Highlights
-
•
No data regarding how epilepsy patients with neurostimulation view exercise exists.
-
•
We surveyed these patients about their barriers, beliefs, and interests in exercise.
-
•
At least 90% noted one or more common barriers to exercise participation.
-
•
Perceived increased activation of autostimulation may prevent exercise participation.
-
•
Only 8% reported knowledge of ILAE recommendations for exercise in epilepsy.
Keywords: Epilepsy, Exercise, Neurostimulation, Survey, Barriers, Beliefs, Guidelines
Abstract
Exercise improves many comorbidities associated with epilepsy in addition to seizure control. Despite the ILAE consensus statement noting the positive effects of exercise in patients with epilepsy (PWE) and individual assessment of risks pertaining to these activities, many healthcare professionals, including neurologists, are unfamiliar with these guidelines. Neurostimulation is an increasingly prevalent treatment option for refractory epilepsy. To date, no literature exists regarding how PWE treated with neurostimulation devices view and participate in exercise. We surveyed 36 adult PWE treated with neurostimulation (11 VNS, 21 RNS, 3 DBS, 1 VNS+RNS) on their barriers, beliefs, activity levels, and interests in exercise. Forty-three percent of patients reported meeting AHA guidelines for physical activity. Ninety percent of participants noted at least one barrier to exercise with transportation being most common. Fear of embarrassment of a seizure during exercise was reported by 44% with 39% endorsing prior seizure while exercising. Device-specific barriers included fear of device damage or avoidance of specific exercises. There was a statistically significant effect on activity level and prior seizure while exercising. Only 8% of participants reported knowledge of exercise guidelines for PWE. This data provides insight into the views of PWE treated with neurostimulation devices on exercise.
1. Introduction
Approximately 3 million adults in the US have active epilepsy described as individuals with a diagnosed seizure disorder who were currently taking medication to control it, had a seizure in the past year, or both [1]. One-third of patients with epilepsy (PWE) are resistant to antiseizure drugs or continue to seize despite the use of two appropriately chosen and tolerated medications [2], [3]. Subsequently, treatment often extends beyond medication and can include resective surgery, laser interstitial thermal therapy (LITT), neurostimulation, or a combination of treatments. Neurostimulation has been shown to improve seizure control, quality of life, and comorbid psychiatric disorders with the use of both FDA-approved [4], [5], [6], [7] and other devices, e.g. subthreshold cortical stimulation (CSCS) [8], [9], [10], transcranial magnetic stimulation (TMS) [11], and transcranial direct stimulation (tDCS) [12], [13]. Widely used FDA-approved therapies consist of vagus nerve stimulation (VNS), deep brain stimulation (DBS), and responsive neurostimulation (RNS).
Exercise has emerged as a potential complementary therapy for epilepsy. Interestingly, PWE reported decreased seizure frequency, improved quality of life, and lower levels of depression [14], [15], [16], [17]. Prior studies have also sought to identify the barriers to and level of activity in these individuals. The major barriers to exercise in PWE were lack of social support, access to facilities, transportation, and fear of injury, seizure activity, or the perception of others [15], [17], [18], [19]. PWE were often sedentary [20] or exercised three or fewer days per week at light intensity [21].
Despite the benefits of exercise in PWE and the increasing prevalence of neurostimulation for treatment, the attitudes, beliefs, and barriers regarding exercise in this population remain unknown. Therefore, our study aimed to investigate exercise in PWE treated with neurostimulation devices with similar goals of assessing their knowledge of the benefits, beliefs, levels of activity, and barriers to exercise. Future efforts can address common misconceptions regarding exercise in epilepsy. However, understanding of novel device-specific barriers and development of exercise regimens and recommendations is of critical importance to improving seizure control and overall quality of life in this population of refractory epilepsy patients.
2. Materials and methods
2.1. Participant recruitment
This study was approved by the Penn State Health Milton S. Hershey Medical Center Institutional Review Board (IRB-00023752). Our potential study participants were screened for inclusion and exclusion criteria prior to contact regarding survey participation. Individuals with RNS and DBS were contacted over the phone due to the low frequency of visits and a database of patients previously presented during our multidisciplinary surgical conference that also includes those with laser ablation and resection. Due to the frequent clinics for our many patients implanted with VNS but no readily available list, these individuals were approached by researchers at the beginning of their regularly scheduled outpatient epilepsy visits. We contacted 38 patients for a total of 36 recruits with an enrollment percentage of 94.7%. Eligibility criteria included an age of 18 years or older, a diagnosis of epilepsy with a FDA-approved device in place, and proficiency in the English language. Individuals were excluded if they had cognitive impairment or severe intellectual disability precluding them from completing the survey, and current pregnancy. Patients with physical limitations were not excluded but allowed to list it as a barrier to exercise. Demographic data for these patients is detailed in Table 1.
