Abstract
Refractory pituitary adenomas are difficult to control tumors that progress through optimal surgical, medical, and radiation management. Repeat surgery is a valuable tool to reduce tumor volume for more effective radiation and/or medical therapy, and to decompress critical neurovascular structures. Advances in surgical techniques and technologies, including minimally invasive cranial approaches, intraoperative MRI suites, and cranial nerve monitoring, have improved surgical outcomes and expanded indications. Today, repeat transsphenoidal surgery has similar complications rates to upfront surgery in historical cohorts. The decision to operate on refractory adenomas should be made with multidisciplinary teams, balancing the benefit of tumor reduction with the potential for complications, including cranial nerve injury, carotid injury, and cerebrospinal fluid leak.
Keywords: Refractory adenoma, Transsphenoidal surgery, Intraoperative guidance, Craniotomy
Introduction
Pituitary adenomas are common intracranial neoplasms, accounting for 10–20% of all primary brain tumors. [1] Transsphenoidal surgery is the first-line treatment for symptomatic or secreting pituitary adenomas, with the general exception of prolactinomas which commonly respond to medical therapy. Gross total resection rates are generally around 60–80% in modern series, but the risk of recurrence is upwards of 50% in those with residual tumor and 7–12% among patients with a complete tumor removal. [2] Recurrent tumors may be treated with radiation or endocrine therapy, but repeat surgery is often considered for tumors that prove to be refractory to radiation and medical management.
In cases of refractory adenomas, repeat surgery is a valuable tool to reduce tumor volume for more effective radiation and/or medical therapy, decompress critical neurovascular structures, and obtain tissue for molecular profiling. [3] Due to the invasive nature of refractory pituitary adenomas, gross total resection is rarely achievable. [3] Though there is a paucity of data in support of this approach in the specific context of refractory adenomas, it is widely acknowledged that surgical debulking with a goal of separating the tumor from the optic apparatus and other critical structures can facilitate the delivery of full doses of radiation. [4] In the absence of clinical trials or prospective data, surgical decisions for recurrent refractory adenomas are driven by symptom management, risk assessment, and surgical expertise. [3].
Advances in surgical techniques and technologies have allowed for safer surgery, as complication rates for repeat transsphenoidal surgeries are now reported to be similar to historical series, at roughly 1–3%.5,6 In this review, we will describe how innovation in surgical technologies and approaches have expanded surgical possibilities while aiming to reduce morbidity in the treatment of refractory adenomas. We will emphasize how anatomical challenges often dictate surgical approaches and drive innovations, thereby improving patient outcomes.
Anatomy of the cavernous sinus
Innovations in surgical techniques have driven improved outcomes for the treatment of pituitary adenomas. In the 1960s, Hardy introduced the intraoperative microscope to improve visualization during pituitary surgery; endoscopic approaches in the 1990s and early 2000s further improved visualization, safety, and outcomes. [5] Today, the majority of pituitary adenomas are resected through an endoscopic endonasal approach, with some studies suggesting improved gross total resection rates for both functioning and non-functioning adenomas, compared to microscopic approaches. [5].
Anatomic considerations drive surgical success, both for new and refractory cases. Large tumors, especially those with cavernous sinus invasion, are nearly impossible to completely resect without significantly increasing the risks of surgery. When visualized endoscopically, the cavernous sinus can be divided into four compartments: (1) superior compartment: superior to the horizontal internal carotid artery (ICA) and anterior to the genu, and comprises the oculomotor nerve; (2) posterior compartment: posterior to the vertical cavernous ICA; it comprises a segment of the abducens nerve and the inferior hypophyseal artery; (3) inferior compartment: inferior to the horizontal and anterior genu of the ICA; it includes the sympathetic plexus and the distal segment of the abducens nerve and (4) lateral compartment: lateral to the anterior genu and horizontal ICA; it contains the third and fourth cranial nerves, and the first division of the trigeminal nerve. Tumors extending into the cavernous sinus present surgical challenges and higher risks of complications, with diminishing opportunities for gross total resection. They are often graded using the Knosp classification which assesses the extent of cavernous invasion based on MRI imaging and the anatomic relationship of the tumor to the supra- and intra-cavernous ICA. [6] For tumors within the lateral compartment of the cavernous sinus, gross total resection rates range from 0–21%.9,10
Resection of tumor in the cavernous sinus
One of the most common reasons for incomplete resection and tumor recurrence is invasion into the cavernous sinus, which encompasses a segment of the internal carotid artery and the associated postganglionic sympathetic plexus, as well as cranial nerves III, IV, VI, V1 and V2. [7] Injury to the internal carotid artery (ICA) is the most dreaded complication of transsphenoidal surgery; its reported incidence ranges from 0.18 to 1.1%. In a recent review, the incidence of ICA injury during transsphenoidal pituitary surgery was reported at 0.2–0.4% in microscopic and endoscopic approaches. [8] Risk factors for ICA injury include extended transsphenoidal surgery, resection of cavernous sinus tumor, previous radiation, growth hormone secreting tumors, prolonged treatment with a dopamine agonist, less experienced surgeons and larger more complex tumors. [7] Radiation-induced vasculopathies, which occur in upwards of 10% of previously radiated patients, can make vessels more susceptible to injury and, independent of surgery, increases the risk of stroke. [9] ICA injuries often involve the cavernous segment of the ICA and less frequently the ophthalmic artery. Outcomes range from fatal events or significant morbidity to successful management using endovascular techniques such as carotid occlusion or stenting and occasionally bypass procedures. Access to endovascular treatment facilities is critical for the successful management of ICA injuries after pituitary surgery.
