Abstract
During aging the inter‐individual variability in both the neural and behavioral functions is likely to be emphasized. Decreased competence particularly in working memory and general executive control compromises many aspects of the quality of life also within the nonclinical population. We aimed, first, to clarify the brain basis of visual working memory and inhibition during multi‐stage natural‐like task performance, and second, to identify associations between variation in task‐related neural activity and relevant cognitive skills, namely inhibition and general working memory capacity. We recorded, using magnetoencephalography (MEG), the neural modulations associated with encoding, maintenance, and retrieval, as well as interference suppression during a visual working memory task in older adults. We quantified the neural correlates of these cognitive processes through two complementary approaches: evoked responses and oscillatory activity. Neural activity during memory retrieval and interference suppression were correlated with behavioral measures of task switching and general executive functions. Our results show that general inhibitory control induced frontocentral neural modulation across a broad range of frequencies whereas domain‐specific inhibition was limited to right posterior areas. Our findings also suggest that modulations particularly in phase‐locked evoked neural activity can be reliably associated with explicit measures of cognitive skills, with better inhibitory control linked with an early neural effect of distractor inhibition during retrieval. In general, we show that exploiting the inherent inter‐individual variability in neural measures and behavioral markers of cognition in aging populations can help establish reliable links between specific brain functions and their behavioral manifestations.
Keywords: aging, behavior, brain, executive control, magnetoencephalography, neurophysiology
We used magnetoencephalography to study the link between cognitive skills and variance in neural dynamics underlying visual working memory and inhibition in elderly individuals. Induced oscillatory activity showed task‐related modulations, but only the evoked response amplitude correlated with behavioral performance.
Practitioner Points.
General inhibitory control engaged broad frequency band fronto‐centrally, while domain specific inhibition was limited to right posterior areas.
Evoked responses, but not oscillatory measures, correlated with performance level.
Better inhibition linked with early effect of distractor inhibition during retrieval.
1. INTRODUCTION
A large body of evidence suggests that aging is associated with a decline in performance in numerous perceptual and cognitive processes (Bherer et al., 2013). Behavioral studies have examined this age‐related cognitive decline mainly through the lens of four distinct domains of cognitive tasks: working memory, inhibitory control, processing speed and long‐term memory (Peich et al., 2013; Pelosi & Blumhardt, 1999; Reuter‐Lorenz & Park, 2010).
Especially working memory and inhibitory control have received extensive attention as they have been shown to be potent core markers of age‐related cognitive decline (e.g., Chao & Knight, 1997; Grandjean & Collette, 2011; Reuter‐Lorenz & Park, 2010; Waters & Caplan, 2001). Working memory, that is, the ability to maintain and consciously manipulate information in short‐term memory crucially involves executive processes that afford the ability to process increasing memory loads, update and manipulate items kept in memory, and inhibit irrelevant sensory inputs (Bherer, 2015; Bherer et al., 2013). Inhibitory control allows gating of sensory information based on their behavioral relevance. As such, it is closely interrelated with working memory processes as efficient inhibitory control ensures successful maintenance of items in memory (Borella et al., 2008). Inhibitory control is also paramount in shifting of attention to relevant sensory inputs and suppressing sensory interference (Neill et al., 1995), and therefore represents a generally important function for coherent behavioral and psychological integrity.
Age‐related declines in working memory and inhibitory control are reflected as impaired performance in standardized neuropsychological tests (Brennan et al., 1997; Nitrini et al., 2004; Waters & Caplan, 2005), such as the Stroop test (Stroop, 1935; West & Alain, 2000) and the CERAD‐battery, i.e. Consortium to Establish a Registry for Alzheimer's Disease (Ehrensperger et al., 2010; Piefke et al., 2012). The general neural mechanisms underlying cognitive decline can be comprehensively documented in two domains. First, numerous reports suggest that a wide range of structural changes take place in the aging brain that are associated with reduced cognitive and memory function (Lupien et al., 1998; Penke et al., 2010): these include losses of grey and white matter volume especially in frontal cortical areas (Penke et al., 2010), less efficient synaptic transmission (Bäckman et al., 2010) and marked reductions in the size of frontal cortical regions and subcortical structures (Lupien et al., 1998). Secondly, numerous reports suggest that cognitive impairments are also associated with modulations in cortical function (e.g., Chao & Knight, 1997; Piefke et al., 2012; Wang et al., 2011). The functional neural markers of age‐related cognitive decline can be summarized as belonging to the following three distinct categories: altered cortical dynamics, most notably involving alterations in activity of frontal areas, slowing down of spontaneous cortical oscillations and compensatory neural activity (Reuter‐Lorenz & Park, 2010).
Importantly, the above‐mentioned age‐related modulations in cognitive capacity and the associated changes in brain structure and function have been found to vary greatly between individuals (Aine et al., 2011; Kimura et al., 2013). Indeed, there is large inter‐individual variability in cognitive abilities within same‐age elderly individuals (Bastin et al., 2012; Bherer, 2015). In fact, the, ongoing brain's state is naturally variable (Faisal et al., 2008) and thus even in healthy, young individuals there is, to some extent, intra‐ and inter‐individual variability in general functional properties of the brain (MacDonald et al., 2006). However, this variability is emphasized in the early and late stages of life. Indeed, in elderly populations, the variability in functional connectivity in resting‐state BOLD activity correlated with general cognitive performance (Li et al., 2017). In children, the highly variable brain activity across individuals is a neural signature of the maturation of neural structures on the path towards adulthood (e.g., Bonte et al., 2013). This variability is concretely evidenced in both the behavioral manifestation and the brain basis of reading speed in children, compared to a control group consisting of adult readers (Parviainen et al., 2006).
We hypothesize that establishing the brain‐behavior link, that is, bridging the measures of neural activity and behavior, is feasible in elderly populations. Specifically, we hypothesize that this inherent inter‐individual variability in both brain function and behavioral measures in the elderly allows one to distinguish, in a most straightforward manner, the neural signatures that are behaviorally meaningful. Considering only the neuroscientific aspect (i.e. using cognitive function only as task requirement) is an approach that is often adopted when addressing a given experimental question: observing the neural modulations resulting from the demands of a given task allows one to reach sound conclusions about neural correlates of specific cognitive functions. However, it has been rightfully argued that theoretical and experimental decomposition of behavior, and focusing on association between specific neural and behavioral measures, may represent the crucial approach to better understand also the brain functional organization (cf. Krakauer et al., 2017). Especially in the neuroscience of aging, it is crucial to integrate both behavioral and neuroscientific aspects when focusing on cognitive capacity as, even though neurocognitive deficits remain mild in most cases, their variability could reveal possible trajectories leading to more severe pathologies such as Alzheimer's disease (e.g., Bäckman et al., 2005). In a broader context, the direct link between brain and behavioral measures informs on the interaction between neuronal wiring and functional output and can thus be utilized in understanding brain function and dysfunction at a more global level, also linking to the genetic correlates of neurophysiological function (Guo, 2004).
In this study, we aim to reveal the brain‐behavior link in the context of complex cognitive task, and specifically the age‐sensitive processes of working memory and inhibitory control that are important markers of the overall course of cognitive decline in elderly. We recorded, using magnetoencephalography (MEG), the task‐related modulations associated with working memory and inhibitory control in the elderly. We quantified the neural correlates of working memory and inhibitory control through two distinct neural markers: evoked responses and neural oscillatory activity. We take into account both neural measures in unison since evoked activity and neural oscillations have been shown to demonstrate differential modulations in their respective characteristic patterns in response to the effect of aging (Ziegler et al., 2010). In addition, evoked activity reflects more short‐term, presumably bottom‐up, neural processing whereas modulations in oscillatory activity are not strictly locked to an external event and can be considered to represent also top‐down influence on a broader temporal scale. Therefore, evoked responses and neural oscillatory activity provide spatiotemporally complementary accounts of brain function during higher‐level cognitive tasks (Laaksonen et al., 2012). The behavioral aspect of working memory and inhibitory control were monitored through standardized, widely used tests including the Stroop test, the CERAD battery of neuropsychological tests and the Trail Making Test (TMT).
We assumed that the encoding and maintenance of a face in memory, as well as the inhibition of a distractor image, would be reflected in modulations of neural oscillatory activity, because these functions strongly build on task‐relevant top‐down processes. As our first objective, we examined the spatiospectral distribution of oscillatory activity engaged in these two distinct stages of processing. The presentation of target image was expected to engage phase‐locked, evoked activity, reflecting bottom‐up driven sequence of visual analysis and memory retrieval. Focusing on evoked response strength, as our second objective we examined the spatiotemporal distribution of target‐evoked memory retrieval. Importantly, the above brain functional measures were chosen to provide robust, individual‐level indicators of neural resources engaged in working memory and inhibition. As our third objective, we tested whether these distinct neural measures, quantifying the essential task‐related neural operations, correlate with behavioral manifestations of working memory and inhibition. These correlation analyses, in turn, would reveal relationships between the aforementioned neural and behavioral markers of cognitive processes and enable us to establish a link between functional cortical properties and the manifestation of higher‐level cognitive functions.
2. MATERIALS AND METHODS
2.1. Participants
Twenty‐eight older individuals (19 females; mean 74.6 years, range 71.1–82.7 years) gave their informed consent to participate in the experiment. The participants were part of a larger sample of older individuals (n = 314, recruited through the Finnish National Registry) partaking to a randomized controlled trial (the PASSWORD). For the larger PASSWORD cohort the aim was to investigate the effects of physical activity and cognitive training interventions on walking speed, executive functioning and the incidence of falls in sedentary individuals (for more details, see Sipilä et al., 2018). Inclusion criteria to the PASSWORD study were the following: 70–85‐year‐old; dwelling in the community; able to walk 500 m without assistance; sedentary or at most moderately active (less than 150 min of walking/week; no attendance in resistance training) and a score of 24 points or higher in the Mini Mental State Examination test. Exclusion criteria were the following: severe chronic condition or medication; other medical, psychiatric, or behavioral factor that may interfere with study participation; excessive alcohol use; severe vision or hearing problem. Ethical approval for the study was received from the review board at the Ethical Committee of Central Finland Health Care District (14/12/2016, ref.: 11/2016).
2.2. Stimuli
The MEG experimental paradigm consisted of a working memory task involving visual images and it was designed to sequentially engage the cognitive processes of short‐term memory, inhibitory control and retrieval. Participants were shown three images: an encoder image representing a male or a female face, a distractor (a face of the same gender to the encoder image or a grey oval shape), and a target image representing either a male or a female face (gender matched to the encoder image). After the target image was presented, the participants' task was to indicate with a button press whether the encoder image and the target image were identical.
