Abstract
Serologic testing to detect antibodies to avian influenza (AI) virus has been an underused tool for the study of these viruses in wild bird populations, which traditionally has relied on virus isolation and reverse transcriptase-polymerase chain reaction (RT-PCR). In a preliminary study, a recently developed commercial blocking enzyme-linked immunosorbent assay (bELISA) had sensitivity and specificity estimates of 82% and 100%, respectively, for detection of antibodies to AI virus in multiple wild bird species after experimental infection. To further evaluate the efficacy of this commercial bELISA and the agar gel immunodiffusion (AGID) test for AI virus antibody detection in wild birds, we tested 2,249 serum samples collected from 62 wild bird species, representing 10 taxonomic orders. Overall, the bELISA detected 25.4% positive samples, whereas the AGID test detected 14.8%. At the species level, the bELISA detected as many or more positive serum samples than the AGID in all 62 avian species. The majority of positive samples, detected by both assays, were from species that use aquatic habitats, with the highest prevalence from species in the orders Anseriformes and Charadriiformes. Conversely, antibodies to AI virus were rarely detected in the terrestrial species. The serologic data yielded by both assays are consistent with the known epidemiology of AI virus in wild birds and published reports of host range based on virus isolation and RT-PCR. The results of this research are also consistent with the aforementioned study, which evaluated the performance of the bELISA and AGID test on experimental samples. Collectively, the data from these two studies indicate that the bELISA is a more sensitive serologic assay than the AGID test for detecting prior exposure to AI virus in wild birds. Based on these results, the bELISA is a reliable species-independent assay with potentially valuable applications for wild bird AI surveillance.
Keywords: AGID, antibodies, avian influenza virus, blocking, enzyme-linked immunosorbent assay, serology, wild birds
INTRODUCTION
Avian influenza (AI) viruses (Orthomyxoviridae) have been isolated from a wide diversity of avian species (Stallknecht and Shane, 1988; Olsen et al., 2006), and wild bird populations represent the natural reservoir for all known hemagglutinin (HA) and neuraminidase subtypes of AI virus (Webster et al., 1992). The prevalence of AI viruses within wild birds, however, varies extensively between taxonomic orders, genera, and, in some cases, related species (Munster et al., 2007). Currently, wild aquatic birds in the orders Anseriformes (ducks, geese, and swans) and Charadriiformes (gulls, terns, and shorebirds) are considered the most important natural reservoirs for AI viruses (Stallknecht and Brown, 2007).
Surveillance for AI virus infection in wild birds, to date, has largely used diagnostic assays to directly detect viral shedding, including virus isolation and reverse transcriptase-polymerase chain reaction (RT-PCR). Both of these diagnostic assays have undeniable benefits for wild bird surveillance and have largely defined our existing knowledge of the epidemiology of AI in wild birds (Webster et al., 1992). There are, however, some limitations to their use, including the high cost and time requirements associated with virus isolation and the need for specialized equipment to perform RT-PCR. An additional limitation of these agent identification assays is that they are dependent on the avian host excreting virus. This limitation can be particularly problematic in the context of wild bird AI surveillance due to the relatively limited window of viral shedding and the marked spatial and temporal variation associated with infection in different wild avian populations (Munster et al., 2007). Consequently, virus isolation and RT-PCR represent efficient diagnostic assays for AI surveillance in wild bird populations in which the epidemiology is well defined, such as dabbling ducks in North America or shorebirds at Delaware Bay, USA (Stallknecht et al., 2007; Hanson et al., 2008). In these situations, existing published data can guide when, where, and how best to sample these populations to detect active infections. These virus-dependent assays are much less efficient when attempting AI surveillance on species in which existing prevalence data are not available.
