute.github.io/picard) was used to eliminate duplicate reads. Calibration was reset, and SNP/indel calling was performed. The resulting alignment was subjected to variant calling and annotation using GATK [6] and ANNOVAR [7], respectively. The called variants were then filtered, annotated, and prioritized based on MAF (minimum allele frequency < 0.1 %) presence in the public genome databases (the gnomAD, Iranome) and internal variant databases, variant pathogenicity and effect using computational tools (MutationTaster, FATHMM-MKL, LRT, and LIST-S2, etc.), clinically relevant variant databases, and previously described associations with the phenotype according to the performed literature review. The variants were classified into different categories, such as pathogenic, likely pathogenic, VUS (variant of uncertain significance), benign, and likely benign, following the ACMG guidelines [8]. From the WES data, a considerable number of variants were detected and filtered for high frequency, leaving a modest number of variants for analysis. Variants were then prioritized if causing splicing region disruption, stop codon , or frameshift mutation. To assess the pathogenicity of the identified variants, in silico analysis was utilized. The prioritized variants were then manually reviewed by a team of experts to identify potential disease-causing mutations.
3.2. Sanger sequencing
To validate the variant identified in the proband, Sanger sequencing was employed. The variant was also examined in the proband's parents for segregation analysis. Sanger sequencing was conducted using the ABI Sequencer 3500XL PE and the BigDye Terminator v3.1 Cycle Sequencing Kit (Life Technologies; Thermo Fisher Scientific, Shanghai, China) (Applied Biosystems, CA, USA). Standard techniques were followed for PCR amplification, purification of the PCR product, and Sanger sequencing. Specific primers targeting exon 4 of the BRAT1 locus and its flanking intronic regions were designed using Gene Runner Software (v 3.05). The obtained sequences were analyzed using Chromas Lite software (Technelysium Pty Ltd, Australia) and compared with the reference sequence in GenBank.
3.3. Prediction analysis
This study utilized several computational tools and databases to predict the potential impact of a genetic variant on protein function and structure. The AlphaFold method was used to predict the protein structure since the experimentally confirmed structure was not available [9]. To visualize the effect of a missense mutation, the PremPS computational tool was used, which analyzes protein structures and predicts the effect of amino acid substitutions on protein function. The results obtained from the PremPS analysis were critical in understanding the impact of the missense mutation on the protein structure and its interactions [10].
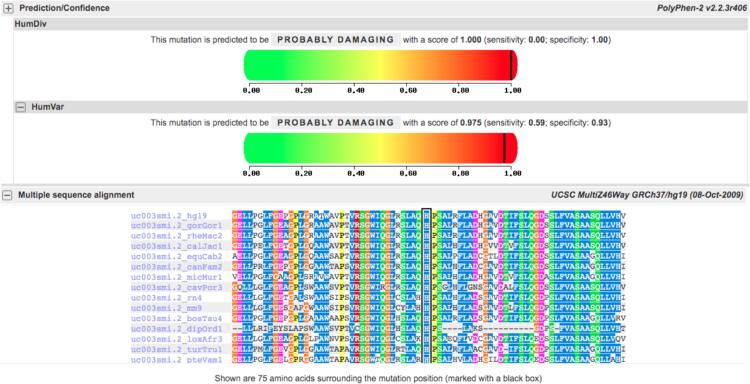
Polyphen-2 was used to assess the evolutionary conservation of the amino acid residue at position 133. I-Mutant was used to estimate the impact of amino acid substitutions on protein stability and aggregation. However, it is important to note that computational predictions provide insights but should be validated through experimental studies, such as functional assays or structural characterization, to confirm the actual impact of the c.398A>G (p.His133Arg) variant on the BRAT1 protein.