The stimulus set consisted of 60 unique images, 30 images of male and 30 images of female faces, taken from the Karolinska Emotional Faces database (Bowie & Harvey, 2006). All facial stimuli represented a neutral facial expression. In order to increase the difficulty of the task, all face stimuli were modified in order to be as neutral in appearance as possible: hair border and any other specific distinguishing features were removed, but basic face characteristics were preserved. Importantly, the experimental task was designed to probe specifically short‐term memory capacity: all face stimuli were unfamiliar to the participants prior to the experiment and hence they could not rely on long‐term memory retrieval during the task. During the experiment, each face was used four times as the encoder image and four times as the distractor image. The distractor image was always different than the encoder image.
2.3. Experimental procedure
Participants performed a total of 240 experimental trials involving both male and female faces (120 trials for each gender). The total number of trials was divided into four blocks of 60 trials. Between blocks participants had an opportunity to rest for a few minutes. A single trial (Figure 1) consisted of a fixation cross presented for 1000 ms (100 ms jitter), followed by the encoder image presented for 500 ms, a second fixation cross presented for 2000 ms (100 ms jitter), the distractor image presented for 500 ms and a third fixation cross presented for 1000 ms (100 ms jitter). Subsequently, the target image was shown for 500 ms. A visually presented question mark prompted the participants to indicate within a 2000‐ms time‐window whether the target image was identical or nonidentical to the encoder image (i.e., there were separate buttons for “yes” and “no” answers). The target image matched the encoder image in 50% of the trials, and in 50% of trials it was another image of the 29 alternative face images of the same gender, but never the distractor image of the same trial. For each trial, the button presses representing the participants' responses to the task were digitally encoded and subsequently used to compute accuracy scores (see the “Behavioral data analysis” section for more information). During the experiment, participants were instructed to remain still and to keep their gaze on a fixation point projected on a screen at ∼1 m from their sitting position.
FIGURE 1.
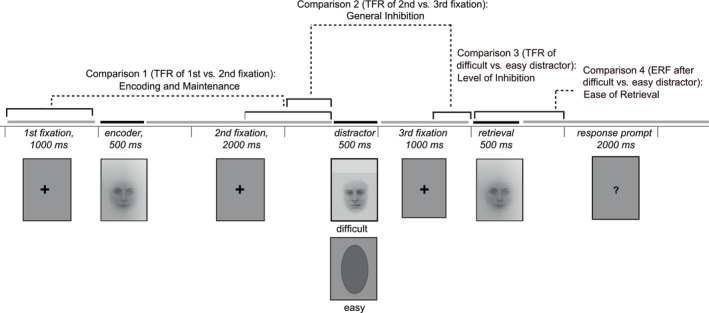
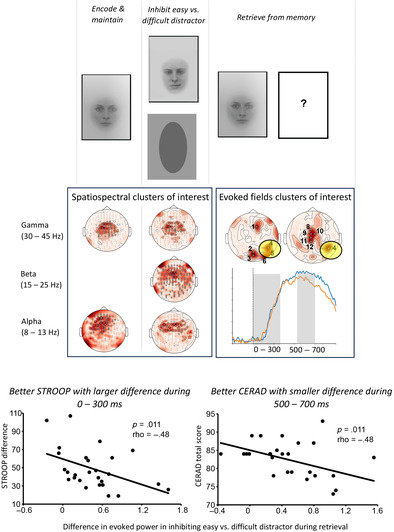
Experimental paradigm and examined contrasts. The encoder, distractor and target images were separated by visual presentation of fixation crosses lasting for 1000–2000 ms. After the target image, a question mark visually presented for 2000 ms prompted the participants to indicate with the button press whether the target image was identical or different than the encoder image. In the analyses of the oscillatory data, we examined neural processes related to encoding and maintenance by comparing the first to the second fixation window (1000 ms time windows), and related to inhibitory control by comparing the second to the third fixation window (500 ms time windows) and the third fixation window preceded by difficult distractor to the third fixation window preceded by easy distractor (500 ms time windows). In the analysis of the evoked data, we examined neural processes related to retrieval by comparing the matching target image preceded by difficult distractor to the matching target image preceded by easy distractor (time windows of 0–300, 300–500, and 500–700 ms after the presentation of the target image). TFR, time–frequency representation.
2.4. MEG data recordings
MEG data was collected using a 306‐channel whole head Elekta Neuromag system (Elekta Oy, Helsinki, Finland) in a magnetically shielded room (VacuumSchmelze GmbH, Hanau, Germany) at the Jyväskylä Centre for Interdisciplinary Brain Research. Data were filtered at 0.1–330 Hz and sampled at 1000 Hz. The participants were seated, with the head covered by the MEG helmet. Each participant's head position with respect to the MEG sensor array was determined by attaching five head position indicator coils to the scalp and briefly energizing them before the measurement. The coil locations were determined in reference to anatomical landmarks (nasion and right/left preauricular points) using a 3‐D digitizer (Isotrak 3S1002, Polhemus Navigation Science). Blinks and eye movements (saccades) during the MEG measurement were monitored using electro‐oculography (EOG).
2.5. Behavioral data recordings
Task accuracy, a metric of cognitive skills specific to the current task, was computed by estimating the proportion of correct responses to the total number of button presses. We obtained accuracy as a percentage by dividing the total number of correct responses to the task with the total number of button presses and multiplying them by a factor of 100. This quantification was conducted across the trials where the subjects had responded after the response cue before the onset of the next trial.
The reaction times of the subjects were determined based on these trials. We also counted for each subject for how many trials they had responded too early (before the cue), responded more than one time, or not responded at all.
The following three behavioral measures were collected in the broader context of PASSWORD study (for more information, see Sipilä et al., 2018) and were subsequently considered in the present experimental paradigm: the CERAD battery of neuropsychological tests, the Stroop test and the TMT.
The CERAD battery of neuropsychological tests consists of the following subtests: Category Verbal Fluency, Modified Boston Naming Test (BNT), Mini Mental State Examination, Word List Memory, and Constructional Praxis. Based on the outcome of these tests, the CERAD total score can be computed by summing scores from the individual CERAD subtests excluding the Mini Mental State Examination into a total composite (maximum score = 100) (Chandler et al., 2005). The CERAD total score provides an index of the overall level of cognitive functioning (Chandler et al., 2005; Paajanen et al., 2010) with higher score representing a higher level of overall cognitive functioning (Ehrensperger et al., 2010).
The Stroop test examines executive function, and more specifically inhibition (Graf et al., 1995; Scarpina & Tagini, 2017). It includes three test conditions. In the first condition, participants are instructed to read aloud 72 words printed in black ink. In the second condition, they are instructed to read aloud the color of 72 colored letter X's. In the third and final condition, they are shown a page with 72 color words printed in incongruent colored inks (e.g., the word “GREEN” printed in yellow ink). Participants are asked to name the color in which the words are printed and ignore the word itself. Participants were asked to do the test as quickly and as accurately as possible. The time taken to complete each condition was recorded and the time difference between the third and the second condition was calculated (Stroop difference). Smaller time differences indicate better performance in the most demanding 3rd condition, and thus the ability to efficiently inhibit cognitive interference (Scarpina & Tagini, 2017).
The TMT comprises two parts, Part A and Part B. It is used to assess executive function, and specifically the ability to efficiently switch between tasks (Bowie & Harvey, 2006). The TMT Part A (TMTA) evaluates psychomotor speed and involves drawing a line connecting circles that contains the numbers 1–25 sequentially. The TMT Part B (TMTB) also evaluates psychomotor speech but is more demanding than TMTA. It consists of circles with numbers and letters; it requires participants to draw a line from 1 to A, A to 2, 2 to B, B to 3, etc., until the letter L. Participants were instructed to perform both tasks as quickly and as correctly as possible. The time to complete each task was recorded and the time difference to complete TMTB and TMTA was calculated (TMT B‐A). Smaller time differences are indicative of a better performance, as a small time difference signifies, in most cases, that the time taken to complete the more challenging TMTB is similar to the time taken to complete the easier TMTA. In subsequent analysis, we only considered TMT B‐A, as it was thought to be the most relevant metrics of task switching abilities and thus executive control.
In the context of the larger PASSWORD cohort, these behavioral tests were performed 6 months (except CERAD total score) and 12 months after the initial measurement following the same behavioral data measurement procedures. MEG data were also collected 6 and 12 months after the initial measurement following the same MEG data recording procedure and the same experimental protocol as for the initial MEG measurements. These data were used to constrain the analyses of link between neural activity and behavior to phenomena that showed stability across the latter two measurement points.
2.6. MEG data analysis
MEG data preprocessing and subsequent analysis was carried out using the MNE Python toolbox implemented in Python computing environment (Gramfort et al., 2013), unless stated otherwise.
2.6.1. Preprocessing
Only gradiometers were included in MEG data analysis as they have a narrow spatial sensitivity pattern and are well suited for recording data from superficial sources; in contrast, magnetometers more readily pick up signals from distant sources, including external artifacts. First, channels visually identified during MEG data collection being contaminated with artifacts or flat were interpolated using MNE Python's built‐in functions. Subsequently, oversampled temporal projection was used in order to suppress noise and interpolate bad channels (Clarke et al., 2020). There were 3.36 ± 0.85 bad channels (mean ± SD; range 1–5 channels) across the subjects. Afterwards, MaxFilter (Elekta Oy) was used to remove external disturbances from the MEG data with spatiotemporal signal space separation and transformation of all participants' head position to one reference head position (Taulu & Simola, 2006). As the reference head position, we used the subject's head position whose position was closest to the median position across the subjects. Finally, independent component analysis (fastICA) (Hyvärinen, 1999) was used in order to reduce artifacts of ocular and cardiac origin. ICA was applied to the data processed with oversampled temporal projection and spatiotemporal signal space separation, and maximally three components were removed both for suppressing the cardiac and ocular artefacts.
Prior to further analysis, we discarded trials in which the button presses occurred too early (before the cue): pressing a button is associated with motor activity and would therefore obscure neural activity relative to the cognitive processes of interest. Subsequently, based on the preprocessed and artifact‐free trials, two complementary neural measures to be considered in the correlations with the behavioral data. These neural measures consisted of both measures reflecting stimulus‐induced (nonphase‐locked) modulation of oscillatory activity (time–frequency representations [TFRs]) and evoked (phase‐locked) neural activity (evoked responses).