Screening for antibodies directed against AI virus is a common method of surveillance used in domestic poultry for detecting prior AI infection on a population level (Spackman et al., 2008). Such serologic testing capabilities, as applied to wild birds, would represent a valuable complement to existing virus isolation- and RT-PCR-based surveillance by providing an additional perspective on AI infection in different avian populations. This information would not only help in the interpretation of virus isolation results, but also could serve as an economical method to identify target populations for more costly and time-consuming surveillance efforts. Traditionally, however, serologic testing has been an underused tool in wild bird AI surveillance. A major reason for this underuse is that existing serologic assays for domestic poultry lack sensitivity or may not provide useful data when applied to wild bird AI surveillance (Cattoli and Capua, 2007; Stallknecht et al., 2007).
The agar gel immunodiffusion (AGID) test is a serologic assay that is commonly used to screen domestic poultry flocks for prior AI virus exposure because it is not host specific and detects immunologic responses to type A influenza viruses regardless of subtype (Swayne et al., 2008). Although this test is relatively inexpensive and simple to perform, it generally is not as sensitive as other serologic assays such as enzyme-linked immunosorbent assays (ELISA; Snyder et al., 1985). Additionally, the AGID test has traditionally been considered to have limited applications for wild bird surveillance due to the lack of precipitating antibodies produced by waterfowl species, particularly ducks, resulting in a poor diagnostic sensitivity (Higgins, 1998).
The hemagglutination inhibition (HI) and neuraminidase inhibition (NI) tests are subtype-specific serologic assays that are frequently used in domestic poultry. These assays can be sensitive and have applications in screening for specific subtypes or further characterizing known positive sera. The HI and NI tests, however, are labor-intensive and not suitable as general screening assays for previous AI virus exposure in wild birds due to the high number of subtype-specific tests that would have to be performed for each sample.
Several commercial ELISAs have been developed for use in domestic poultry, both in indirect and blocking formats. The indirect ELISAs reportedly can be more sensitive than the AGID test in gallinaceous birds (Snyder et al., 1985; Spackman et al., 2008), but these commercial assays were developed with species-specific secondary antibodies; consequently, they have little applications for AI surveillance in taxonomically diverse wild avian populations (Spackman et al., 2008). The commercial blocking ELISAs (bELISA) use mouse-derived monoclonal antibodies to compete with serum antibodies for binding to the antigen-labeled test kit and should perform well in multiple avian species. In a recent study, investigators evaluated the performance of a commercial bELISA and the AGID test on sera from multiple wild avian species experimentally infected with AI viruses (Brown et al., 2009). The bELISA was more sensitive than the AGID test and was a robust assay that performed well across a wide range of avian taxa. These results, however, were based on experimental trials in which the postinoculation serum samples were collected ≤21 days after inoculation with AI virus under controlled conditions. It remains undetermined how the commercial bELISA would perform on field samples, where additional variables and unknowns related to viral infection could affect the detectable host immune response and the performance of the test. The goal of this study was to evaluate the ability of a commercial bELISA and the AGID test to detect antibodies to AI viruses in field serum samples collected from a large diversity of wild avian species.
MATERIALS AND METHODS
Serum samples: collection and processing
Serum samples (n = 2,249) were collected from 62 wild avian species representing 10 taxonomic orders (Table 1). Birds were captured via standard methods, including cannon netting, mist netting, or trapping, in cooperation with state, federal, and private wildlife organizations in Alaska, California, Delaware, Georgia, Michigan, Minnesota, Mississippi, New Jersey, New York, North Carolina, Texas, and Virginia, USA, under federal permit MB779238–3. Whole blood (0.5–1.0% of blood volume based on body weight) was collected via jugular, medial metatarsal, or basilic veins, as appropriate for each species. Blood samples were placed into Microtainer serum separator tubes (BD Biosciences, Franklin Lanes, New Jersey, USA), allowed to clot, and stored on ice in the field. Within 24 hr, blood samples were centrifuged and serum was transferred to individual screw-cap tubes. The tubes were stored at 4 C or frozen in liquid nitrogen for transport back to the laboratory, where the samples were held in a −20 C freezer until serologic testing was conducted. The duration of storage for these banked serum samples ranged from <1 to 9 yr, but most were tested within the year of collection. Samples were thawed once, and both serologic assays were performed.