As more surgeons acquire advanced endoscopic surgery skills and techniques, there is a growing trend towards more aggressive exploration of the cavernous sinus, especially in repeat surgeries. This has led to improved rates of gross total resection over time. In fact, aggressive surgical approaches have progressed over the past two decades to the point that revision endoscopic surgery has comparable outcomes and complications to upfront surgery in historical cohorts. [10, 7] Even in cases without clear radiographic invasion into the sinus, microscopic invasion of the medial cavernous sinus wall is often identified upon histopathological evaluation. [11] Some groups propose resection of the medial cavernous sinus wall for functioning pituitary adenomas, and have reported reduced rates of recurrence and improved endocrinological control. [11] This approach does increase risks of ICA injury and damage to cranial nerves within the cavernous sinus, especially the abducens nerve, which is injured 2–3% of the time in large series. [12, 13] Additional surgical concerns include the incidence of spinal fluid leak, typically around 2–3%, which increases with more aggressive exposure (e.g. extended transsphenoidal) and dissection. [14] Advances in skull base reconstruction techniques have led to improved success rates of repair of spinal fluid leaks; they commonly involve the use of a pedicled nasoseptal flap to cover defects, effective in over 90% of leaks, and lumbar drain for spinal fluid diversion in the acute postoperative period. [14, 15]
Another challenge with adenomas, in general, and refractory adenomas is tumor consistency. The texture of pituitary tumors is a major determinant in how well and safely they can be removed, since resection relies on gentle dissection and loosening of tumor lobules with curettes. Sharp resection and more aggressive tumor removal devices, such as ultrasonic aspirators or even electrical coagulation, are used very sparingly in view of the risks of injury to critical structures. A change to a more firm texture is often seen in heavily treated prolactinomas or after radiation, leading to a firmer consistency and adherence to delicate neurovascular structures within or around the cavernous sinus. This makes surgery both more challenging and increases surgical risk to delicate neurovascular stuctures. [16].
Electromyographic monitoring (EMG) of extraocular cranial nerves during endoscopic transsphenoidal surgery is a technological adjunct requiring needle electrode insertion in extraocular muscles transorbitally, that may reduce rates of cranial nerve complications during cavernous sinus exploration. [17] Free-run EMG of extraocular muscles allows surgeons to identify early unwanted activation of cranial nerves, often encased in tumors, and adjust surgical strategies to maximize cranial nerve preservation while exploring the cavernous sinus.
Role of craniotomy in the management of invasive pituitary adenomas
Tumor in the lateral cavernous sinus compartment, [18] a common site of tumor recurrence, is often inaccessible safely, with some groups reporting 0% gross total resection rates via midline endoscopic approaches. [19, 20] Craniotomies for pituitary adenoma resection fell out of favor decades ago after the introduction of transsphenoidal techniques. Craniotomies can represent the best surgical approach in select cases, such as accessing tumor within the lateral cavernous sinus, or tumors with significant suprasellar and intracranial extent. For large complex tumors invading the cavernous sinus, surgery can be staged with an endoscopic approach to resect midline and medial cavernous tumor and, at a later point, a craniotomy to remove residual tumor lateral to the carotid arteries. Innovations in minimally invasive neurosurgical approaches have led to reduced recovery times for craniotomies approaching the lateral cavernous. Recently introduced transorbital endoscopic techniques allow access to the cavernous sinus for various pathologies via an eyelid incision with minimal bony removal. While technically interesting, these approaches are not the only options for tumors lateral to the cavernous sinus, do not have wide-spread adoption, and potentially carry a higher risk of complications. [21].