2.6.2. Computation of TFRs
TFRs were computed in the 1–90 Hz frequency range (based on Morlet wavelets; half a cycle per Hz, i.e., at 10 Hz a wavelet with the width of 5 cycles was used). The range from 1 to 31 Hz was sampled at 1 Hz steps, whereas the range from 32 to 90 Hz was sampled at 2 Hz steps. In the encoding/maintenance analyses (comparison 1), TFRs were computed for the 1000 ms‐long fixation time‐windows preceding and succeeding the encoder image, where the preceding window was immediately before image presentation and the succeeding window was placed between 1000 and 2000 ms from image onset. In the analyses of general inhibition (comparison 2), TFRs were computed for the last 500 ms of the fixation time‐window preceding and succeeding the distractor image. In the analyses of level of inhibition (comparison 3), TFRs were computed for the last 500 ms of the fixation time‐window following the distractor image. All TFR time‐windows were at the end of the fixation periods so that the evoked neural activity due to the presentation of the face images would not bias the estimation of the induced responses.
2.6.3. Computation of evoked responses
The evoked neural activity associated with the ease of retrieval (following inhibition of a difficult vs. an easy distractor) was examined by computing evoked responses in the time‐window following the presentation of the target image (see Figure 1, comparison 4). Specifically, the evoked responses were computed in the −200 to 700 ms time‐window, with −200 to 0 ms as a baseline, where zero represents target image onset. For each subject, responses were averaged across trials and visualized after low‐pass filtering at 40 Hz.
2.6.4. Statistical analyses of the TFR MEG data
TFR data was used to examine the neural basis of encoding and maintenance (comparison 1, Figure 1), inhibitory control (comparison 2, Figure 1), as well as level of inhibition (comparison 3, Figure 1). For each of these comparisons, statistically significant differences were evaluated using a cluster‐based permutation test (1000 permutations; statistical threshold: alpha = 0.05; clustering threshold: F = 8.0) that was implemented in distinct frequency bands of interest (1–30, 8–13, 15–25, 35–45, 60–90 Hz). In the cluster‐based permutation testing each gradiometer was treated separately and the neighborhood information of between the gradiometers was determined based on the template connectivity matrix within MNE‐Python. Statistical testing for each of the comparisons was done by averaging across each frequency band of interest, thus obtaining one value per frequency range across all time points of interest (referred as time‐sensitive TFR statistical analysis). Here, the values that were averaged were the time‐resolved spectral power estimates obtained by multiplying the wavelet transformation with its complex conjugate. This analysis allows the identification of time‐intervals in which the whole frequency bands of interest show modulation of neural activity across conditions. The extraction of values of interest can be done also by averaging across all time points in each time‐window of interest, yielding one value per frequency bin across the frequency range of interest (referred as frequency‐sensitive TFR statistical analysis). This analysis allows the identification relevant frequency components within the frequency bands of interest. The results of this analysis are provided as the Supplementary material.
Subsequently, any potential correlation effects were estimated within spatiospectral clusters of interest (COIs) identified based on the time‐sensitive TFR statistical analysis (for the frequency‐sensitive TFR analysis, see the Supplementary material). Specifically, the COIs enabled us to define time‐windows, frequency bands and sensors of interest that reflect spatiospectral profiles of neural activity associated with the two cognitive processes of interest in the TFR analysis: first, encoding and maintenance of an image (before vs. after encoder) and second, inhibitory control (for the neural correlates of general inhibitory control we contrasted before versus after distractor; for the neural effects due to level of inhibition, we contrasted difficult versus easy distractor) (cf. Figure 1). Once COI identification for each of the three statistical contrasts of relevance was completed, we extracted the difference in TFRs for each of the three contrasts of relevance to probe the relationship between neural modulations and behavioral data. Here, we first identified for each contrast and cluster the sensor showing the largest difference between the two conditions and extracted for each subject the TFR values within the frequency‐bands of interest across the significant time‐points. We then averaged these subject‐specific values across time. This yielded, for each contrast‐specific COI, a single power value extracted from a single‐sensor for each subject for each frequency band.
To minimize the influence of outliers, this vector of power values was subsequently normalized by an individual normalization factor (the standard deviation of neural activity for each individual participant averaged across the experimental conditions of interest, as well as across time‐points [e.g., 1000 ms], frequency bands [e.g., 1–30 Hz] and sensors in which the statistically significant modulation in MEG signal power was observed). These normalized values of neural oscillatory activity were considered in the elucidation of the brain‐behavior link, that is, the correlation analysis between neural activity measures and behavioral measures.
2.6.5. Statistical analyses of the evoked response MEG data
To evaluate the neural correlates associated with the ease of retrieval, we contrasted evoked responses time‐locked to a matching target image after a difficult versus an easy distractor in the 0–300, 300–500 and 500–700 ms time‐windows post‐stimulus (maximum statistics based permutation t‐test on all 204 gradiometers; 1000 permutations; statistical p‐threshold = .05). These time‐windows covered the task‐related sensory‐perceptual processes and processes related to memory‐based retrieval and enabled coarse timing of the effects. This procedure yielded a list of sensors that demonstrated statistically significant differences in evoked activity in each of the three time‐windows of interest. For each of these sensors, we computed, for each subject, the difference in evoked activity between the two conditions being contrasted. This yielded, for each MEG sensor, a single evoked activity value for each participant.
Similarly to power values derived from TFR analysis, to minimize the influence of outliers, the evoked activity values were subsequently normalized by an individual normalization factor (the standard deviation of evoked activity for each individual participant averaged across the two conditions of interest, as well as across time‐points [e.g., 0–300 ms] in which the significant effect was observed). These normalized values of evoked activity were considered in the correlation analysis between neural activity measures and behavioral measures.
2.7. Correlations between the neural activity and behavioral measures
As described above we obtained, for each participant, a single value for each contrast‐specific COI (COIs specific either to encoding and maintenance, general inhibition, level of inhibition) or sensor (ease of retrieval) representing neural activity associated with the cognitive processes of working memory and inhibitory control. We subsequently examined the correlations between these neural activity measures and behavioral measures of cognitive skills and executive control.
Correlation analysis was carried out in IBM SPSS Statistics for Mac, version 26 (IBM Corp., Armonk, N.Y., USA). Using Spearman's nonparametric correlation method (alpha = 0.05), we performed all‐to‐all correlations between the neural activation measures described above and the behavioral data collected in the context of the present experiment (see the “Behavioral data” section). Specifically, we correlated neural activity measures with the following behavioral measures: CERAD total score, Stroop difference and TMT B‐A.
The considerably large number of neural and behavioral measures of interest resulted to an even larger number of possible correlation tests (69 correlation tests; 15 ERF and eight TFR values and three distinct behavioral measures). To limit the number of conducted tests, we applied a masking procedure based on the fact that MEG and behavioral data were available for two additional measurement sessions, carried out 6 and 12 months, after the MEG and behavioral measurement of interest (baseline; see the sections “MEG data recordings” and “Behavioral data recordings” for more details). Our correlation‐test mask was thus based on correlations observed between neural and behavioral measures at these two additional measurement sessions. These additional correlations were derived in the COIs defined through the time‐sensitive TFR analysis (gradiometer with largest neural effect in each COI) and the sensors demonstrating significant modulations in evoked activity for the contrasts of interest (for frequency‐sensitive TFR analysis, see Supplementary material). For these specific COIs and sensors of interest, we extracted power and evoked response values for MEG data measured 6 and 12 months after the initial measurement session, following the same procedure described above for the MEG data from the measurement of interest. We then correlated these measures of neural activity with the behavioral data collected at the corresponding measurement sessions (carried out at 6 and 12 months). For behavioral measures where data were available for all three measurement points (Stroop, TMT B‐A), we tested for significant correlations (p < .05) at baseline if the correlation values at 6 months and 12 months had the same sign between them and each of the two correlation values had an absolute rho value of at least .1. For CERAD total score there were only two measurement points available (baseline and 12 months), and the correlation masking criteria involved testing for significant correlations (p < .05) at baseline if the correlation value at 12 months had an absolute rho value of at least .15. This correlation masking criteria was instrumental in limiting correlation testing at the measurement point of interest to a reasonable number of tests. Specifically, it yielded the following limited set of testable correlations between neural and behavioral data: seven ERF tests (five CERAD, two Stroop) and eight TFR tests (three CERAD, five Stroop). The statistical significance (p < .05) of these correlations at baseline were evaluated separately for ERF and TFR, corrected for multiple comparisons using the False Discovery Rate correction.
3. RESULTS
3.1. Task performance
Overall, the subjects were able to perform the task well, with an accuracy of 81 ± 7% (mean ± SD, range 61%–90%). The mean response time of the subjects was 563 ± 225 ms (range 287–1147 ms). The subjects responded within the correct time interval (after the response cue but before the next trial) in 225 ± 20 trials (range 176–250). For some trials the subjects responded too early (8 ± 12 trials, range 0–35) or not at all/not before the next trial (6 ± 13 trials, range 0–59). In individual trials the subjects responded more than one time before the next trial (1 ± 2 trials, range 0–7). In the MEG analyses, only trials in which the subjects had responded only within the correct time interval and only once were considered.
3.2. MEG results
3.2.1. TFRs: Encoding and maintenance of an image in memory, general inhibitory control, and the level of inhibition
We found statistically significant modulations in TFRs specifically in the alpha, beta and low gamma frequency bands in specific time‐windows reflecting the encoding of an image to be kept in short‐term memory, inhibition of an irrelevant visual input, and processing of a difficult, compared to an easy distractor (see Figure 2). Overall, the outcome of the TFR analysis emphasized frequencies below 45 Hz, covering the theta, alpha, beta and low gamma frequency bands.
FIGURE 2.
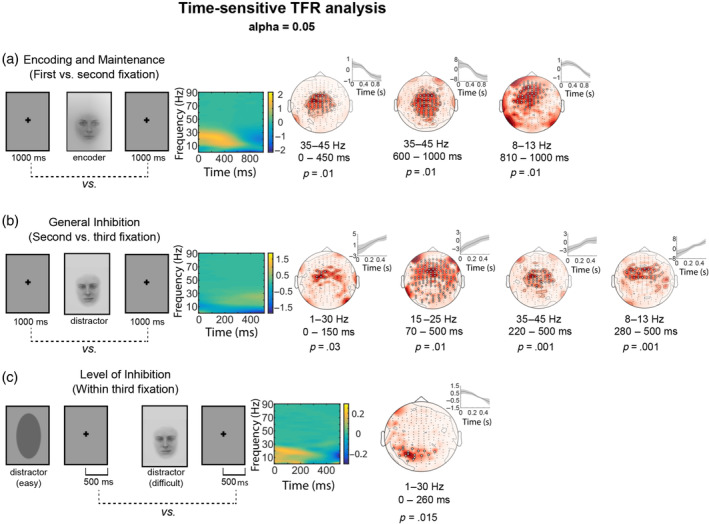
Statistically significant results of the cluster‐based permutation analysis for the time‐sensitive time–frequency representation (TFR) statistical analysis. The information on the contrasts of interest (left) is followed by the mean TFR difference (normalized values, AU) across all gradiometers and topoplots of the significant effects. The small insets next to the topoplots represent the contrast‐specific modulation of neural activity across time (normalized values, AU; mean ± 2 × SEM) within the identified cluster for the frequency‐band showing the significant effect. Each topoplot represents one statistically significant cluster. White circles indicate MEG channels that demonstrate statistically significant modulations in MEG signal power. The frequency bands and time‐windows in which the statistically significant effect is observed, as well as the p‐values are reported underneath each topoplot. (a) Statistically significant modulations in magnetoencephalography (MEG) signal power for the first versus second fixation contrast (before vs. after encoder image). (b), statistically significant modulations in MEG signal power for the second versus third fixation contrast (before vs. after distractor image). (c) Statistically significant modulations in MEG signal power for the within third fixation contrast (after an easy vs. a difficult distractor).