Table 1.
Prevalence of antibodies to avian influenza virus in 62 avian species from multiple states in the USA by using the agar gel immunodiffusion (AGID) test and a commercial blocking enzyme-linked immunosorbent assay (bELISA; S/N = serum-sample-to–negative-control absorbance values).
| Species | Sample date | Sample locationa,b | No. tested | AGID no. positive (%) | bELISA no. positive (%) | Positive S/N mean (range) | Negative S/N mean (range) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Order Gaviiformes | |||||||
| Pacific Loon (Gavia pacifica) | March 2006 | Solano, CA | 3 | 0 (0.0) | 0 (0.0) | NAc | 1.17 (1.05, 1.26) |
| Common Loon (Gavia immer) | May 2006 | Solano, CA | 3 | 0 (0.0) | 0 (0.0) | NA | 0.95 (0.84, 1.08) |
| Order Podicipediformes | |||||||
| Pied-billed Grebe (Podilymbus podiceps) | March 2006 | Solano, CA | 1 | 0 (0.0) | 0 (0.0) | NA | 1.67 |
| Red-necked Grebe (Podiceps grisegena) | August 2008 | Marshall, MN | 2 | 0 (0.0) | 0 (0.0) | NA | 0.93 (0.90, 0.95) |
| Western Grebe (Aechmophorus occidentalis) | March–July 2006 | Solano, CA | 3 | 0 (0.0) | 0 (0.0) | NA | 1.47 (1.37, 1.57) |
| Clark’s Grebe (Aechmophorus clarkii) | June 2006 | Solano, CA | 2 | 0 (0.0) | 0 (0.0) | NA | 1.42 (1.25, 1.60) |
| Order Pelecaniformes | |||||||
| Brandt’s Cormorant (Phalacrocorax penicillatus) | May 2006 | Solano, CA | 1 | 0 (0.0) | 0 (0.0) | NA | 0.91 |
| Double-crested Cormorant (Phalacrocorax auritus) | April–July 2006 | Solano, CA | 8 | 0 (0.0) | 0 (0.0) | NA | 0.99 (0.89, 1.17) |
| Order Anseriformes | |||||||
| Canada Goose (Branta canadensis) | 106 | 9 (8.5) | 25 (23.6) | 0.30 (0.08, 0.49) | 0.90 (0.50, 1.34) | ||
| June 2008 | Chatham, GA | 56 | 2 | 7 | |||
| June 2008 | Ramsey, MN | 40 | 4 | 17 | |||
| July 2008 | Pine, MN | 10 | 3 | 1 | |||
| Tundra Swan (Cygnus columbianus) | July–August 2008 | Northwest arctic, AK | 60 | 0 (0.0) | 16 (26.7) | 0.31 (0.16, 0.48) | 0.74 (0.51, 0.96) |
| Wood Duck (Aix sponsa) | 3 | 0 (0.0) | 0 (0.0) | NA | 0.85 (0.77, 0.94) | ||
| September 2007 | Marshall, MN | 1 | 0 | 0 | |||
| September 2008 | Marshall, MN | 2 | 0 | 0 | |||
| American Wigeon (Anas americana) | July–September 2007 | Marshall, MN | 37 | 9 (24.3) | 12 (32.4) | 0.23 (0.09, 0.47) | 0.85 (0.58, 1.07) |
| Mallard (Anas platyrhynchos) | 176 | 57 (32.4) | 81 (46.0) | 0.28 (0.11, 0.49) | 0.80 (0.50, 1.31) | ||
| September 2004 | Tyrell, NC | 25 | 0 | 4 | |||
| July–September 2007 | Chippewa, MI | 9 | 3 | 4 | |||
| July–September 2007 | Marshall, MN | 140 | 54 | 73 | |||
| September 2008 | Burlington, NJ | 2 | 0 | 0 | |||
| Domestic Mallard (Anas platyrhynchos) | 25 | 11 (44.0) | 17 (68.0) | 0.22 (0.08, 0.43) | 0.73 (0.52, 0.96) | ||