4. Results
Among the WES results, a novel homozygous variant was identified as c.398A>G (p.His133Arg), located in exon 4 of BRAT1 (NM_152743.4). This variant had not been previously reported in general genome databases [11], [12] and is considered a variant of uncertain significance (VUS) according to the American College of Medical Genetics and Genomics (ACMG) guidelines [8]. However, in silico tools including VARITY [13], DANN [14], MetaRNN [15], BayesDel_addAF [16], EVE [17], Likelihood ratio test (LRT), PROVEAN, SIFT (sorts intolerant from tolerant), and MutationTaster [18] suggest that the variant is likely to be pathogenic. To validate the presence of the variant, Sanger sequencing was performed on the probands exhibiting suspected clinical manifestations, and heterozygous genotypes were segregated and confirmed in their parents.
The results of the various computational prediction analyses suggest that the p.His133Arg variant in the BRAT1 protein may have pathogenic implications. The AlphaFold predicted structure of the protein indicates that the missense mutation may impact protein interactions, as observed through the PremPS computational tool (Fig. 1). Additionally, the highly conserved histidine at position 133 and the predicted impact on protein stability and aggregation as suggested by I-Mutant further imply that the variant may have a functional impact on the protein. The substitution of histidine to arginine at this position is predicted to be potentially functionally impactful, as arginine is a structurally and chemically different amino acid than histidine and may alter the protein's function. The results from Polyphen-2 suggest that the substitution at position 133 may have a significant impact on the protein's function (Fig. 2). Therefore, this alteration may cause changes in protein structure and function, potentially leading to impaired RNA metabolism and impacting normal brain development. While these computational predictions provide valuable insights, experimental studies such as functional assays or structural characterization are necessary to confirm the actual impact of the variant on the protein. Nonetheless, the results of this study suggest that the c.398A>G (p.His133Arg) variant could be a pathogenic mutation in the BRAT1 protein, and further investigation is warranted.
Fig. 2.
Predicted Pathogenicity Score for BRAT1 p.His133Arg Variant by PolyPhen-2 and Conservation Analysis of His133 Residue across Different Species.
5. Discussion
BRAT1 is a highly conserved gene that encodes a protein involved in RNA metabolism, DNA repair, and telomere maintenance [19]. Variants in BRAT1 can lead to a disruption in protein function, potentially affecting various cellular processes. Understanding the genotype-phenotype correlations in BRAT1-related disorders is crucial for accurate diagnosis and genetic counseling [2]. This discussion focuses on extending the genotype and phenotype of the BRAT1 by introducing a novel biallelic variant, c.398A>G (p.His133Arg), and its potential implications based on the literature review, along with its probable association with clubfoot.
In a recent study conducted by Camille Engel et al. (2023), the authors reviewed previously reported cases of BRAT1-related disorders, which amounted to a total of 40 individuals. Furthermore, the study collected clinical and molecular data from an additional 57 cases, allowing for the study of a large cohort of 97 individuals. The study's findings provided valuable insight into the clinical presentation and genetic underpinnings of BRAT1-related disorders, enablingthe researchers to draw phenotype-genotype correlations [2].
Affected patients with BRAT1 mutations can present with two distinct clinical phenotypes: the severe BRAT1-related RMFSL phenotype and the milder BRAT1-related NEDCAS phenotype. Biallelic null variants are generally associated with the severe phenotype, while missense variants are associated with the milder phenotype. Previous studies highlighted the importance of considering BRAT1-related neurodevelopmental disorders in the differential diagnosis of epileptic encephalopathy with rigidity and suggested that it could provide novel therapeutic perspectives [2].
In the previous study, all Iranian patients exhibited the severe form of the condition (RMFSL phenotype) [2]. However, our present study reports an Iranian case with a milder phenotype (NEDCAS), the first case of its kind in Iran. The novel biallelic variant, c.398A>G (p.His133Arg), may disrupt DNA repair processes, potentially contributing to the observed neurodevelopmental phenotype.
Engel et al. previously reported four individuals with BRAT1 mutations from Algeria, France, Italy, and Egypt who presented with clubfoot [2]. The Egyptian family had two affected individuals, with the younger girl having clubfoot while the older girl did not. From the four cases with clubfoot reported by Engel et al., three cases were male and exhibited the severe RMFSL phenotype, while the fourth case, from Egypt, was female and showed the milder NEDCAS phenotype. Our study reported the first documented instance of a male with the NEDCAS phenotype who also has clubfoot. It is important to note that clubfoot is not a typical symptom of BRAT1-related disorders.