The spatiospectral patterns of these effects demonstrated different characteristics for encoding and maintenance versus inhibition stages. The encoding and maintenance of an image in memory was associated with modulations in neural activity in MEG sensors mostly located at the vertex. This effect highlighted a special emphasis on the low gamma band (35–45 Hz) at the central channels and alpha band (8–13 Hz) in more frontally distributed channels (Figure 2a).
General inhibitory control was associated with two distinct patterns of activation: spatially and spectrally delimited effects in central‐to‐frontal areas at alpha and low gamma bands as well as broader spatiospectral patterns of neural activation specifically at beta‐band (Figure 2b). Finally, neural modulations associated with the level of inhibition emphasized more posterior cortical regions, with the significant effects being delimited within the 1–30 Hz frequency range (Figure 2c). The frequency‐sensitive TFR analysis results (Figure S1) were in line with the above, time‐sensitive results, but revealed statistically significant modulations in MEG signal power that were less spatially and spectrally specific, especially for the encoding and maintenance stage as well as for general inhibitory control. The neural correlates of the level of inhibition, in turn, aligned well with the time‐sensitive analysis but decomposed the findings into several low‐frequency posterior clusters. See Figure 2 (time‐sensitive TFR analysis) and Figure S1 (frequency‐sensitive TFR analysis) for a complete list of the frequency bands, and time‐windows in which statistically significant effects are observed. The p‐values associated with these significant findings are also displayed.
3.2.2. Evoked responses: Level of inhibition on retrieval
We found statistically significant differences in evoked responses to the matching target image after a difficult versus an easy distractor, interpreted to indicate the required level of inhibition for the preceding distractor image and thus ease of retrieval (Figure 3). These differences were observed for all three time‐windows of interest: 0–300, 300–500 and 500–700 ms. Spatially, there was an emphasis on MEG sensors located above the right posterior brain regions. In addition, in the late time‐window (500–700 ms), we observed statistically significant differences also in centrally located MEG sensors. It is noteworthy that the direction of difference indicated higher amplitudes for the difficult distractor compared to the easy distractor for some of the sensors whereas for other sensors we observed the opposite trend. However, for the right posterior cluster, the amplitudes were always higher for difficult than easy distractor.
FIGURE 3.
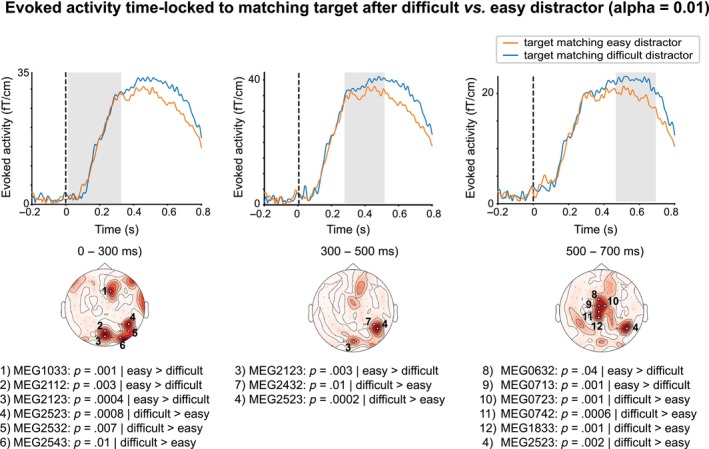
Statistically significant differences between evoked responses time‐locked to a matching target stimulus after a difficult versus an easy distractor image. Evoked activity for each of the two contrasted conditions (y‐axis: evoked response amplitude, in ft/cm; x‐axis: time, in ms) is plotted for the magnetoencephalography (MEG) sensors demonstrating statistically significant differences between the two conditions for each of the three time‐windows (0–300 ms; 300–500 ms and 500–700 ms) in which the significant effects were observed. The topoplots depict significant MEG sensors as white circles; significant p‐values and names of significant MEG sensors (Elekta Neuromag) are reported separately for each‐time window under each topoplot.
3.3. Correlation results between MEG data and behavioral data
The statistically significant modulations in neural activity described above, defined in specific COIs (TFRs) or sensors (ERFs) and specific time‐windows (TFRs/ERFs) and/or frequency bands (TFRs) were tested for significant correlations with behavioral data using the correlation tests mask as described in “Correlations between neural activity and behavioral measures” in the Methods section (see Table 1). Significant correlations between neural activity and behavioral data were exclusively detected for evoked activity, i.e., no correlations were found between TFR measures and behavioral measures.
TABLE 1.
Rho‐values for the 15 correlation tests that were performed on the basis of our masking procedure.
| CERAD | STROOP | Time‐window | |
|---|---|---|---|
| Ease of retrieval: Evoked response at matching target after difficult versus easy distractor | |||
| Ch 1, frontocentral | −0.22 | 0.0–0.3 s | |
| Ch 5, r occ/temporal | −0.26 | 0.0–0.3 s | |
| Ch 6, r occ/temporal | −0.16 | 0.0–0.3 | |
| Ch 4, r occ/temporal | −/−0.36/−0.48* | −0.48*/−/− | 0–0.3/0.3–0.5/0.5–0.7 s |
| Ch 11, central | −0.16 | 0.5–0.7 s | |
| CERAD | STROOP | |
|---|---|---|
| Encoding and maintenance: MEG signal power before versus after encoder | ||
| 8–13 Hz | 0.13 | −0.24 |
| 35–45 Hz (cluster 2) | −0.27 | |
| General inhibition: MEG signal power before versus after distractor | ||
| 15–25 Hz | 0.26 | |
| 35–45 Hz | −0.037 | |
| 1–30 Hz | −0.2 | 0.23 |
| Level of inhibition: MEG signal power for difficult versus easy distractor | ||
| 1–30 Hz | −0.16 | |
Note: Values are shown for all tested correlations (that survived the masking procedure), and the tests that reached statistical significance are highlighted in bold. The locations of the channels in which evoked responses were examined are shown in Figure 3 and the time–frequency representation clusters in Figure 2.
Abbreviation: MEG, magnetoencephalography
We observed a statistically significant negative correlation between CERAD total score and difference in evoked activity in response to a matching target image after difficult versus easy distractor in one MEG sensor in the right posterior cluster (Channel 4 in Table 1 and Figure 3), which reflects persistent activity and higher amplitude for retrieval image after difficult than after easy distractor. The correlation emerged in the time‐windows of 500–700 ms (Figure 4a; MEG2523: p = .011, rho = −.48). This correlation signifies that the smaller the difference in evoked responses to a matching target image after a difficult versus easy distractor, the larger the CERAD total score (which indicates a higher level of overall cognitive functioning).
FIGURE 4.
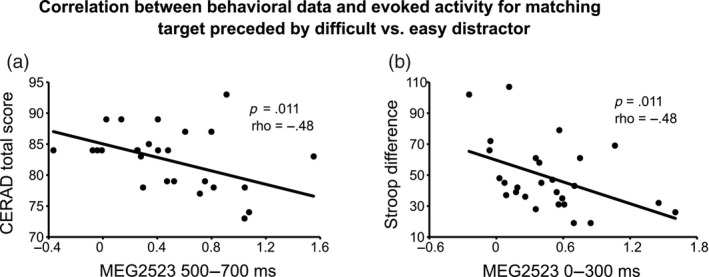
Scatterplots demonstrating the statistically significant correlations between neural activity measures and behavioral measures. Significant correlations were detected exclusively for evoked activity reflecting ease of retrieval, specifically for the difference in strength for the target‐evoked response after a difficult versus an easy distractor. For each scatterplot, we report the significant p‐value and Spearman's rho. (a) Scatterplot between neural measures (x‐axis; in ft/cm) and CERAD scores (y‐axis). (b) Scatterplot between neural measures (x‐axis; in ft/cm) and the Stroop difference (y‐axis; in s). MEG, magnetoencephalography.
We also found a statistically significant negative correlation between the Stroop difference and difference in evoked activity in response to matching target image after difficult versus easy distractor in the 0–300 ms time‐window (Figure 4b; MEG2523: p = .011, rho = −.48). This negative correlation suggests that the smaller the difference in evoked activity in response to matching target image after difficult versus easy distractor the larger the Stroop difference (which indicates weaker performance in the most demanding third condition).
4. DISCUSSION
Our study clarified the existence and robustness of the brain‐behavior link in elderly subjects, focusing on temporally varying neuronal activity involved in visual working memory and inhibitory control. The neural dynamics demonstrated distinct spatiospectral pattern of oscillatory activity underlying visual working‐memory and inhibition task stages mainly within the expected fronto‐central areas, as well as robust evoked activity to target‐triggered visual memory retrieval in the posterior visual areas. Interestingly, our results suggest that although the oscillatory activity shows task‐relevant modulation, the brain‐behavior link can be only identified in the evoked response‐derived measures. Specifically, we showed that individual‐level capacity for executive control as well as general cognitive skills are linked with evoked response amplitude reflecting the level of processing engaged in retrieval and manipulation of memory‐based items. The present findings highlight that, when considering the complementary markers of neural activity (evoked responses and modulation of oscillatory activity), and standardized tests of cognitive function (CERAD total score, TMT, Stroop difference), it is possible to associate brain measures with the individual level behavioral competence.