| September 2008 | Atlantic, NJ | 1 | 0 | 0 | |||
| September 2008 | Burlington, NJ | 1 | 0 | 0 | |||
| September 2008 | Cape May, NJ | 1 | 1 | 1 | |||
| September 2008 | Middlesex, NJ | 22 | 10 | 16 | |||
| Blue-winged Teal (Anas discors) | July–September 2007 | Marshall, MN | 15 | 3 (20.0) | 4 (26.7) | 0.19 (0.14, 0.32) | 0.76 (0.56, 0.92) |
| Northern Pintail (Anas acuta) | 32 | 9 (28.1) | 20 (62.5) | 0.26 (0.11, 0.44) | 0.70 (0.50, 0.97) | ||
| July–September 2007 | Marshall, MN | 4 | 2 | 3 | |||
| September 2008 | Marshall, MN | 28 | 7 | 17 | |||
| American Green-winged Teal (Anas crecca) | 69 | 20 (29.0) | 33 (47.8) | 0.29 (0.06, 0.48) | 0.80 (0.54, 1.43) | ||
| July–September 2007 | Marshall, MN | 1 | 0 | 1 | |||
| July–September 2008 | Marshall, MN | 68 | 20 | 32 | |||
| Redhead (Aythya americana) | August 2008 | Marshall, MN | 2 | 1 (50.0) | 1 (50.0) | 0.08 | 0.70 |
| Ring-necked Duck (Aythya collaris) | August 2008 | Marshall, MN | 1 | 0 (0.0) | 0 (0.0) | NA | 0.86 |
| Order Falconiformes | |||||||
| Black Vulture (Coragyps atratus) | March 2007 | Lowndes, MS | 184 | 0 (0.0) | 0 (0.0) | NA | 0.92 (0.66, 1.34) |
| Peregrine Falcon (Falco peregrinus) | 299 | 0 (0.0) | 2 (0.7) | 0.45 (0.41, 0.49) | 0.78 (0.51, 2.15) | ||
| October 2001 | Kenedy, TX | 74 | 0 | 0 | |||
| October 2002 | Accomack, VA | 5 | 0 | 0 | |||
| April 2003 | Kenedy, TX | 2 | 0 | 0 | |||
| September–October 2003 | Kenedy, TX | 127 | 0 | 2 | |||
| October 2003 | Accomack, VA | 81 | 0 | 0 | |||
| April 2004 | Kenedy, TX | 10 | 0 | 0 | |||
| Order Gruiformes | |||||||
| American Coot (Fulica americana) | 32 | 7 (21.9) | 11 (34.4) | 0.20 (0.08, 0.47) | 0.85 (0.54, 1.00) | ||
| August 2008 | Marshall, MN | 11 | 0 | 0 | |||
| January 2009 | Lincoln, GA | 21 | 7 | 11 | |||
| Order Charadriiformes | |||||||
| Ruddy Turnstone (Arenaria interpres) | 223 | 100 (44.8) | 145 (65.0) | 0.18 (0.04, 0.45) | 0.78 (0.50, 1.52) | ||
| May 2007 | Cape May, NJ | 71 | 36 | 43 | |||
| May 2007 | Cumberland, NJ | 34 | 19 | 25 | |||
| May 2008 | Cape May, NJ | 98 | 32 | 63 | |||
| May 2008 | Cumberland, NJ | 20 | 13 | 14 | |||
| Red Knot (Calidris canutus) | 151 | 45 (29.8) | 81 (53.6) | 0.24 (0.05, 0.47) | 0.82 (0.50, 1.53) | ||
| May 2007 | Cape May, NJ | 20 | 9 | 12 | |||
| May 2007 | Cumberland, NJ | 27 | 8 | 13 | |||
| May 2008 | Cape May, NJ | 65 | 20 | 39 | |||
| May 2008 | Cumberland, NJ | 35 | 7 | 16 | |||
| May 2008 | Sussex, DE | 4 | 1 | 1 | |||
| Sanderling (Calidris alba) | 128 | 2 (1.6) | 4 (3.1) | 0.30 (0.11, 0.49) | 1.20 (0.57, 1.77) | ||
| May 2007 | Cape May, NJ | 15 | 2 | 1 | |||