Adjuvant technologies in endonasal pituitary surgery
Several new, assistive technologies are enabling safer surgery with greater ability to resect recurrent adenomas. One example is the difficulty of differentiating normal pituitary gland or fibrotic scarred tissue from tumor, whereby a mistaken resection of normal gland leads to pituitary insufficiency. Contact endoscopy uses high magnification endoscopes to allow surgeons to differentiate between normal gland and adenoma at the level of cellular organization. [22] With magnification 150 times more powerful than the human eye, surgeons can see the disorganized structure of pituitary adenomas in real time endoscopically. Similarly, intraoperative Raman histology can provide rapid, within minutes, histopathological evaluation of minute tissue samples during surgery. This allows surgeons to receive accurate, reliable pathological analysis to guide surgery in real time, without the prolonged wait times required for formal intraoperative diagnosis. Both technologies are experimental at this time.
Several investigational intraoperative fluorescing agents allow increased tumor visualization, which may improve a surgeon’s ability to distinguish normal gland from tumor. [23] Among these, OTL38, a folate-indole-cyanine green-like conjugate to folate receptor alpha, has shown promise as a selective agent that increasingly binds normal pituitary over non-functional adenomas. This increases tumor visualization, allowing surgeons to distinguish between brightly fluorescing normal gland and tumor more readily. Indocyanine green, another agent, is used for ensuring vascularization of nasoseptal flaps, considered critical in the repair of cerebrospinal fluid leaks.
Assistive radiographic tools have similarly progressed over the past two decades to improve surgical decision making. Intraoperative guidance, whereby high-definition, pre-operative imaging can enable surgeons to identify key anatomical structures in real-time in the operating room, is widely available and leads to improved resection rates. [24] Intra-operative MRI suites allow surgeons to identify residual tumor, in real-time during surgery, leading to improved rates of tumor resection and endocrinological remission (Fig. 1). [25].
Fig. 1.
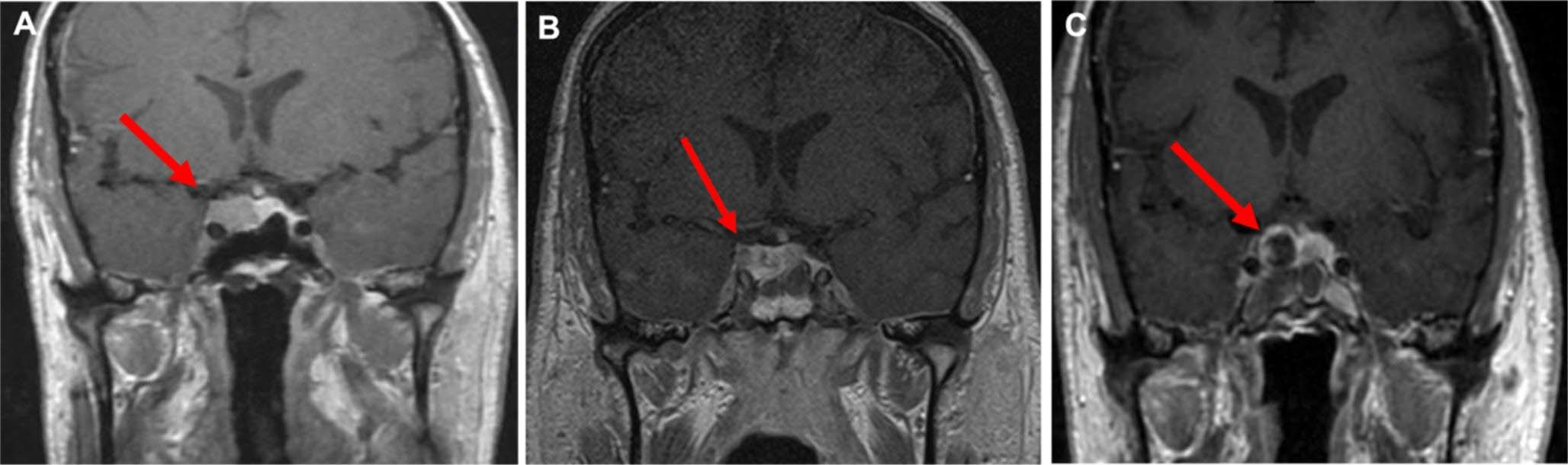
Intraoperative MRI for identifying residual tumor. This is a 73-year-old woman with a non-secretory pituitary adenoma. A Pre-operative coronal sequences show a hypo-enhancing pituitary lesion with invasion into the cavernous sinus (red arrow). B After initial resection, intraoperative MRI demonstrates a small, hypo-enhancing residual in the cavernous sinus (red arrow) that was subsequently removed (C, red arrow)
Conclusion
Surgery plays an important role in the management of refractory adenomas through debulking tumors to allow more successful adjunct treatments, including chemotherapy and radiation. [3, 4, 26] The benefits of repeat surgery must be carefully balanced against the risks, which includes spinal fluid leak (2–3%), neurovascular injury (< 1%), and injury to cranial nerves (1–2%).5,6,10,14 There is a trend towards a decrease in the rate of surgical morbidity and improvement in patient outcomes, associated with enhanced neurosurgical expertise and the availability of surgical adjuncts. This is particularly the case with repeated transsphenoidal surgeries whereby risks for repeat surgery approach the same rate as for initial surgery. [27, 28] Surgery will continue to play a role in the management of complex, refractory pituitary adenomas as future innovations improve surgical safety and effectiveness.