Our starting point in bridging brain and behavior was to obtain better understanding of the neural correlates of cognitive processes that are central to psychological wellbeing and functional capacity, especially in elderly individuals. Indeed, inhibitory control, understood from broad perspective, has been indicated as one of the key domains predicting individual wellbeing and cognitive competence (Ganesan & Steinbeis, 2021; Moffitt et al., 2011). In line with existing evidence on cognitive function of younger individuals (Honkanen et al., 2015; Palva et al., 2010), and confirming our hypotheses, oscillatory power in the alpha, beta and gamma frequency bands was modulated by task periods requiring working memory and inhibitory control. However, contrary to our expectations, these oscillatory phenomena did not show significant correlations with the behavioral measures. The evoked activity in response to target triggered retrieval of the memorized item, in turn, showed associations with individually defined level of behavioral performance in executive processes, although only in restricted area in the right posterior occipital cortex. This result is compatible with the interpretation of oscillatory versus evoked activity, where modulation of oscillatory rhythms, particularly at alpha and beta‐bands, subserve the general top‐down driven (perhaps less specified) milieu (e.g. attention allocation) for processing while evoked responses may be more strictly bound to the specific bottom‐up driven pathway directly associated with task‐specific requirements. Stronger specificity could explain why evoked responses indicate more intimate link to behavioral variance. Indeed, evoked response amplitude in response to target‐ or feedback trials at around 300 ms (such as P300) has shown individually robust association with behavioral measures of inhibitory control (Rueda‐Delgado et al., 2021) to a degree to be useful also in context of neurofeedback applications (e.g. Zioga et al., 2019). On the other hand, the frequency of oscillations, namely alpha peak frequency, has been shown to reflect the level of cognitive engagement (Haegens et al., 2014). However, even though the alpha frequency was reported to increase with increasing cognitive demands at individual level, it did not predict the performance level across individuals.
The brain functional basis of cognitive processes, specifically executive skills, has been extensively studied across the life‐span, and our findings are in general agreement with this literature. In line with the present findings regarding the stage of encoding and maintenance, these cognitive functions have been repeatedly shown to engage the vertex and frontally distributed oscillations at alpha (Jensen et al., 2002) and gamma bands (Honkanen et al., 2015; Lozano‐Soldevilla et al., 2014; Lundqvist et al., 2016; Meeuwissen et al., 2011; Morgan et al., 2011). Alpha and beta frequencies, in particular, have also been shown to provide the relevant computational basis for inhibition (Bonnefond & Jensen, 2012, 2013; Klimesch, 2012; Klimesch et al., 2007; Schaum et al., 2021). Thus, our results bring confirmation to the established importance of alpha and gamma‐bands for working memory processes, and to the evidence of the role of beta‐band oscillations for active anticipatory inhibition (Solís‐Vivanco et al., 2021) also outside motor control. Interestingly, while the contrast indicating general engagement of resources for inhibiting distracting visual input reflected mainly central (vertex and frontal) brain regions, the more specific contrast sensitive to the required level of inhibition (namely easy vs. difficult visual distractor) evidenced exclusively posterior (visual) localization. Indeed, specifically in tasks where the process of working memory maintenance is distracted by information within the same (visual) modality, it is likely that domain specific suppression of processing is required besides the domain‐general inhibitory control.
In elderly, the brain basis of cognitive skills is usually studied from the perspective of general decrease in performance level, which is characterized by age‐related differences both in evoked responses and in brain oscillations (Dustman et al., 1996; Friedman et al., 2007; Reuter‐Lorenz & Park, 2010). Aging has been associated with changes in the task‐related (induced) modulations in signal power of oscillatory activity measured using MEG and electroencephalography—more specifically, attenuations and increasing spatial uniformity in neural activity at alpha band (Rossini et al., 2007) in context of working memory, alterations in beta band (Rossiter et al., 2014) during motor inhibition, and attenuation in higher frequencies (>30 Hz) (Kurimoto et al., 2012) with relevance for working memory processes (Lisman, 2010). The spectral distribution of these effects clearly overlaps with the current results. Although our interest was not in contrasting young and old individuals, but rather on individual variability, together these findings suggest that aging is associated with broad changes in oscillatory dynamics, of which our results suggest that low gamma (35–45 Hz) modulation in vertex area underlies the encoding and maintenance of visual information, and to some extent also inhibition, and broadly distributed beta‐band (15–25 Hz) modulation links especially with inhibitory control. Alpha‐band seem to relate to both working memory and inhibitory stages. From a methodological point of view, it is important to note, however, that the specific frequencies showing significance varied somewhat depending on whether the time‐varying nature of frequencies was taken into account or not (cf. time‐sensitive vs. frequency‐sensitive analysis; Figure 3 vs. Figure S1).
The general time‐course of target‐evoked activity in our data is in line with earlier literature, and the robust evoked response overlaps in time with the P3 complex. Indeed, the target‐related task requirements are likely to evoke similar processes to P3, broadly associated with stimulus evaluation and decision making, as the ongoing visual information processing needs to be compared against the encoder image simultaneously suppressing the distractor image. Particularly relevant for the present study, P300 has been shown to differ between groups with typical aging versus memory disorders (Polich & Corey‐Bloom, 2005; Rossini et al., 2007). In general, the time‐window where behaviorally relevant contrasts in evoked responses were shown in the current study match with the time‐window of age‐related effects in evoked activity reported earlier. Specifically, declines in working memory capacity in ageing population are associated with increases in (early) evoked response amplitude (Nowak et al., 2021), shifts of amplitude maxima and altered amplitude distribution at later time‐windows (Pelosi & Blumhardt, 1999). Impaired inhibitory control in the elderly has also been associated with enhanced evoked responses time‐locked to irrelevant stimuli, suggested to reflect decreased capacity to suppress unnecessary inputs (Vallesi et al., 2009). To reach behaviorally meaningful markers of brain activity, we focused on task‐relevant contrast also in evoked activity, which showed an expected shift from posterior and right lateralized distribution prior to 500 ms to more vertex‐emphasized distribution at later latencies.
The conventional approach in neuroimaging has followed the tradition of experimental psychology, where the focus is on the neural correlates of specific cognitive tasks and individual variability is consider as noninformative noise. However, in past 10 years, the variability in neural activity has been increasingly considered as a tool to reach a deeper understanding about key cognitive processes and individual patterning of spontaneous oscillatory rhythms (e.g., Haegens et al., 2014) or evoked response measures (Duarte et al., 2013). Importantly, neurophysiological studies of cognitive processing in elderly have revealed marked inter‐individual variability in neural activity. The variability in cognitive aging is further associated with increases in moment‐to‐moment fluctuations that drive behavioral mechanisms (Lindenberger et al., 2006; Lindenberger & von Oertzen, 2006; MacDonald et al., 2006). While variability, even in younger populations where it is less pronounced, is often considered as a caveat in MEG data analysis (e.g., Olivetti et al., 2014), the present study was founded on the assumption that variability is instead a valuable resource that can reveal meaningful connections between behavioral and brain measures.
In the present study, we considered the behavioral correlations with the modulations in TFR's for encoding, maintenance and inhibition task periods, and evoked responses to retrieval task period. The selection of these specific neural measures was based on the substantial earlier literature evidencing their relevance for the cognitive operations engaged by our task requirements (see, e.g., Palva et al., 2011). We found correlation between evoked response measures and behavioral measures, but surprisingly, no significant correlations between neural oscillatory activity and behavior. Specifically, differences in evoked activity supposedly reflecting level of inhibition that is engaged during mental manipulation of memorized (encoder image) and interference (distractor image) items correlated with behavioral measures. In other words, larger difference in evoked activity in the 0–300 ms time‐window after difficult versus easy distractor was associated with more efficient executive control (as evidenced by smaller Stroop difference). We also observer that a smaller difference in evoked activity in the 500–700 ms time‐window after difficult versus easy distractor was associated with higher level of overall cognitive functioning (as indexed by a higher CERAD total score). These results may be interpreted as indexing the behavioral relevance of the brain's capability to internally attend to relevant inputs (encoder face), and interindividual variability in the ability to suppress competing stimuli of the same category (difficult distractor, i.e. another face stimulus) or a different category (easy distractor, i.e. an oval shape) (Chen & Huang, 2016; Pulvermüller et al., 1996). The evoked response amplitude across time‐windows could thus reflect the ability to mobilize inhibitory control resources to efficiently switch between tasks (Bowie & Harvey, 2006), or the ability to focus internal attention to task‐relevant information in memory, evidenced earlier by using retro‐cues (Barth & Schneider, 2018). Indeed, Duarte and colleagues (Duarte et al., 2013) reported reduced evoked activity in old versus young individuals indicative of impaired resources in the old for retrospective attention to modulate working memory content. According to our results, there may be individual variance even among the older individuals in this neurocognitive capacity, that link also more broadly to executive skills. Importantly, as the evoked response was measured to the presentation of the target stimulus, i.e. following the (presumable) identification of the matching visual input after the distractor image, this increase in amplitude must in our study be interpreted as an indicator of successful inference suppression while performing mental comparison between encoder and target images.
It is noteworthy, and somewhat puzzling, that our correlation findings exclusively emphasize the relationship between evoked responses and behavior, and there were no significant correlations involving neural oscillatory activity. This observation may be due to the small sample size combined with the fact that neural oscillatory activity presents a lower signal‐to‐noise ratio than evoked responses. It may also be, that stimulus‐induced change in power is not the best choice to capture individually varying characteristics in oscillatory dynamics. Indeed, some earlier studies suggest that for example phase locking or other synchronization measures either during rest (Jauny et al., 2022) or task performance (Hinault et al., 2020; López et al., 2014) are associated with the individually measured cognitive task performance. However, our findings highlight the sensitivity of evoked response measure to the individual variance in cognitive capacity. The validity of evoked responses in reflecting individually meaningful information is also demonstrated by the brain computer interfaces that are built on the P300 response component. This time‐window between 350 and 600 ms has shown to robustly reflect attention, error awareness and memory of the target stimuli. Partly in line with our correlational findings, specifically for the later 500–700 ms time‐window, stronger amplitude of P300 has been associated with superior performance in these tasks (Polich, 2007), and at group level Alzheimer patients show diminished P300 amplitude in comparison with typically aging individuals. Although the focus on oscillatory (the periods of encoding, maintenance and inhibition) versus evoked (target‐based retrieval) activity was based on characteristics of the utilized experimental design and literature‐based assumption regarding the best neural markers for the given cognitive function, it is important to clarify in the future whether the relevance of target‐related activation for behavioral capacity would also be present in oscillatory activity.