| May 2007 | Cumberland, NJ | 14 | 0 | 0 | |||
| May–June 2008 | Cape May, NJ | 99 | 0 | 3 | |||
| Dunlin (Calidris alpine) | 17 | 0 (0.0) | 6 (35.3) | 0.35 (0.20, 0.49) | 0.76 (0.54, 1.02) | ||
| May 2007 | Cape May, NJ | 2 | 0 | 1 | |||
| May 2008 | Cape May, NJ | 3 | 0 | 1 | |||
| May 2008 | Cumberland, NJ | 12 | 4 | ||||
| Short-billed Dowitcher (Limnodromus giseus) | 20 | 0 (0.0) | 1 (5.0) | 0.21 | 0.75 (0.52, 0.87) | ||
| May 2007 | Cape May, NJ | 1 | 0 | 0 | |||
| May 2008 | Cape May, NJ | 4 | 0 | 0 | |||
| May 2008 | Cumberland, NJ | 15 | 0 | 1 | |||
| Laughing Gull (Leucophaeus atricilla) | 217 | 54 (24.9) | 91 (41.9) | 0.26 (0.05, 0.49) | 0.80 (0.50, 1.83) | ||
| June–August 2005 | Chatham, GA | 85 | 8 | 19 | |||
| May 2008 | Cape May, NJ | 124 | 46 | 70 | |||
| September 2008 | Cape May, NJ | 8 | 0 | 2 | |||
| Franklin’s Gull (Leucophaeus pipixcan) | May 2008 | Marshall, MN | 11 | 2 (18.2) | 9 (81.8) | 0.35 (0.14, 0.49) | 0.65 (0.56, 0.74) |
| Ring-billed Gull (Larus delarensis) | May 2008 | Cumberland, NJ | 3 | 1 (33.3) | 3 (100.0) | 0.28 (0.16, 0.47) | NA |
| Herring Gull (Larus argentatus) | 7 | 1 (14.3) | 3 (42.9) | 0.33 (0.21, 0.47) | 0.63 (0.50, 0.82) | ||
| May 2008 | Cape May, NJ | 3 | 1 | 2 | |||
| May 2008 | Cumberland, NJ | 4 | 0 | 1 | |||
| Great Black-backed Gull (Larus marinus) | May 2008 | Cape May, NJ | 2 | 1 (50.0) | 2 (100.0) | 0.24 (0.19, 0.28) | NA |
| Common Tern (Sterna hirundo) | June 2008 | Suffolk, NY | 155 | 0 (0.0) | 2 (1.3) | 0.30 (0.23, 0.36) | 0.96 (0.61, 1.64) |
| Common Murre (Uria aalge) | March–July 2006 | Solano, CA | 9 | 0 (0.0) | 2 (22.2) | 0.39 (0.36, 0.43) | 0.89 (0.58, 1.12) |
| Order Piciformes | |||||||
| Downy Woodpecker (Picoides pubescens) | January 2009 | Madison, GA | 1 | 0 (0.0) | 0 (0.0) | NA | 1.03 |
| Red-bellied Woodpecker (Melanerpes carolinus) | January 2009 | Madison, GA | 1 | 0 (0.0) | 0 (0.0) | NA | 0.94 |
| Order Columbiformes | |||||||
| Mourning Dove (Zenaida macroura) | 6 | 0 (0.0) | 0 (0.0) | NA | 0.93 (0.83, 0.99) | ||
| April 2006 | Clarke, GA | 2 | 0 | 0 | |||
| January 2009 | Oconee, GA | 3 | 0 | 0 | |||
| February 2009 | Oglethorpe, GA | 1 | 0 | 0 | |||
| Order Passeriformes | |||||||
| Eastern Phoebe (Sayornis phoebe) | 3 | 0 (0.0) | 0 (0.0) | NA | 1.01 (0.94, 1.09) | ||
| January 2009 | Madison, GA | 1 | 0 | 0 | |||
| January 2009 | Oconee, GA | 2 | 0 | 0 | |||
| American Crow (Corvus brachyrhyncos) | 48 | 0 (0.0) | 0 (0.0) | NA | 1.03 (0.63, 1.44) | ||
| May 2000 | Greene, GA | 2 | 0 | 0 | |||
| May 2000 | Oglethorpe, GA | 2 | 0 | 0 | |||
| May–October 2000 | Wilkes, GA | 5 | 0 | 0 | |||
| June 2000 | Ware, GA | 8 | 0 | 0 | |||