Despite extensive research, the precise mechanisms underlying clubfoot formation remain elusive. Numerous genetic and environmental factors have been implicated, such as gene mutations, maternal smoking, and intrauterine constraints. However, the intricate interactions and specific contributions of these factors to clubfoot pathogenesis require further investigation.
Molecularly, one hypothesis is that a disruption in the normal muscle–tendon-bone interactions during fetal development may contribute to the development of clubfoot. Another hypothesis is that abnormal development of the nervous system, particularly the motor neurons that control muscle movement, may play a role in clubfoot development. Additionally, abnormalities in the connective tissue, such as collagen, may contribute to the development of clubfoot.
Variants in BRAT1 have been associated with a range of neurodevelopmental disorders, suggesting that this gene plays a crucial role in brain development. It is possible that alterations in the BRAT1 gene could impact the development of the motor neurons that control muscle movement, leading to clubfoot. However, further research is needed to investigate this potential mechanism. Overall, our findings underscore the importance of considering both genetic and clinical factors in the evaluation and management of clubfoot. By better understanding the underlying mechanisms that contribute to this condition, we may be able to develop more effective treatments and improve patient outcomes.
Expanding the genotype spectrum of BRAT1 is crucial for improving diagnostic accuracy and understanding the full range of clinical manifestations associated with BRAT1-related disorders. The identification of the novel biallelic variant c.398A>G (p.His133Arg) further emphasizes the importance of comprehensive genetic testing and the need to consider BRAT1 mutations in patients with intellectual disability and epilepsy.
The phenotypic presentation associated with BRAT1 pathogenic variants is highly variable, as highlighted in the literature review. Individuals with BRAT1 mutations commonly present with intellectual disability, epilepsy, speech delay, motor impairments, and autistic features. However, additional features, such as microcephaly, have also been observed in some cases. We then investigated whether the novel variant c.398A>G (p.His133Arg) is associated with a distinct clinical phenotype or if it aligns with the previously reported spectrum of BRAT1-associated disorders.
One possible explanation for the variability in phenotypes associated with mutations in BRAT1 is that the location and type of mutation can have different effects on protein function. For example, mutations that occur in the highly conserved regions of the gene may have a greater impact on protein function, leading to more severe phenotypes, whereas mutations that occur in less conserved regions may have less significant effects and result in milder phenotypes.
In addition, the specific functions of the BRAT1 protein may also contribute to the variability in phenotypes. BRAT1 is involved in multiple cellular processes, including RNA metabolism, DNA repair, and telomere maintenance. Mutations that affect one or more of these functions may lead to different phenotypes, depending on the specific cellular processes that are disrupted.
The variability in phenotypes associated with mutations in BRAT1 is likely due to a combination of factors, including the location and type of mutation, the specific functions of the protein, and the influence of other genetic and environmental factors. For example, mutations in other genes or exposure to environmental toxins may interact with BRAT1 mutations to modulate the severity or type of phenotype observed. According to Engel et al., the type of mutation is important in the severity of the phenotype, where biallelic nonsense, frameshift or inframe deletion/insertion variants cause a severe form of the disease (RMFSL phenotype). On the contrary, genotypes with at least one missense were more likely associated with the NEDCAS phenotype. Our findings also align with previous observations, and back up the genotype-phenotype correlation suggested by Engel et al. [2]. This genotype-phenotype correlation is particularly important in genetic counseling in clinical settings.
In conclusion, mutations in the BRAT1 gene have been associated with a wide range of phenotypes. The novel biallelic variant c.398A>G (p.His133Arg) in this gene may disrupt DNA repair processes, leading to genomic instability and contributing to the observed neurodevelopmental phenotype. The variability in phenotypes associated with BRAT1 mutations is likely due to a combination of factors, including the location and type of mutation, the specific functions of the protein, and the influence of other genetic and environmental factors. Further clinical and functional studies are needed to fully understand the underlying mechanisms and factors that contribute to the phenotypic variability associated with BRAT1 mutations.