Funding
No funding was used for this manuscript.
Footnotes
Competing interests The authors declare no competing interests.
References
- 1.Delgado-López PD, Pi-Barrio J, Dueñas-Polo MT, Pascual-Llorente M, Gordón-Bolaños MC (2018) Recurrent non-functioning pituitary adenomas: a review on the new pathological classification, management guidelines and treatment options. Clin Transl Oncol 20(10):1233–1245. 10.1007/s12094-018-1868-6 [DOI] [PubMed] [Google Scholar]
- 2.Roelfsema F, Biermasz NR, Pereira AM (2012) Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: a structured review and meta-analysis. Pituitary 15(1):71–83. 10.1007/s11102-011-0347-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X, Dai C, Feng M, Li M, Chen G, Wang R (2021) Diagnosis and treatment of refractory pituitary adenomas: a narrative review. Gland Surg 10(4):1499–1507. 10.21037/gs-20-873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forster N, Warnick R, Takiar V, Pater L, Breneman J (2018) Debulking surgery of pituitary adenoma as a strategy to facilitate definitive stereotactic radiosurgery. J Neurooncol 138(2):335–340. 10.1007/s11060-018-2801-0 [DOI] [PubMed] [Google Scholar]
- 5.Guo S, Wang Z, Kang X, Xin W, Li X (2021) A Meta-analysis of endoscopic vs. microscopic transsphenoidal surgery for non-functioning and functioning pituitary adenomas: comparisons of efficacy and safety. Front Neurol 12(March):1–11. 10.3389/fneur.2021.614382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knosp E, Steiner E, Kitz K, Matula C (1993) Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33(4):610–618. 10.1227/00006123-199310000-00008 [DOI] [PubMed] [Google Scholar]
- 7.Micko ASG, Wöhrer A, Wolfsberger S, Knosp E (2015) Invasion of the cavernous sinus space in pituitary adenomas: endoscopic verification and its correlation with an MRI-based classification. J Neurosurg 122(4):803–811. 10.3171/2014.12.JNS141083 [DOI] [PubMed] [Google Scholar]
- 8.Perry A, Graffeo CS, Meyer J et al. (2019) Beyond the learning curve: comparison of microscopic and endoscopic Incidences of Internal Carotid Injury in a series of highly experienced operators. World Neurosurg 131:e128–e135. 10.1016/j.wneu.2019.07.074 [DOI] [PubMed] [Google Scholar]
- 9.van Westrhenen A, Muskens IS, Verhoeff JJC, Smith TRS, Broekman MLD (2017) Ischemic stroke after radiation therapy for pituitary adenomas: a systematic review. J Neurooncol 135(1):1–11. 10.1007/s11060-017-2530-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong X, Zhuo Y, Yuan H et al. (2022) Outcome of endoscopic transsphenoidal surgery for recurrent or residual pituitary adenomas and comparison to non-recurrent or residual cohort by propensity score analysis. Front Endocrinol (Lausanne) 13(April):1–10. 10.3389/fendo.2022.837025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishida A, Shiramizu H, Yoshimoto H et al. (2022) Resection of the Cavernous Sinus Medial Wall 00(00):1–7 [DOI] [PubMed] [Google Scholar]
- 12.Kalinin PL, Sharipov OI, Pronin IN et al. (2016) Endoscopic transsphenoidal resection of pituitary adenomas invading the cavernous sinus. Zh Vopr Neirokhir Im N N Burdenko 80(4):63–74. 10.17116/neiro201680463-74 [DOI] [PubMed] [Google Scholar]
- 13.Ouyang T, Zhang N, Xie S et al. (2021) Outcomes and complications of aggressive resection strategy for Pituitary Adenomas in Knosp Grade 4 with Transsphenoidal Endoscopy. Front Oncol 11:693063. 10.3389/fonc.2021.693063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kessler RA, Garzon-Muvdi T, Kim E, Ramanathan M, Lim M (2019) Utilization of the Nasoseptal flap for repair of Cerebrospinal Fluid Leak after Endoscopic Endonasal Approach for Resection of Pituitary Tumors. Brain Tumor Res Treat 7(1):10. 10.14791/btrt.2019.7.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zwagerman NT, Wang EW, Shin SS et al. Does lumbar drainage reduce postoperative cerebrospinal fluid leak after endoscopic endonasal skull base surgery? A prospective, randomized controlled trial. J Neurosurg. October 2018:1–7. doi: 10.3171/2018.4.JNS172447 [DOI] [PubMed] [Google Scholar]