| July 2000 | Camden, GA | 5 | 0 | 0 | |||
| July 2000 | Chatham, GA | 3 | 0 | 0 | |||
| July 2000 | Jenkins, GA | 4 | 0 | 0 | |||
| October 2000 | Habersham, GA | 4 | 0 | 0 | |||
| October–November 2000 | Madison, GA | 6 | 0 | 0 | |||
| December 2000 | Chatham, GA | 2 | 0 | 0 | |||
| December 2000 | Glynn, GA | 2 | 0 | 0 | |||
| December 2000 | Wayne, GA | 1 | 0 | 0 | |||
| March 2004 | Oconee, GA | 4 | 0 | 0 | |||
| Blue Jay (Cyanocitta cristata) | January 2009 | Madison, GA | 1 | 0 (0.0) | 0 (0.0) | NA | 1.07 |
| Eastern Tufted Titmouse (Baeolophus bicolor) | 17 | 0 (0.0) | 0 (0.0) | NA | 1.18 (1.01, 1.58) | ||
| January 2009 | Madison, GA | 6 | 0 | 0 | |||
| January–March 2009 | Oconee, GA | 4 | 0 | 0 | |||
| January–February 2009 | Clarke, GA | 7 | 0 | 0 | |||
| Carolina Wren (Thryothorus ludovicianus) | 4 | 0 (0.0) | 0 (0.0) | NA | 1.14 (0.99, 1.25) | ||
| April 2006 | Clarke, GA | 1 | 0 | 0 | |||
| January 2009 | Oconee, GA | 1 | 0 | 0 | |||
| January–February 2009 | Clarke, GA | 2 | 0 | 0 | |||
| Eastern Bluebird (Sialis sialis) | 3 | 0 (0.0) | 0 (0.0) | NA | 1.32 (1.20, 1.52) | ||
| January 2009 | Madison, GA | 1 | 0 | 0 | |||
| January 2009 | Oconee, GA | 2 | 0 | 0 | |||
| Swainson’s Thrush (Catharus ustulatus) | April 2008 | Clarke, GA | 1 | 0 (0.0) | 0 (0.0) | NA | 0.83 |
| American Robin (Turdus migratorius) | February 2004 | Clarke, GA | 3 | 0 (0.0) | 0 (0.0) | NA | 1.22 (1.02, 1.42) |
| Gray Catbird (Dumetella carolinensis) | April 2008 | Clarke, GA | 1 | 0 (0.0) | 0 (0.0) | NA | 0.93 |
| Northern Mockingbird (Mimus polyglottus) | January 2009 | Madison, GA | 1 | 0 (0.0) | 0 (0.0) | NA | 1.64 |
| Brown Thrasher (Toxostoma rufum) | January 2009 | Madison, GA | 1 | 0 (0.0) | 0 (0.0) | NA | 1.34 |
| Brown-headed Cowbird (Molothrus alter) | 8 | 0 (0.0) | 0 (0.0) | NA | 1.02 (0.89, 1.18) | ||
| March 1999 | Clarke, GA | 1 | 0 | 0 | |||
| March 1999 | Madison, GA | 1 | 0 | 0 | |||
| June 2006 | Clarke, GA | 6 | 0 | 0 | |||
| Purple Finch (Carpodacus purpureus) | 9 | 0 (0.0) | 0 (0.0) | NA | 1.00 (0.95, 1.05) | ||
| January 2009 | Madison, GA | 4 | 0 | 0 | |||
| February 2009 | Oglethorpe, GA | 5 | 0 | 0 | |||
| House Finch (Carpodacus mexicanus) | 7 | 0 (0.0) | 0 (0.0) | NA | 1.01 (0.86, 1.20) | ||
| January 2009 | Clarke, GA | 3 | 0 | 0 | |||
| January 2009 | Madison, GA | 1 | 0 | 0 | |||
| February–March 2009 | Oglethorpe, GA | 3 | 0 | 0 | |||
| House Sparrow (Passer domesticus) | 5 | 0 (0.0) | 0 (0.0) | NA | 1.17 (1.13, 1.21) | ||
| January 2009 | Madison, GA | 4 | 0 | 0 | |||
| February 2009 | Oconee, GA | 1 | 0 | 0 | |||
Sampling location in Alaska provided as borough, state.