It should also be noted that it is not straightforward to interpret the correlation measures. We observed significant correlations in two distinct time‐windows. In the early time‐window (0–300 ms) a larger difference between the conditions (difficult versus easy distractor) was associated with better performance whereas in the late time‐window (500–700 ms) better performance was associated with a smaller difference between the conditions. Correlation was measured between behavioral performance and a task contrast in the brain. As the evoked response amplitude reflects changing cognitive operations along its progression (Galer et al., 2015; Parviainen & Kujala, 2023; Wen et al., 2019), it is possible that the amplitude values (hence synchronization of neural activity) show distinct associations with behaviour in distinct stages of the processing. Indeed, our results suggest that a pattern of a larger difference between task conditions in the early time‐window and a smaller difference between task conditions in the later time‐window is linked with better performance in inhibitory and working‐memory functions. This pattern would be in line with benefit of early emerging inhibition, perhaps overlapping with perceptual judgement, and thus successfully directing of internal attention to relevant memory content. If the inhibitory contrast emerges only later (as was the case for individuals performing more poorly), the timing may be too late to support successful retrieval. This interpretation is supported by Andersen and Müller (2010), who showed that in selective visual attention task suppression of unattended visual stimuli and enhancement of responses to visual targets commenced in neural activity already before 300 ms, and the behavioral performance (reaction times) was best explained not by the absolute amplitudes, but the relative difference between these activations to attended versus nonattended stimuli. It must be noted, however, that we only analysed successfully retrieved trials. Moreover, it is also possible that, especially at the later time‐window also processes related to motor preparation influence the evoked response amplitude. Further studies are needed to clarify the core neural measure underlying the evoked response effects.
An important future direction for the current study is to expand the focus from correlations to causal relationships between neural activity and behavioral measures. This would afford a more direct proof that neural measures that correlate with behavioral measures are indeed the generators of the behaviors of interest. Recent interest in decoding cognitive operations from electrophysiological recordings during attentional selection (Wen et al., 2019) could be applied also to inform about the contribution of different neural processes on memory‐based decision making (cf. Foster et al., 2016). Moreover, modulating the neural activity through stimulation techniques and observing the associated changes in behavior can achieve a novel, causality‐based view on the brain‐behavior link. Notably, transcranial magnetic stimulation has been shown to transiently alter neural dynamics on a stimulation‐dependent manner. It could thus be used in conjunction with functional neuroimaging measures that emphasize correlational relationships and further consolidate the observed brain‐behavioral link through the angle of causality (Silvanto & Pascual‐Leone, 2012).
These findings are of relevance in the more general context of developmental neuroscience (Lindenberger et al., 2006), that involves mapping the link between brain and behavior across the lifespan. The relationship between individual level behavioral performance and specific brain measures is particularly complex, as this link dynamically changes as a function of time both on the time‐scale of cognitive performance itself, and in more larger time‐scale of individual life‐span, and is subject to gene–environment influences. In order to reach any stable, age‐invariant neural level markers of the level of behavioral performance, it would be beneficial to examine the similar brain‐behavior link in children, another population in which inter‐individual variability is large (Bonte et al., 2013; Parviainen et al., 2011). Appending additional data points in the continuously evolving association between brain and behavioral measures could strengthen the understanding of how neural activity ultimately contributes to behavioral performance. This emerging understanding of the interconnection between neural activation and behavioral patterns at individual level is of particular relevance in neurogenomics and psychopathology, where genetic predispositions are considered in conjunction to inter‐individual variability in brain function and the subsequently observed behaviors (Bogdan et al., 2013).
5. CONCLUSIONS
The question of whether specific patterns of neural activity are individually meaningful to certain behavior or cognitive performance has remained poorly understood and pertain to an important next step in understanding the brain basis of cognitive functions. The present findings suggest that neural activity can be associated with individually defined performance at cognitive tasks involving working memory and inhibitory control. Importantly, although induced oscillatory activity showed task‐related modulation in expected frequency bands, presumably providing the top‐down driven frame for mental manipulation of memorized items, only the evoked response amplitude, bound to the bottom‐up information processing pathway, correlated with behavioral performance. Further studies are needed to extract the core elements reflected by this effect, but our findings, together with earlier evidence of, e.g., P3 effects, evidence the robustness of evoked activity measures also at individual level.
Supporting information
Data S1 Supporting information.
Figure S1. Statistically significant results of the cluster‐based permutation analysis for the frequency‐sensitive TFR statistical analysis. Each topoplot represents one statistically significant cluster. White circles indicate MEG channels that demonstrate statistically significant modulations in MEG signal power. The frequency bands in which the statistically significant effect is observed as well as the p‐values are reported underneath each topoplot. (A) Statistically significant modulations in MEG signal power for the first vs. second fixation (before vs. after encoder). (B) Statistically significant modulations in MEG signal power for the second vs. third fixation (before vs. after distractor image). (C) Statistically significant modulations in MEG signal power for the within third fixation contrast (after an easy vs. a difficult distractor).
ACKNOWLEDGMENTS
We thank Anna Tirkkonen (MSc) for coordinating the PASSWORD‐project and Sini Hentilä, Tiina Autio, Tiina Mikkonen and Arttu Perello for assisting in MEG data acquisition. Funding: This work was supported by the Academy of Finland (Grant numbers 296843, 311877). The authors have no conflicts of interest to declare.
Parviainen, T. , Alexandrou, A. M. , Lapinkero, H.‐M. , Sipilä, S. , & Kujala, J. (2024). The link between executive skills and neural dynamics during encoding, inhibition, and retrieval of visual information in the elderly. Human Brain Mapping, 45(12), e26755. 10.1002/hbm.26755
Tiina Parviainen and Anna Maria Alexandrou contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Aine, C. J. , Sanfratello, L. , Adair, J. C. , Knoefel, J. E. , Caprihan, A. , & Stephen, J. M. (2011). Development and decline of memory functions in normal, pathological and healthy successful aging. Brain Topography, 24, 323–339. 10.1007/s10548-011-0178-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, S. K. , & Müller, M. M. (2010). Behavioral performance follows the time course of neural facilitation and suppression during cued shifts of feature‐selective attention. Proceedings of the National Academy of Sciences of the United States of America, 107(31), 13878–13882. 10.1073/pnas.1002436107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman, L. , Jones, S. , Berger, A. K. , Laukka, E. J. , & Small, B. J. (2005). Cognitive impairment in preclinical Alzheimer's disease: A meta‐analysis. Neuropsychology, 19, 520–531. 10.1037/0894-4105.19.4.520 [DOI] [PubMed] [Google Scholar]
- Bäckman, L. , Lindenberger, U. , Li, S. C. , & Nyberg, L. (2010). Linking cognitive aging to alterations in dopamine neurotransmitter functioning: Recent data and future avenues. Neuroscience and Biobehavioral Reviews, 34, 670–677. 10.1016/j.neubiorev.2009.12.008 [DOI] [PubMed] [Google Scholar]
- Barth, A. , & Schneider, D. (2018). Manipulating the focus of attention in working memory: Evidence for a protection of multiple items against perceptual interference. Psychophysiology, 55, e13062. 10.1111/PSYP.13062 [DOI] [PubMed] [Google Scholar]
- Bastin, C. , Yakushev, I. , Bahri, M. A. , Fellgiebel, A. , Eustache, F. , Landeau, B. , Scheurich, A. , Feyers, D. , Collette, F. , Chételat, G. , & Salmon, E. (2012). Cognitive reserve impacts on inter‐individual variability in resting‐state cerebral metabolism in normal aging. NeuroImage, 63, 713–722. 10.1016/J.NEUROIMAGE.2012.06.074 [DOI] [PubMed] [Google Scholar]
- Bherer, L. (2015). Cognitive plasticity in older adults: Effects of cognitive training and physical exercise. Annals of the new York Academy of Sciences, 1337, 1–6. 10.1111/nyas.12682 [DOI] [PubMed] [Google Scholar]
- Bherer, L. , Erickson, K. I. , & Liu‐Ambrose, T. (2013). A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. Journal of Aging Research, 2013, 657508. 10.1155/2013/657508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan, R. , Hyde, L. W. , & Hariri, A. R. (2013). A neurogenetics approach to understanding individual differences in brain, behavior, and risk for psychopathology. Molecular Psychiatry, 18, 288–299. 10.1038/MP.2012.35 [DOI] [PubMed] [Google Scholar]
- Bonnefond, M. , & Jensen, O. (2012). Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Current Biology, 22, 1969–1974. 10.1016/j.cub.2012.08.029 [DOI] [PubMed] [Google Scholar]
- Bonnefond, M. , & Jensen, O. (2013). The role of gamma and alpha oscillations for blocking out distraction. Communicative & Integrative Biology, 6, e22702. 10.4161/CIB.22702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonte, M. , Frost, M. A. , Rutten, S. , Ley, A. , Formisano, E. , & Goebel, R. (2013). Development from childhood to adulthood increases morphological and functional inter‐individual variability in the right superior temporal cortex. NeuroImage, 83, 739–750. 10.1016/J.NEUROIMAGE.2013.07.017 [DOI] [PubMed] [Google Scholar]