Table 1.
Demographic Data and Epilepsy Characteristics with Activity Level. Demographic data is listed based on code number for patient. ASM no. = Number of antiseizure medications taken per day. Seizures/mo. = estimate of seizure frequency per month. *Refers to survey question of an average 150 min of moderate-intensity aerobic activity or 75 min of vigorous aerobic activity per week.
|
Demographic Data and Epilepsy Characteristics with Activity Level | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | Sex | Age | Race | Epilepsy Classification | Device | ASMno. |
Seizures/ mo. |
Active* | Other surgery |
| 1 | F | 45 | White | Generalized onset | VNS | 0 | 0 | Y | |
| 2 | M | 36 | Two or more | Focal onset | VNS | 3 | 0 | Y | |
| 3 | M | 65 | Black | Focal onset, unaware | VNS | 3 | 0 | N | |
| 4 | M | 37 | White | Bitemporal, focal onset | RNS | 2 | 60 | N | Left temporal lobectomy |
| 5 | F | 51 | White | Right frontotemporal, focal onset | RNS | 3 | 0 | Y | Right temporal laser ablation |
| 6 | F | 60 | White | Bitemporal, focal onset | RNS | 3 | 30 | N | |
| 7 | F | 49 | White | Right temporal, focal onset | RNS | 3 | 1 | N | |
| 8 | M | 24 | White | Right parietotemporal, focal onset | VNS | 3 | 0 | N | |
| 9 | F | 53 | White | Bitemporal, focal onset | RNS+VNS | 2 | 3 | Y | |
| 10 | M | 40 | White | Left temporal, focal onset | RNS | 3 | 5 | Y | Left anterior temporal lobectomy |
| 11 | F | 57 | White | Bitemporal, focal onset | RNS | 3 | 0 | N | |
| 12 | M | 25 | White | Generalized onset | VNS | 3 | 1 | N | |
| 13 | F | 42 | White | Right temporal, focal onset | RNS | 3 | 2 | Y | |
| 14 | F | 27 | White | Generalized onset | VNS | 2 | 8 | N | |
| 15 | F | 21 | White | Generalized onset | VNS | 0 | 0 | N | |
| 16 | F | 41 | White | Left temporal, focal onset | VNS | 3 | 16 | N | |
| 17 | F | 27 | White | Generalized onset | VNS | 1 | 5 | N | |
| 18 | F | 45 | White | Right temporal, focal onset | VNS | 4 | 3 | N | |
| 19 | M | 77 | White | Focal onset | VNS | 3 | 0 | N | |
| 20 | M | 53 | White | Bitemporal, focal onset | RNS | 2 | 1 | N | |
| 21 | F | 23 | White | Right temporal, focal onset | RNS | 1 | 12 | Y | |
| 22 | F | 48 | White | Right temporal, focal onset | RNS | 1 | 5 | Y | |
| 23 | F | 39 | Other | Left frontotemporal, focal onset | RNS | 3 | 3 | Y | |
| 24 | F | 32 | White | Bitemporal, focal onset | RNS | 1 | 0 | Y | |
| 25 | M | 36 | White | Bitemporal, focal onset | RNS | 2 | 5 | Y | |
| 26 | M | 60 | White | Bitemporal, focal onset | RNS | 3 | 61 | N | |
| 27 | M | 44 | White | Right frontal, focal onset | RNS | 3 | 6 | N | Right temporal lobectomy |
| 28 | M | 54 | White | Left frontal, focal onset | RNS | 3 | 1 | Y | |
| 29 | F | 41 | White | Right frontotemporal, focal onset | RNS | 2 | 1 | N | |
| 30 | M | 36 | Other | Left temporal, focal onset | RNS | 2 | 7 | N | |
| 31 | F | 44 | Other | Left temporal, focal onset | RNS | 2 | 3 | Y | |
| 32 | F | 25 | Other | Generalized onset | DBS | 3 | Unknown | N | |
| 33 | F | 50 | White | Focal onset | DBS | 4 | 2 | N | |
| 34 | F | 39 | White | Left temporal, focal onset | RNS | 3 | 17 | Y | Right temporal lobectomy |
| 35 | M | 52 | White | Left temporal, focal onset | RNS | 4 | 2 | N | |
| 36 | M | 19 | White | Bilateral frontal, focal onset | DBS | 4 | 3 | Y | |
2.2. Demographic data collection
VNS patients were screened prior to their regularly scheduled appointments, whereas RNS and DBS patients were extracted from an epilepsy surgical database. From their medical record we collected age at the time of survey completion, sex assigned at birth, race, type of epilepsy, current anti-seizure medications (ASMs) and dosage, date of device implantation, seizure frequency per month, and prior epilepsy surgeries.