CA = California; MN = Minnesota; GA = Georgia; AK = Alaska; NC = North Carolina; MI = Michigan; NJ = New Jersey; MS = Mississippi; TX = Texas; VA = Virginia; DE = Delaware; NY = New York.
NA = not applicable.
Serologic assay
Serum samples from 62 species (n = 2,249) were tested with the AGID and a commercial bELISA (FlockCheck AI MultiS-Screen Antibody Test Kit, IDEXX Laboratories, Westbrook, Maine, USA). The AGID testing was performed following standard protocols (Swayne et al., 2008) and using reagents supplied by the National Veterinary Services Laboratories (US Department of Agriculture, Animal and Plant Health Inspection Service, Ames, Iowa, USA). Results were observed at 24 hr and again within 48 hr, if necessary, for verification. Samples were determined to be negative, weak positive, or positive for the AGID test. Both weak positives and positives were classified as “positive,” except for the purpose of comparison with bELISA serum-sample-to-negative-control (S/N) absorbance values, as described below in the data analysis section.
The bELISA was performed according to the manufacturer’s instructions, with the following exception. Due to expense and time associated with testing large numbers of serum samples, most samples were tested with the bELISA using a single well rather than duplicate wells as recommended by the IDEXX protocol. To assess the repeatability of the bELISA, we performed duplicate testing on a subset of 225 serum samples, which included five samples from Common Terns (Sterna hirundo), one from a Great Black-backed Gull (Larus marinus), four from Franklin’s Gulls (Leucophaeus pipixcan), 47 from Laughing Gulls (Leucophaeus atricilla), 72 from Ruddy Turnstones (Arenaria interpres), 19 from Sanderlings (Calidris alba), 18 from American Green-winged Teals (Anas crecca), 49 from Mallards (Anas platyrhynchos), and 10 from Northern Pintails (Anas acuta). Absorbance values for the bELISA were read with a Benchmark Microplate Reader (Bio-Rad Laboratories, Hercules, California, USA) at 650 nm, and S/N values were calculated for each sample. Samples with S/N values ≥0.50 were considered negative for antibodies to AI virus, and samples with S/N values <0.50 were considered positive.
Data analysis
The percent agreement and kappa statistic (κ) with 95% confidence intervals (CIs) were calculated to estimate agreement between the bELISA and AGID test. McNemars χ2 test was used to determine whether there was a significant difference between proportions of positive results yielded by the two assays. The three semiquantitative AGID test result categories (negative, weak positive, or positive) were compared with bELISA S/N absorbance values using a one-way analysis of variance. The 225 serum samples tested in duplicate were analyzed by calculating percent agreement and κ, and by determining the mean coefficient of variation between individual tests. A plot of the within-pair differences versus the within-pair means was also constructed to further evaluate repeatability of duplicate bELISA testing (Bland and Altman, 1986). All analyses were performed using commercially available statistical software (Stata 10.1; Stata Corporation, College Station, Texas, USA).