- 16.Kamimura K, Nakajo M, Bohara M et al. (2021) Consistency of pituitary adenoma: prediction by pharmacokinetic dynamic contrast-enhanced mri and comparison with histologic collagen content. Cancers (Basel) 13(15). 10.3390/cancers13153914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thirumala P, Mohanraj S, Habeych M et al. (2013) Value of Free-Run Electromyographic monitoring of Extraocular cranial nerves during expanded endonasal surgery (EES) of the Skull Base. J Neurol Surg Reports 74(01):043–050. 10.1055/s-0033-1346975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez-Miranda JC, Zwagerman NT, Abhinav K et al. (2018) Cavernous sinus compartments from the endoscopic endonasal approach: anatomical considerations and surgical relevance to adenoma surgery. J Neurosurg 129(2):430–441. 10.3171/2017.2.JNS162214 [DOI] [PubMed] [Google Scholar]
- 19.Hayhurst C, Taylor PN, Lansdown AJ, Palaniappan N, Rees DA, Davies JS (2020) Current perspectives on recurrent pituitary adenoma: the role and timing of surgery vs adjuvant treatment. Clin Endocrinol (Oxf) 92(2):89–97. 10.1111/cen.14127 [DOI] [PubMed] [Google Scholar]
- 20.Park HH, Kim EH, Ku CR, Lee EJ, Kim SH (2018) Outcomes of Aggressive Surgical Resection in Growth hormone–secreting Pituitary Adenomas with Cavernous Sinus Invasion. World Neurosurg 117:e280–e289. 10.1016/j.wneu.2018.06.012 [DOI] [PubMed] [Google Scholar]
- 21.Vural A, Carobbio ALC, Ferrari M et al. (2021) Correction to: transorbital endoscopic approaches to the skull base: a systematic literature review and anatomical description (neurosurgical review, (2021), 10.1007/s10143-020-01470-5). Neurosurg Rev 44(5):2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fung K, Szeto C, Wehrli B et al. (2011) Contact endoscopy as a novel technique in the detection and diagnosis of mucosal lesions in the head and neck: A brief review. J Oncol.;2011. doi: 10.1155/2011/196302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vergeer RA, Theunissen REP, van Elk T et al. (2022) Fluorescence-guided detection of pituitary neuroendocrine tumor (PitNET) tissue during endoscopic transsphenoidal surgery available agents, their potential, and technical aspects. Rev Endocr Metab Disord 23(3):647–657. 10.1007/s11154-022-09718-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung TK, Riley KO, Woodworth BA (2015) The use of image-guidance during transsphenoidal pituitary surgery in the United States. Am J Rhinol Allergy 29(3):215–220. 10.2500/ajra.2015.29.4166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juthani RG, Reiner AS, Patel AR et al. (2021) E 1824 134(June):1824–1835. 10.3171/2020.4.JNS20178.J [DOI] [Google Scholar]
- 26.Cooper O, Bonert V, Liu NA, Mamelak AN (2021) Treatment of aggressive pituitary adenomas: a case-based narrative review. Front Endocrinol (Lausanne) 12(November). 10.3389/fendo.2021.725014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almeida JP, Ruiz-Treviño AS, Liang B et al. (2018) Reoperation for growth hormone–secreting pituitary adenomas: report on an endonasal endoscopic series with a systematic review and meta-analysis of the literature. J Neurosurg 129(2):404–416. 10.3171/2017.2.JNS162673 [DOI] [PubMed] [Google Scholar]
- 28.Benveniste RJ, King WA, Walsh J, Lee JS, Delman BN, Post KD (2005) Repeated transsphenoidal surgery to treat recurrent or residual pituitary adenoma. J Neurosurg 102(6):1004–1012. 10.3171/jns.2005.102.6.1004 [DOI] [PubMed] [Google Scholar]