2.3. Survey procedure
Patients were approached either by phone or at the clinic visit according to the participant recruitment process. The survey began with a detailed informed consent document and after the patient verbally agreed over the phone or signed the consent form, the survey began. We asked questions regarding barriers, beliefs, current activity levels, interests in future exercise participation, and any contributing thoughts for a total of 20 questions.
Survey questions relevant to barriers patients face with exercise were adapted from “reasons for inactivity questionnaire” from Roth et al [17]. These were largely yes/no questions with one question of multiple choice regarding common barriers and barriers specific to epilepsy such as fear of seizure during exercise. Additionally, questions regarding barriers specific to devices were included for this patient population (Table 2). The percentage of patients responding yes are presented except for the multiple-choice question which is expressed as those selecting one or more answer choices.
Table 2.
Barriers to Exercise in Patients with Epilepsy and Exercise. Survey Participants responded yes or no to the questions. Corresponding percentages reflect those that selected yes. *This question is further represented in Fig. 1. **Percentages of this question reflect one or more selected options.
| Question | Percent answering yes |
|---|---|
| What barriers to exercise do you have? (listed options include interest in exercise, access to exercise facilities, transportation, time, someone to exercise with, or other)* | 91% (VNS), 95% (RNS), 100% (DBS), 100% (RNS+VNS)** |
| Are you afraid of how others perceive you while exercising? | 18% (VNS), 10% (RNS), 0% (DBS), 100% (RNS+VNS) |
| Do you have other health problems, besides epilepsy, that prevent you from exercising? | 55% (VNS), 35% (RNS), 67% (DBS), 0% (RNS+VNS) |
| Are you afraid that exercise may lead to health problems? | 18% (VNS), 15% (RNS), 67% (DBS), 0% (RNS+VNS) |
| Have you previously experienced a seizure while exercising? | 36% (VNS), 45% (RNS), 0% (DBS), 100% (RNS+VNS) |
| Are you afraid you may be embarrassed if you have a seizure while exercising? | 55% (VNS), 40% (RNS), 33% (DBS), 100% (RNS+VNS) |
| Have you been told to avoid exercise by a physician? | 0% (VNS), 5% (RNS), 0% (DBS), 100% (RNS+VNS) |
| Are you concerned that exercise will interfere with your anti-seizure medications? | 9% (VNS), 5% (RNS), 0% (DBS), 0% (RNS+VNS) |
| Have you been discouraged from exercising by family/friends? | 9% (VNS), 5% (RNS), 33% (DBS), 0% (RNS+VNS) |
| Before you underwent device placement, did you exercise on average more per week? | 9% (VNS), 50% (RNS), 33% (DBS), 100% (RNS+VNS) |
| Do you believe your device might become damaged with exercise? | 18% (VNS), 10% (RNS), 33% (DBS), 0% (RNS+VNS) |
| Do you avoid certain exercises now that you have a device in place? | 27% (VNS), 15% (RNS), 67% (DBS), 100% (RNS+VNS) |
| Have you been told by family/friends you should avoid certain exercises now that you have a device in place? | 27% (VNS), 10% (RNS), 67% (DBS), 0% (RNS+VNS) |
Survey questions pertaining to patients’ beliefs about the effects of exercise on epilepsy were adapted from a survey developed by Arida et al, which was designed to assess the attitudes and perceptions of neurologists [22]. We specifically surveyed patients’ perceptions about exercise reducing seizure frequency, improving cognitive function, and decreasing psychiatric comorbidity. This section also included knowledge of the ILAE’s recommendation on exercise in epilepsy.