- Borella, E. , Carretti, B. , & De Beni, R. (2008). Working memory and inhibition across the adult life‐span. Acta Psychologica, 128, 33–44. 10.1016/j.actpsy.2007.09.008 [DOI] [PubMed] [Google Scholar]
- Bowie, C. R. , & Harvey, P. D. (2006). Administration and interpretation of the trail making test. Nature Protocols, 1, 2277–2281. 10.1038/nprot.2006.390 [DOI] [PubMed] [Google Scholar]
- Brennan, M. , Welsh, M. C. , & Fisher, C. B. (1997). Aging and executive function skills: An examination of a community‐dwelling older adult population. Perceptual and Motor Skills, 84, 1187–1197. 10.2466/pms.1997.84.3c.1187 [DOI] [PubMed] [Google Scholar]
- Chandler, M. J. , Lacritz, L. H. , Hynan, L. S. , Barnard, H. D. , Allen, G. , Deschner, M. , Weiner, M. F. , & Cullum, C. M. (2005). A total score for the CERAD neuropsychological battery. Neurology, 65, 102–106. 10.1212/01.wnl.0000167607.63000.38 [DOI] [PubMed] [Google Scholar]
- Chao, L. L. , & Knight, R. T. (1997). Prefrontal deficits in attention and inhibitory control with aging. Cerebral Cortex, 7, 63–69. 10.1093/CERCOR/7.1.63 [DOI] [PubMed] [Google Scholar]
- Chen, Y. , & Huang, X. (2016). Modulation of alpha and beta oscillations during an n‐back task with varying temporal memory load. Frontiers in Psychology, 6, 2031. 10.3389/FPSYG.2015.02031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, M. , Larson, E. , Tavabi, K. , & Taulu, S. (2020). Effectively combining temporal projection noise suppression methods in magnetoencephalography. Journal of Neuroscience Methods, 341, 108700. 10.1016/J.JNEUMETH.2020.108700 [DOI] [PubMed] [Google Scholar]
- Duarte, A. , Hearons, P. , Jiang, Y. , Delvin, M. C. , Newsome, R. N. , & Verhaeghen, P. (2013). Retrospective attention enhances visual working memory in the young but not the old: An ERP study. Psychophysiology, 50, 465–476. 10.1111/PSYP.12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustman, R. E. , Emmerson, R. Y. , & Shearer, D. E. (1996). Life span changes in electrophysiological measures of inhibition. Brain and Cognition, 30, 109–126. 10.1006/BRCG.1996.0007 [DOI] [PubMed] [Google Scholar]
- Ehrensperger, M. M. , Berres, M. , Taylor, K. I. , & Monsch, A. U. (2010). Early detection of Alzheimer's disease with a total score of the German CERAD. Journal of the International Neuropsychological Society, 16, 910–920. 10.1017/S1355617710000822 [DOI] [PubMed] [Google Scholar]
- Faisal, A. A. , Selen, L. P. J. , & Wolpert, D. M. (2008). Noise in the nervous system. Nature Reviews. Neuroscience, 9, 292–303. 10.1038/NRN2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, J. J. , Sutterer, D. W. , Serences, J. T. , Vogel, E. K. , & Awh, E. (2016). The topography of alpha‐band activity tracks the content of spatial working memory. Journal of Neurophysiology, 115(1), 168–177. 10.1152/jn.00860.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, D. , Nessler, D. , & Johnson, R. (2007). Memory encoding and retrieval in the aging brain. Clinical EEG and Neuroscience, 38, 2–7. 10.1177/155005940703800105 [DOI] [PubMed] [Google Scholar]
- Galer, S. , Op De Beeck, M. , Urbain, C. , Bourguignon, M. , Ligot, N. , Wens, V. , Marty, B. , Van Bogaert, P. , Peigneux, P. , & De Tiège, X. (2015). Investigating the neural correlates of the stroop effect with magnetoencephalography. Brain Topography, 28(1), 95–103. 10.1007/s10548-014-0367-5 [DOI] [PubMed] [Google Scholar]
- Ganesan, K. , & Steinbeis, N. (2021). Development and plasticity of executive functions: A value‐based account. Current Opinion in Psychology, 44, 215–219. 10.1016/J.COPSYC.2021.09.012 [DOI] [PubMed] [Google Scholar]
- Graf, P. , Uttl, B. , & Tuokko, H. (1995). Color‐ and picture‐word stroop tests: Performance changes in old age. Journal of Clinical and Experimental Neuropsychology, 17, 390–415. 10.1080/01688639508405132 [DOI] [PubMed] [Google Scholar]
- Gramfort, A. , Luessi, M. , Larson, E. , Engemann, D. A. , Strohmeier, D. , Brodbeck, C. , Goj, R. , Jas, M. , Brooks, T. , Parkkonen, L. , & Hämäläinen, M. (2013). MEG and EEG data analysis with MNE‐python. Frontiers in Neuroscience, 7, 267. 10.3389/fnins.2013.00267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean, J. , & Collette, F. (2011). Influence of response prepotency strength, general working memory resources, and specific working memory load on the ability to inhibit predominant responses: A comparison of young and elderly participants. Brain and Cognition, 77, 237–247. 10.1016/J.BANDC.2011.08.004 [DOI] [PubMed] [Google Scholar]
- Guo, S. (2004). Linking genes to brain, behavior and neurological diseases: What can we learn from zebrafish? Genes, Brain, and Behavior, 3, 63–74. 10.1046/J.1601-183X.2003.00053.X [DOI] [PubMed] [Google Scholar]
- Haegens, S. , Cousijn, H. , Wallis, G. , Harrison, P. J. , & Nobre, A. C. (2014). Inter‐ and intra‐individual variability in alpha peak frequency. Neuroimage, 92, 46–55. 10.1016/j.neuroimage.2014.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinault, T. , Kraut, M. , Bakker, A. , Dagher, A. , & Courtney, S. M. (2020). Disrupted neural synchrony mediates the relationship between white matter integrity and cognitive performance in older adults. Cerebral Cortex, 30, 5570–5582. [DOI] [PubMed] [Google Scholar]
- Honkanen, R. , Rouhinen, S. , Wang, S. H. , Palva, J. M. , & Palva, S. (2015). Gamma oscillations underlie the maintenance of feature‐specific information and the contents of visual working memory. Cerebral Cortex, 25, 3788–3801. 10.1093/cercor/bhu263 [DOI] [PubMed] [Google Scholar]
- Hyvärinen, A. (1999). Fast and robust fixed‐point algorithms for independent component analysis. IEEE Transactions on Neural Networks, 10, 626–634. 10.1109/72.761722 [DOI] [PubMed] [Google Scholar]
- Jauny, G. , Eustache, F. , & Hinault, T. (2022). Connectivity dynamics and cognitive variability during aging. Neurobiology of Aging, 118, 99–105. [DOI] [PubMed] [Google Scholar]
- Jensen, O. , Gelfand, J. , Kounios, J. , & Lisman, J. E. (2002). Oscillations in the alpha band (9‐12 Hz) increase with memory load during retention in a short‐term memory task. Cerebral Cortex, 12, 877–882. [DOI] [PubMed] [Google Scholar]
- Kimura, K. , Yasunaga, A. , & Wang, L. Q. (2013). Correlation between moderate daily physical activity and neurocognitive variability in healthy elderly people. Archives of Gerontology and Geriatrics, 56, 109–117. 10.1016/J.ARCHGER.2012.10.004 [DOI] [PubMed] [Google Scholar]
- Klimesch, W. (2012). Alpha‐band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences, 16, 606–617. 10.1016/j.tics.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch, W. , Sauseng, P. , & Hanslmayr, S. (2007). EEG alpha oscillations: The inhibition‐timing hypothesis. Brain Research Reviews, 53, 63–88. 10.1016/J.BRAINRESREV.2006.06.003 [DOI] [PubMed] [Google Scholar]
- Krakauer, J. W. , Ghazanfar, A. A. , Gomez‐Marin, A. , MacIver, M. A. , & Poeppel, D. (2017). Neuroscience needs behavior: Correcting a reductionist bias. Neuron, 93, 480–490. 10.1016/J.NEURON.2016.12.041 [DOI] [PubMed] [Google Scholar]
- Kurimoto, R. , Ishii, R. , Canuet, L. , Ikezawa, K. , Iwase, M. , Azechi, M. , Aoki, Y. , Ikeda, S. , Yoshida, T. , Takahashi, H. , Nakahachi, T. , Kazui, H. , & Takeda, M. (2012). Induced oscillatory responses during the Sternberg's visual memory task in patients with Alzheimer's disease and mild cognitive impairment. NeuroImage, 59, 4132–4140. 10.1016/J.NEUROIMAGE.2011.10.061 [DOI] [PubMed] [Google Scholar]
- Laaksonen, H. , Kujala, J. , Hultén, A. , Liljeström, M. , & Salmelin, R. (2012). MEG evoked responses and rhythmic activity provide spatiotemporally complementary measures of neural activity in language production. NeuroImage, 60, 29–36. 10.1016/j.neuroimage.2011.11.087 [DOI] [PubMed] [Google Scholar]
- Li, R. , Yin, S. , Zhu, X. , Ren, W. , Yu, J. , Wang, P. , Zheng, Z. , Niu, Y. N. , Huang, X. , & Li, J. (2017). Linking inter‐individual variability in functional brain connectivity to cognitive ability in elderly individuals. Frontiers in Aging Neuroscience, 9, 385. 10.3389/FNAGI.2017.00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger, U. , Li, S. C. , & Bäckman, L. (2006). Delineating brain‐behavior mappings across the lifespan: Substantive and methodological advances in developmental neuroscience. Neuroscience and Biobehavioral Reviews, 30, 713–717. 10.1016/J.NEUBIOREV.2006.06.006 [DOI] [PubMed] [Google Scholar]
- Lindenberger, U. , & von Oertzen, T. (2006). Variability in cognitive aging: From taxonomy to theory. In Bialystok E. & Craik F. I. M. (Eds.), Lifespan cognition: Mechanisms of change (pp. 297–314). Oxford University Press. [Google Scholar]
- Lisman, J. (2010). Working memory: The importance of theta and gamma oscillations. Current Biology, 20, R490–R492. 10.1016/J.CUB.2010.04.011 [DOI] [PubMed] [Google Scholar]
- López, M. E. , Aurtenetxe, S. , Pereda, E. , Cuesta, P. , Castellanos, N. P. , Bruña, R. , Niso, G. , Maestú, F. , & Bajo, R. (2014). Cognitive reserve is associated with the functional organization of the brain in healthy aging: A MEG study. Frontiers in Aging Neuroscience, 6, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano‐Soldevilla, D. , ter Huurne, N. , Cools, R. , & Jensen, O. (2014). GABAergic modulation of visual gamma and alpha oscillations and its consequences for working memory performance. Current Biology, 24, 2878–2887. 10.1016/j.cub.2014.10.017 [DOI] [PubMed] [Google Scholar]
- Lundqvist, M. , Rose, J. , Herman, P. , Brincat, S. L. , Buschman, T. J. , & Miller, E. K. (2016). Gamma and Beta bursts underlie working memory. Neuron, 90, 152–164. 10.1016/j.neuron.2016.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien, S. J. , de Leon, M. , de Santi, S. , Convit, A. , Tarshish, C. , Nair, N. P. V. , Thakur, M. , McEwen, B. S. , Hauger, R. L. , & Meaney, M. J. (1998). Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neuroscience, 1, 69–73. 10.1038/271 [DOI] [PubMed] [Google Scholar]
- MacDonald, S. W. S. , Nyberg, L. , & Bäckman, L. (2006). Intra‐individual variability in behavior: Links to brain structure, neurotransmission and neuronal activity. Trends in Neurosciences, 29, 474–480. 10.1016/J.TINS.2006.06.011 [DOI] [PubMed] [Google Scholar]
- Meeuwissen, E. B. , Takashima, A. , Fernández, G. , & Jensen, O. (2011). Evidence for human Fronto‐central gamma activity during long‐term memory encoding of word sequences. PLoS One, 6, e21356. 10.1371/JOURNAL.PONE.0021356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt, T. E. , Arseneault, L. , Belsky, D. , Dickson, N. , Hancox, R. J. , Harrington, H. L. , Houts, R. , Poulton, R. , Roberts, B. W. , Ross, S. , Sears, M. R. , Thomson, W. M. , & Caspi, A. (2011). A gradient of childhood self‐control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences of the United States of America, 108, 2693–2698. 10.1073/PNAS.1010076108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, H. M. , Muthukumaraswamy, S. D. , Hibbs, C. S. , Shapiro, K. L. , Bracewell, R. M. , Singh, K. D. , & Linden, D. E. J. (2011). Feature integration in visual working memory: Parietal gamma activity is related to cognitive coordination. Journal of Neurophysiology, 106, 3185–3194. 10.1152/JN.00246.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill, W. T. , Valdes, L. A. , & Terry, K. M. (1995). Selective attention and the inhibitory control of cognition. In Dempster F. N. & Brainerd C. J. (Eds.), Interference and inhibition in cognition (pp. 207–261). Academic Press. [Google Scholar]