Ethical approval and consent to participate
This study was approved by the Research Ethics Committee of the Faculty of Medicine, Shahid Beheshti University of Medical Sciences, and was conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from adult participants to participate in the study. Written informed consent was obtained from parents for all participants aged under 18.
Funding
No external funding was used for this study.
CRediT authorship contribution statement
Mohammad-Reza Ghasemi: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Sahand Tehrani Fateh: Visualization, Software, Investigation, Data curation. Farzad Hashemi-Gorji: Writing – review & editing, Validation, Investigation, Data curation. Morteza Sheikhi Nooshabadi: Investigation, Data curation. Sahar Alijanpour: Investigation, Data curation. Ali Mardi: Investigation. Mohammad Miryounesi: Writing – review & editing, Validation, Supervision, Resources, Project administration, Methodology, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Sincere gratitude to the families for their participation in this study.
Data availability
The data that support the findings of this study are available from the corresponding authors, upon request.
References
- 1.Parenti I., et al. Neurodevelopmental disorders: from genetics to functional pathways. Trends Neurosci. 2020;43(8):608–621. doi: 10.1016/j.tins.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Engel C., et al. BRAT1–related disorders: phenotypic spectrum and phenotype-genotype correlations from 97 patients. Eur J Hum Genet. 2023:1–9. doi: 10.1038/s41431-023-01410-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MWer S., Dykes D., Polesky H. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H., et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKenna A., et al. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang K., Li M., Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richards S., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–423. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jumper J., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y., et al. PremPS: predicting the impact of missense mutations on protein stability. PLoS Comput Biol. 2020;16(12):e1008543. doi: 10.1371/journal.pcbi.1008543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auton A., et al. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The GenomeAsia 100K Project enables genetic discoveries across Asia %J Nature. 2019; 576(7785):106-111. [DOI] [PMC free article] [PubMed]
- 13.Wu Y., et al. Improved pathogenicity prediction for rare human missense variants. Am J Hum Genet. 2021;108(10):1891–1906. doi: 10.1016/j.ajhg.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quang D., Chen Y., Xie X. DANN: a deep learning approach for annotating the pathogenicity of genetic variants. Bioinformatics. 2014;31(5):761–763. doi: 10.1093/bioinformatics/btu703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C., et al. MetaRNN: differentiating rare pathogenic and rare benign missense SNVs and InDels using deep learning. Genome Med. 2022;14(1):115. doi: 10.1186/s13073-022-01120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian Y., et al. REVEL and BayesDel outperform other in silico meta-predictors for clinical variant classification. Sci Rep. 2019;9(1):12752. doi: 10.1038/s41598-019-49224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frazer J., et al. Disease variant prediction with deep generative models of evolutionary data. Nature. 2021;599(7883):91–95. doi: 10.1038/s41586-021-04043-8. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz J.M., et al. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7(8):575–576. doi: 10.1038/nmeth0810-575. [DOI] [PubMed] [Google Scholar]
- 19.Cihlarova Z., et al. BRAT1 links Integrator and defective RNA processing with neurodegeneration. Nat Commun. 2022;13(1):5026. doi: 10.1038/s41467-022-32763-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanes I., Kozenko M., Callen D.J. Lethal neonatal rigidity and multifocal seizure syndrome—a misnamed disorder? Pediatr Neurol. 2015;53(6):535–540. doi: 10.1016/j.pediatrneurol.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Puffenberger E.G., et al. Genetic mapping and exome sequencing identify variants associated with five novel diseases. PLoS One. 2012;7(1):e28936. doi: 10.1371/journal.pone.0028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuovo S., et al. Clinical variability at the mild end of BRAT1-related spectrum: evidence from two families with genotype–phenotype discordance. Hum Mutat. 2022;43(1):67–73. doi: 10.1002/humu.24293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors, upon request.