To assess if patients were active, we asked if they average 150 minutes of moderate-intensity aerobic activity or 75 minutes of vigorous aerobic activity per week which is recommended by the American Heart Association (AHA). Patients were also asked if they were interested in joining an exercise program for patients with epilepsy and any additional questions.
2.4. Statistical analysis
To evaluate self-reported activity levels of patients as active (meeting AHA recommendations) and inactive as well as correlation with differing variables, t-tests assuming equal variances were used for numerical data. Nominal data was analyzed with Fisher’s exact t-test. We specifically evaluated the following variables: number of ASMs, prior seizure while exercising, seizure frequency per month, autostimulation for VNS, and number of barriers including total, epilepsy, and device specific.
3. Results
Over a five-month period, a total of 36 PWE and a neurostimulation device for treatment including VNS (11, 31%), RNS (21, 58%), DBS (3, 8%), and VNS with RNS (1, 3%) were surveyed regarding exercise beliefs, barriers, activity levels and interests. Specific clinical characteristics for each patient are provided in Table1. The mean age of the population was 42 years ± 13.4 (range 19–77) with 58% being female (21). Patients self-identified as White (30, 83%), Other (4, 11%), Black (1, 3%), and two or more races (1, 3%). Most patients were diagnosed with focal epilepsy (31, 86%) and taking a mean of 2.5±1.0 (range 0–4) antiseizure medications. The mean duration of neurostimulation was 5.75 ± 3.5 years (range 1–17). Sixteen patients reported achieving the target level of aerobic activity per week recommended by the AHA consisting of two with VNS, 12 with RNS, one with DBS, and one with dual systems.
3.1. Barriers to exercise
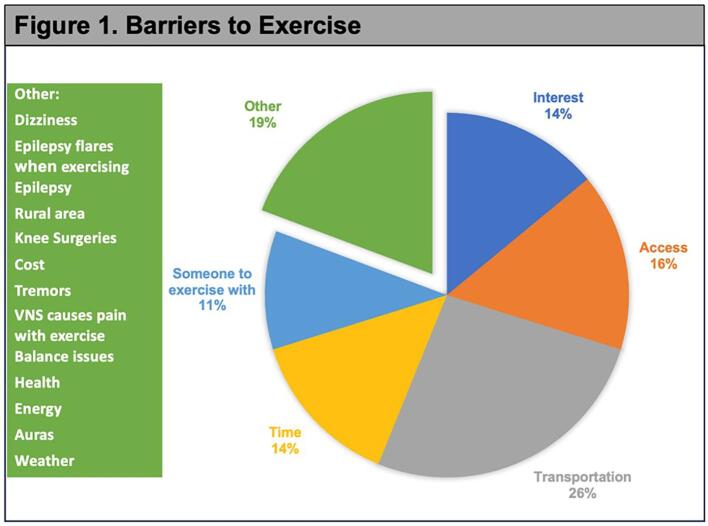
Participants were asked about common barriers to exercise with at least 90% reporting the same difficulties. Transportation (26%) was identified as the most common hindrance followed by access to facilities (16%), interest in exercise (14%), time (14%), and someone to exercise with (11%). Additional barriers noted by participants are listed in Fig. 1.
Fig. 1.
Barriers to Exercise. Participants were asked a multiple-choice question regarding common exercise barriers with an option to write in other barriers which are listed on the right-hand table. Intended for color reproduction on the Web.
Other health problems preventing exercise included migraine, brittle diabetes, anxiety, Parkinson’s disease, post-traumatic stress disorder (PTSD), generalized anxiety disorder, attention deficit hyperactivity disorder, prediabetes, Lyme disease, arthritis, Ehlers Danlos syndrome, heart problems, multiple sclerosis, hypertension, asthma, blindness, fibromyalgia, and shoulder surgery. Those patients listing PTSD as a health problem may suffer from comorbid psychogenic nonepileptic spells (PNES). Four study participants had a concurrent diagnosis of PNES with two responding to the questions about their activity level. One self-reported as active while the other registered as inactive (Fig. 2).