- Nitrini, R. , Caramelli, P. , Herrera, E. , et al. (2004). Performance of illiterate and literate nondemented elderly subjects in two tests of long‐term memory. Journal of the International Neuropsychological Society, 10, 634–638. 10.1017/S1355617704104062 [DOI] [PubMed] [Google Scholar]
- Nowak, K. , Costa‐Faidella, J. , Dacewicz, A. , Escera, C. , & Szelag, E. (2021). Altered event‐related potentials and theta oscillations index auditory working memory deficits in healthy aging. Neurobiology of Aging, 108, 1–15. 10.1016/J.NEUROBIOLAGING.2021.07.019 [DOI] [PubMed] [Google Scholar]
- Olivetti, E. , Kia, S. M. , & Avesani, P. (2014). MEG decoding across subjects. In 2014 international workshop on pattern recognition in neuroimaging (pp. 1–4). IEEE. [Google Scholar]
- Paajanen, T. , Hänninen, T. , Tunnard, C. , Mecocci, P. , Sobow, T. , Tsolaki, M. , Vellas, B. , Lovestone, S. , Soininen, H. , & AddNeuroMed Consortium . (2010). CERAD neuropsychological battery Total score in multinational mild cognitive impairment and control populations: The AddNeuroMed study. Journal of Alzheimer's Disease, 22, 1089–1097. 10.3233/JAD-2010-100459 [DOI] [PubMed] [Google Scholar]
- Palva, J. M. , Monto, S. , Kulashekhar, S. , & Palva, S. (2010). Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proceedings of the National Academy of Sciences of the United States of America, 107, 7580–7585. 10.1073/pnas.0913113107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva, S. , Kulashekhar, S. , Hämäläinen, M. , & Palva, J. M. (2011). Localization of cortical phase and amplitude dynamics during visual working memory encoding and retention. The Journal of Neuroscience, 31, 5013–5025. 10.1523/JNEUROSCI.5592-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parviainen, T. , Helenius, P. , Poskiparta, E. , Niemi, P. , & Salmelin, R. (2006). Cortical sequence of word perception in beginning readers. The Journal of Neuroscience, 26, 6052–6061. 10.1523/JNEUROSCI.0673-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parviainen, T. , Helenius, P. , Poskiparta, E. , Niemi, P. , & Salmelin, R. (2011). Speech perception in the child brain: Cortical timing and its relevance to literacy acquisition. Human Brain Mapping, 32, 2193–2206. 10.1002/hbm.21181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parviainen, T. , & Kujala, J. (2023). Event‐related potentials (ERPs) and event‐related fields (ERFs). In Grimaldi M., Brattico E., & Shtyrov Y. (Eds.), Language electrified. Neuromethods (Vol. 202). Humana. 10.1007/978-1-0716-3263-5_7 [DOI] [Google Scholar]
- Peich, M. C. , Husain, M. , & Bays, P. M. (2013). Age‐related decline of precision and binding in visual working memory. Psychology and Aging, 28, 729–743. 10.1037/A0033236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi, L. , & Blumhardt, L. D. (1999). Effects of age on working memory: An event‐related potential study. Brain Research. Cognitive Brain Research, 7, 321–334. 10.1016/S0926-6410(98)00035-4 [DOI] [PubMed] [Google Scholar]
- Penke, L. , Munoz Maniega, S. , Murray, C. , et al. (2010). A general factor of brain white matter integrity predicts information processing speed in healthy older people. The Journal of Neuroscience, 30, 7569–7574. 10.1523/JNEUROSCI.1553-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piefke, M. , Onur, Ö. A. , & Fink, G. R. (2012). Aging‐related changes of neural mechanisms underlying visual‐spatial working memory. Neurobiology of Aging, 33, 1284–1297. 10.1016/J.NEUROBIOLAGING.2010.10.014 [DOI] [PubMed] [Google Scholar]
- Polich, J. (2007). Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology, 118, 2128–2148. 10.1016/j.clinph.2007.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich, J. , & Corey‐Bloom, J. (2005). Alzheimer's disease and P300: Review and evaluation of task and modality. Current Alzheimer Research, 2, 515–525. 10.2174/156720505774932214 [DOI] [PubMed] [Google Scholar]
- Pulvermüller, F. , Eulitz, C. , Pantev, C. , Mohr, B. , Feige, B. , Lutzenberger, W. , Elbert, T. , & Birbaumer, N. (1996). High‐frequency cortical responses reflect lexical processing: An MEG study. Electroencephalography and Clinical Neurophysiology, 98, 76–85. 10.1016/0013-4694(95)00191-3 [DOI] [PubMed] [Google Scholar]
- Reuter‐Lorenz, P. A. , & Park, D. C. (2010). Human neuroscience and the aging mind: A new look at old problems. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 65, 405–415. 10.1093/GERONB/GBQ035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini, P. M. , Rossi, S. , Babiloni, C. , & Polich, J. (2007). Clinical neurophysiology of aging brain: From normal aging to neurodegeneration. Progress in Neurobiology, 83, 375–400. 10.1016/J.PNEUROBIO.2007.07.010 [DOI] [PubMed] [Google Scholar]
- Rossiter, H. E. , Davis, E. M. , Clark, E. V. , Boudrias, M. H. , & Ward, N. S. (2014). Beta oscillations reflect changes in motor cortex inhibition in healthy ageing. NeuroImage, 91, 360–365. 10.1016/J.NEUROIMAGE.2014.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda‐Delgado, L. M. , O'Halloran, L. , Enz, N. , Ruddy, K. L. , Kiiski, H. , Bennett, M. , Farina, F. , Jollans, L. , Vahey, N. , & Whelan, R. (2021). Brain event‐related potentials predict individual differences in inhibitory control. International Journal of Psychophysiology, 163, 22–34. 10.1016/J.IJPSYCHO.2019.03.013 [DOI] [PubMed] [Google Scholar]
- Scarpina, F. , & Tagini, S. (2017). The stroop color and word test. Frontiers in Psychology, 8, 557. 10.3389/FPSYG.2017.00557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaum, M. , Pinzuti, E. , Sebastian, A. , Lieb, K. , Fries, P. , Mobascher, A. , Jung, P. , Wibral, M. , & Tüscher, O. (2021). Right inferior frontal gyrus implements motor inhibitory control via beta‐band oscillations in humans. eLife, 10, e61679. 10.7554/ELIFE.61679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto, J. , & Pascual‐Leone, A. (2012). Why the assessment of causality in brain‐behavior relations requires brain stimulation. Journal of Cognitive Neuroscience, 24, 775–777. 10.1162/JOCN_A_00193 [DOI] [PubMed] [Google Scholar]
- Sipilä, S. , Tirkkonen, A. , Hänninen, T. , Laukkanen, P. , Alen, M. , Fielding, R. A. , Kivipelto, M. , Kokko, K. , Kulmala, J. , Rantanen, T. , Sihvonen, S. E. , Sillanpää, E. , Stigsdotter‐Neely, A. , & Törmäkangas, T. (2018). Promoting safe walking among older people: The effects of a physical and cognitive training intervention vs. physical training alone on mobility and falls among older community‐dwelling men and women (the PASSWORD study): Design and methods of a randomi. BMC Geriatrics, 18, 215. 10.1186/s12877-018-0906-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solís‐Vivanco, R. , Jensen, O. , & Bonnefond, M. (2021). New insights on the ventral attention network: Active suppression and involuntary recruitment during a bimodal task. Human Brain Mapping, 42, 1699–1713. 10.1002/HBM.25322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop, J. R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18, 643–662. 10.1037/h0054651 [DOI] [Google Scholar]
- Taulu, S. , & Simola, J. (2006). Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Physics in Medicine and Biology, 51, 1759–1768. [DOI] [PubMed] [Google Scholar]
- Vallesi, A. , Stuss, D. T. , McIntosh, A. R. , & Picton, T. W. (2009). Age‐related differences in processing irrelevant information: Evidence from event‐related potentials. Neuropsychologia, 47, 577–586. 10.1016/J.NEUROPSYCHOLOGIA.2008.10.018 [DOI] [PubMed] [Google Scholar]
- Wang, M. , Gamo, N. J. , Yang, Y. , Jin, L. E. , Wang, X. J. , Laubach, M. , Mazer, J. A. , Lee, D. , & Arnsten, A. F. T. (2011). Neuronal basis of age‐related working memory decline. Nature, 476, 210–213. 10.1038/NATURE10243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, G. , & Caplan, D. (2005). The relationship between age, processing speed, working memory capacity, and language comprehension. Memory, 13, 403–413. 10.1080/09658210344000459 [DOI] [PubMed] [Google Scholar]
- Waters, G. S. , & Caplan, D. (2001). Age, working memory, and on‐line syntactic processing in sentence comprehension. Psychology and Aging, 16, 128–144. 10.1037/0882-7974.16.1.128 [DOI] [PubMed] [Google Scholar]
- Wen, T. , Duncan, J. , & Mitchell, D. J. (2019). The time‐course of component processes of selective attention. NeuroImage, 199, 396–407. 10.1016/j.neuroimage.2019.05.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, R. , & Alain, C. (2000). Age‐related decline in inhibitory control contributes to the increased stroop effect observed in older adults. Psychophysiology, 37, 179–189. [PubMed] [Google Scholar]
- Ziegler, D. A. , Pritchett, D. L. , Hosseini‐Varnamkhasti, P. , Corkin, S. , Hämäläinen, M. , Moore, C. I. , & Jones, S. R. (2010). Transformations in oscillatory activity and evoked responses in primary somatosensory cortex in middle age: A combined computational neural modeling and MEG study. NeuroImage, 52, 897–912. 10.1016/J.NEUROIMAGE.2010.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zioga, I. , Hassan, R. , & Luft, C. D. B. (2019). Success, but not failure feedback guides learning during neurofeedback: An ERP study. NeuroImage, 200, 26–37. 10.1016/J.NEUROIMAGE.2019.06.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting information.
Figure S1. Statistically significant results of the cluster‐based permutation analysis for the frequency‐sensitive TFR statistical analysis. Each topoplot represents one statistically significant cluster. White circles indicate MEG channels that demonstrate statistically significant modulations in MEG signal power. The frequency bands in which the statistically significant effect is observed as well as the p‐values are reported underneath each topoplot. (A) Statistically significant modulations in MEG signal power for the first vs. second fixation (before vs. after encoder). (B) Statistically significant modulations in MEG signal power for the second vs. third fixation (before vs. after distractor image). (C) Statistically significant modulations in MEG signal power for the within third fixation contrast (after an easy vs. a difficult distractor).
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