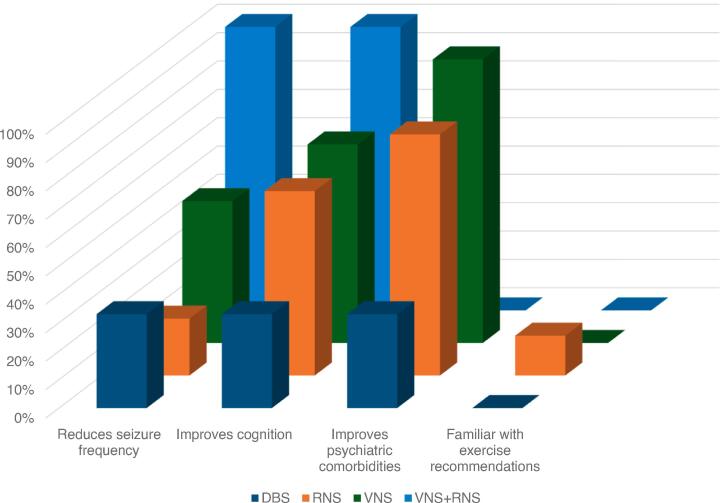
Fig. 2.
Beliefs on Exercise in Epilepsy. Percentages are those that responded yes listed by device. Questions asked if patients believed that exercise reduces seizure frequency, improves cognition, and improves psychiatric comorbidities such as anxiety and depression. Most participants were not familiar with the ILAE guidelines for exercise in patients with epilepsy. Intended for color reproduction on the Web.
Epilepsy specific barriers were listed as prior seizure while exercising, fear of embarrassment if a seizure occurs while exercising, advice to avoid exercising by a physician, interference of exercise with ASM, and discouragement of exercise by family and friends. Of these, the most frequently reported barrier was embarrassment of having a seizure while exercising at 44%. Nearly 40% of all participants experienced a seizure while exercising in the past, which had a statistically significant effect on their activity level (p = 0.032). The mean number of ASMs did not significantly differ between active and inactive patients (p = 0.10) nor did the frequency of seizures (p = 0.26). A minor group of patients were discouraged from engaging in exercise by physicians (5.6%) or family and friends (8.3%).
Patients responded to survey questions about device-specific barriers to exercise which included device implantation, fear of damage to the device, avoidance of certain exercises, and discouragement by family or friends. Thirty-seven percent of patients reported reduced exercise after device placement. This group was stratified into 50% of RNS, 33% of DBS, 9% of VNS, and 100% of dual system patients (Table 2). The VNS device contains a special cardiac-based seizure detection that triggers automated stimulation (autostimulation) for increases in the heart rate at a set percentage. Autostimulation was enabled in eleven (92%) of the patients with VNS with only one of them being active. The lone patient without this feature enabled was inactive. There was no significant difference in activity level for VNS specific patients compared to other devices (p = 0.13). No significant differences for the number of total barriers, consisting of the common and those specific to exercise or the device, were found between the active and inactive participants (p = 0.29).
3.2. Beliefs regarding exercise
Participants believed that exercise improved cognitive function and psychiatric comorbidities such as anxiety and depression at 61% and 81%, respectively. However, only 31% of patients believe that exercise can reduce seizure frequency, which was comprised of 20% RNS, 50% VNS, 33% DBS, and 100% RNS+VNS patients. Only 8% of patients reported familiarity with exercise in epilepsy recommendations and none could accurately convey these guidelines.
3.3. Future interest
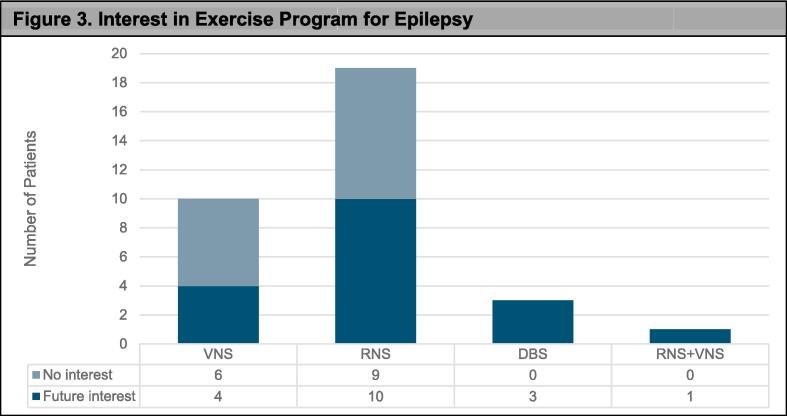
Fifty-five percent of participants reported interest in an exercise program for epilepsy including 53% of RNS, 40% of VNS, 100% DBS, and 100% of the VNS+RNS patients. See Fig. 3 for responses per device type.
Fig. 3.
Interest in Exercise Program for Epilepsy. Overall majority of patients are interested in participating in an exercise program for those with epilepsy. Table below graph details breakdown of the number of patients responding yes or no. Intended for color reproduction on the Web.
3.4. Other concerns
Participants were asked if there were questions that they wished we asked in our survey. We obtained the following responses: What types of activities cause adverse reactions (example vigorous cardio causes seizures but lifting fine)? What recommended exercises? If there is a epilepsy group that exercises in rural areas. Obstacles to exercise that are specifically related to exercise. Other notes from patients included “patients who are encouraged to exercise should also be reminded to be mindful of how they are feeling before they do so” and “very interested in epilepsy exercise group”.
4. Discussion
Our study found several barriers to exercise in patients diagnosed with epilepsy who are treated with neurostimulation devices. We identified common barriers to PWE who are not treated with neurostimulation including lack of transportation, fear of seizure during exercise, and discouragement from physicians and family or friends. Patients also endorsed device specific concerns which is reflected by the low activity level as only 43% engaged in physical exercise. Most of the patients believe that exercise had positive effects on cognitive and psychiatric comorbidities yet only half were interested in exercise. Lastly, only a small subset of patients was aware of the ILAE guidelines regarding exercise. There are several important conclusions that can be drawn from this study.
Less than half of patients reported achieving the recommended activity level by the AHA. Prior literature indicates that 51% of PWE engaged in physical activity [23] and were about half as active as patients without epilepsy [24]. However, patients are often discouraged from participating in physical activity and sports by both friends or family members and physicians [23]. Arida et al demonstrated that approximately 60% of surveyed neurologists in Latin America were unaware of the ILAE guidelines pertaining to exercise in epilepsy but 92.5% advised against participation [22]. Our study findings coincide with this data noting that 19% were specifically advised to avoid certain exercises after device implantation but device companies only advise against contact sports with no specific restrictions found in the literature.
A major barrier to exercise reported by our patients was fear of a seizure while exercising. Forty four percent noted fear of embarrassment if a seizure occurred during exercise which correlates to prior literature where 45% of PWE endorsed the same concern [23]. However, almost 40% of our participants experienced a seizure when exercising in the past, which vastly differs from PWE alone as 84% never had a seizure during exercise [23]. This is most likely due to our special population as seizures were refractory to medication and thus more difficult to treat and possibly more likely to occur further implied by the need for neurostimulation.
Occurrence of seizures during exercise in the past did have a significant difference on activity level. This may account for the overall low activity level for our population at 43% with 37% of patients reporting more frequent exercise prior to device placement. Additionally, there may be device-specific concerns with enabling the autostimulation feature as patients could experience increased device activation resulting in painful stimulation even in the absence of seizure activity during exercise [25]. While in our study population all but one VNS patient had the feature enabled, the majority were inactive. The sample size was underpowered to sufficiently detect such changes and our patients were not asked about perceived device activation during exercise.
Our data demonstrates that patients lack knowledge regarding ILAE guidelines pertaining to exercise in epilepsy as 92% were not familiar with the recommendations and none provided accurate descriptions. The guidelines emphasize the importance of engaging in physical exercise for PWE, stratifying physical activity based on seizure characteristics [26]. The limited awareness of these recommendations represents a target area for education amongst patients and health care providers.
4.1. Limitations
There are several potential limitations to this study. First, our study only includes 36 patients over a five-month period thereby limiting generalizability. The small sample size consists of a heterogenous population as patients treated with three varying devices were questioned. Therefore, it is difficult to detect differences amongst the device subgroups. Finally, the applicability of our findings to other populations may be limited, given the low representation of ethnic and racial minorities within the sample, compared with the overall U.S. population.
5. Conclusion
In this study, PWE who are treated with neurostimulation were surveyed regarding their activity level, beliefs, barriers, and interests in exercise at a level IV epilepsy center. Our study is the first to investigate exercise in PWE treated with neurostimulation. Known barriers such as lack of transportation and embarrassment or fear of seizure while exercising in addition to limited knowledge regarding ILAE recommendations for exercise parallel findings from the literature about PWE. However, barriers such as fear of damage to the device, prior seizure while exercising significantly impacting activity level, and varying activity levels amongst the devices used for treatment were newly identified. Additionally, perceived increased activation of the autostimulation feature may further restrict or prevent participation in exercise and requires further investigation. Future research can first address these barriers and then develop exercise regimens specifically targeted to this population followed by actively educating, encouraging, and engaging these patients and providers to improve their seizure control and quality of life.
The work described in the manuscript “Exercise in patients with epilepsy and neurostimulation devices- physical activity levels, barriers, and beliefs” has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
CRediT authorship contribution statement
Sarah Mauney: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Papul Chalia: Project administration. Justine Julien: Project administration. Tiffany Fisher: Writing – review & editing, Supervision, Methodology, Conceptualization, Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Sarah Mauney, Email: smauney@pennstatehealth.psu.edu.
Papul Chalia, Email: pchalia@pennstatehealth.psu.edu.
Justine Julien, Email: jjulien@pennstatehealth.psu.edu.
Tiffany Fisher, Email: tfisher9@pennstatehealth.psu.edu.
References
- 1.Zack M.M., Kobau R. National and State Estimates of the Numbers of Adults and Children with Active Epilepsy - United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(31):821–825. doi: 10.15585/mmwr.mm6631a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwan P., Arzimanoglou A., Berg A.T., Brodie M.J., Allen Hauser W., Mathern G., et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 3.Sultana B., Panzini M.A., Veilleux Carpentier A., Comtois J., Rioux B., Gore G., et al. Incidence and prevalence of drug-resistant epilepsy: a systematic review and meta-analysis. Neurology. 2021;96(17):805–817. doi: 10.1212/WNL.0000000000011839. [DOI] [PubMed] [Google Scholar]
- 4.Englot D.J., Chang E.F., Auguste K.I. Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. J Neurosurg. 2011;115(6):1248–1255. doi: 10.3171/2011.7.JNS11977. [DOI] [PubMed] [Google Scholar]
- 5.Fisher R., Salanova V., Witt T., Worth R., Henry T., Gross R., et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 6.Heck C.N., King-Stephens D., Massey A.D., Nair D.R., Jobst B.C., Barkley G.L., et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. 2014;55(3):432–441. doi: 10.1111/epi.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meador K.J., Kapur R., Loring D.W., Kanner A.M., Morrell M.J. RNS System Pivotal Trial Investigators. Quality of life and mood in patients with medically intractable epilepsy treated with targeted responsive neurostimulation. Epilepsy Behav. 2015;45:242–247. doi: 10.1016/j.yebeh.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Child N.D., Stead M., Wirrell E.C., Nickels K.C., Wetjen N.M., Lee K.H., et al. Chronic subthreshold subdural cortical stimulation for the treatment of focal epilepsy originating from eloquent cortex. Epilepsia. 2014;55(3):e18–e21. doi: 10.1111/epi.12525. [DOI] [PubMed] [Google Scholar]
- 9.Lundstrom B.N., Van Gompel J., Britton J., Nickels K., Wetjen N., Worrell G., et al. Chronic Subthreshold Cortical Stimulation to Treat Focal Epilepsy. JAMA Neurol. 2016;73(11):1370–1372. doi: 10.1001/jamaneurol.2016.2857. [DOI] [PubMed] [Google Scholar]
- 10.Lundstrom B.N., Worrell G.A., Stead M., Van Gompel J.J. Chronic subthreshold cortical stimulation: a therapeutic and potentially restorative therapy for focal epilepsy. Expert Rev Neurother. 2017;17(7):661–666. doi: 10.1080/14737175.2017.1331129. [DOI] [PubMed] [Google Scholar]
- 11.Walton D, Spencer DC, Nevitt SJ, Michael BD. Transcranial magnetic stimulation for the treatment of epilepsy. Cochrane Database Syst Rev 2021;4(4):CD011025. [DOI] [PMC free article] [PubMed]
- 12.Fregni F., Thome-Souza S., Nitsche M.A., Freedman S.D., Valente K.D., Pascual-Leone A. A controlled clinical trial of cathodal DC polarization in patients with refractory epilepsy. Epilepsia. 2006;47(2):335–342. doi: 10.1111/j.1528-1167.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 13.Auvichayapat N., Rotenberg A., Gersner R., Ngodklang S., Tiamkao S., Tassaneeyakul W., et al. Transcranial direct current stimulation for treatment of refractory childhood focal epilepsy. Brain Stimul. 2013;6(4):696–700. doi: 10.1016/j.brs.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Dunabeitia I., Bidaurrazaga-Letona I., Diz J.C., Colon-Leira S., Garcia-Fresneda A., Ayan C. Effects of physical exercise in people with epilepsy: A systematic review and meta-analysis. Epilepsy Behav. 2022;137(Pt A) doi: 10.1016/j.yebeh.2022.108959. [DOI] [PubMed] [Google Scholar]
- 15.Nakken K.O. Physical exercise in outpatients with epilepsy. Epilepsia. 1999;40(5):643–651. doi: 10.1111/j.1528-1157.1999.tb05568.x. [DOI] [PubMed] [Google Scholar]
- 16.McAuley J.W., Long L., Heise J., Kirby T., Buckworth J., Pitt C., et al. A Prospective Evaluation of the Effects of a 12-Week Outpatient Exercise Program on Clinical and Behavioral Outcomes in Patients with Epilepsy. Epilepsy Behav. 2001;2(6):592–600. doi: 10.1006/ebeh.2001.0271. [DOI] [PubMed] [Google Scholar]
- 17.Roth D.L., Goode K.T., Williams V.L., Faught E. Physical exercise, stressful life experience, and depression in adults with epilepsy. Epilepsia. 1994;35(6):1248–1255. doi: 10.1111/j.1528-1157.1994.tb01796.x. [DOI] [PubMed] [Google Scholar]
- 18.Collard S.S., Ellis-Hill C. How do you exercise with epilepsy? Insights into the barriers and adaptations to successfully exercise with epilepsy. Epilepsy Behav. 2017;70(Pt A):66–71. doi: 10.1016/j.yebeh.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Shawahna R., Abdelhaq I. Exploring perceived benefits, motives, barriers, and recommendations for prescribing yoga exercises as a nonpharmacological intervention for patients with epilepsy: A qualitative study from Palestine. Epilepsy Behav. 2020;106 doi: 10.1016/j.yebeh.2020.107041. [DOI] [PubMed] [Google Scholar]
- 20.Green R., Abe C., Denney D.A., Zhang R., Doyle A., Gadelmola K., et al. Physical activity status and quality of life in patients with epilepsy - Survey from level four epilepsy monitoring units. Epilepsy Res. 2021;173 doi: 10.1016/j.eplepsyres.2021.106639. [DOI] [PubMed] [Google Scholar]
- 21.Ablah E., Haug A., Konda K., Tinius A.M., Ram S., Sadler T., et al. Exercise and epilepsy: a survey of Midwest epilepsy patients. Epilepsy Behav. 2009;14(1):162–166. doi: 10.1016/j.yebeh.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Arida R.M., Sales E., Teixeira-Machado L., Prado G.F.D., Gutierre R.C., Carrizosa J. Neurologists' knowledge of and attitudes toward physical exercise for people with epilepsy in Latin America. Epilepsy Behav. 2022;131(Pt A) doi: 10.1016/j.yebeh.2022.108705. [DOI] [PubMed] [Google Scholar]
- 23.Arida R.M., Scorza F.A., de Albuquerque M., Cysneiros R.M., de Oliveira R.J., Cavalheiro E.A. Evaluation of physical exercise habits in Brazilian patients with epilepsy. Epilepsy Behav. 2003;4(5):507–510. doi: 10.1016/s1525-5050(03)00184-7. [DOI] [PubMed] [Google Scholar]
- 24.Bjorholt P.G., Nakken K.O., Rohme K., Hansen H. Leisure time habits and physical fitness in adults with epilepsy. Epilepsia. 1990;31(1):83–87. doi: 10.1111/j.1528-1157.1990.tb05364.x. [DOI] [PubMed] [Google Scholar]
- 25.Fisher R.S., Afra P., Macken M., Minecan D.N., Bagić A., Benbadis S.R., et al. Automatic vagus nerve stimulation triggered by ictal tachycardia: Clinical outcomes and device performance-The U.S. E-37 Trial. Neuromodulation. 2016 Feb;19(2):188–195. doi: 10.1111/ner.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capovilla G., Kaufman K.R., Perucca E., Moshe S.L., Arida R.M. Epilepsy, seizures, physical exercise, and sports: a report from the ILAE Task Force on Sports and Epilepsy. Epilepsia. 2016;57(1):6–12. doi: 10.1111/epi.13261. [DOI] [PubMed] [Google Scholar]