RESULTS
The serologic test results for all species are summarized in Table 1. Of the 2,249 samples tested with both assays, the proportion of positive results was significantly higher for the bELISA than for the AGID (25.4% vs. 14.8%; McNemar’s χ2, P<0.001). At a species level, the bELISA detected as many or more positive samples than the AGID in all 62 avian species. The majority of antibody-positive serum samples, as detected by both assays, were from species in the orders Anseriformes, Charadriiformes, and Gruiformes. Within these taxonomic orders, the bELISA detected antibodies to AI virus in 39.7% (209/526), 37.0% (349/943), and 34% (11/32) of serum samples, respectively, whereas the AGID only detected 22.6% (119/526), 21.8% (206/943), and 22% (7/32). Nearly all species associated with terrestrial habitats were negative for antibodies to AI virus by both assays, including Black Vultures (Coragyps atratus), woodpeckers (Picidae), Mourning Doves (Zenaida macroura), and passerines. Two of the 299 (0.7%) samples from Peregrine Falcons (Falco peregrinus) were positive via the bELISA.
The results of the bELISA for birds with different AGID test result classifications are summarized in Table 2. Birds with a positive AGID test result had significantly lower S/N values than birds that were classified as having either a weak positive or negative test result. Samples with a weak positive test result had significantly lower S/N values than birds with negative test results.
Table 2.
Summary of blocking enzyme-linked immunosorbent assay serum-sample-to-negative-control (S/N) absorbance values by agar gel immunodiffusion (AGID) test result category, with sample sizes (n) and standard errors (SE) for paired testing of serum for avian influenza virus antibodies in 2,249 wild birds.
| AGID test result | n | Mean S/N ratioa | SE |
|---|---|---|---|
|
| |||
| Negative | 1,917 | 0.82(a) | 0.01 |
| Weak positive | 193 | 0.27(b) | 0.02 |
| Positive | 139 | 0.18(c) | 0.02 |
Means with different letters in parentheses were significantly different at a level of significance of 5% over all comparisons when using the Bonferroni multiple comparison procedure.
The overall agreement between the AGID test and bELISA on the 2,249 samples was 87.1%, with a κ value of 0.604 (95% CI=0.565, 0.643). Based on the Landis and Koch interpretation of κ, this value would fall between the ranges of moderate and substantial agreement (Landis and Koch, 1977). Test agreement varied for individual species and is summarized in Table 3 for a subset of species with samples sizes ≥50 and with five or more positive samples.
Table 3.
Agreement between the agar gel immunodiffusion (AGID) test and a commercial blocking enzyme-linked immunosorbent assay (bELISA) for detection of antibodies to avian influenza virus in species with 50 or more birds sampled and five or more positive results on either test.
| No. positive (%) |
||||||
|---|---|---|---|---|---|---|
| Species | No. birds | bELISA | AGID | p a | % agreement | κ (95% CI) |
|
| ||||||
| American Green-winged Teal | 69 | 33 (47.8) | 20 (29.0) | 0.001 | 78.3 | 0.56 (0.34, 0.78) |
| Canada Goose | 106 | 25 (23.6) | 9 (8.5) | 0.002 | 77.4 | 0.19 (0.03, 0.35) |
| Laughing Gull | 217 | 91 (41.9) | 54 (24.9) | <0.001 | 77.4 | 0.51 (0.39, 0.63) |
| Mallard | 176 | 81 (46.0) | 57 (32.4) | <0.001 | 78.4 | 0.56 (0.41, 0.70) |
| Red Knot | 151 | 81 (53.6) | 45 (29.8) | <0.001 | 73.5 | 0.49 (0.34, 0.63) |
| Ruddy Turnstone | 223 | 145 (65.0) | 100 (44.8) | <0.001 | 76.2 | 0.54 (0.42, 0.66) |
| Tundra Swan | 60 | 16 (26.7) | 0 (0.0) | <0.001 | 73.3 | NCb |
Exact McNemar’s test comparing the proportions of positive test results.
Not calculated because the number of positive AGID results was zero.
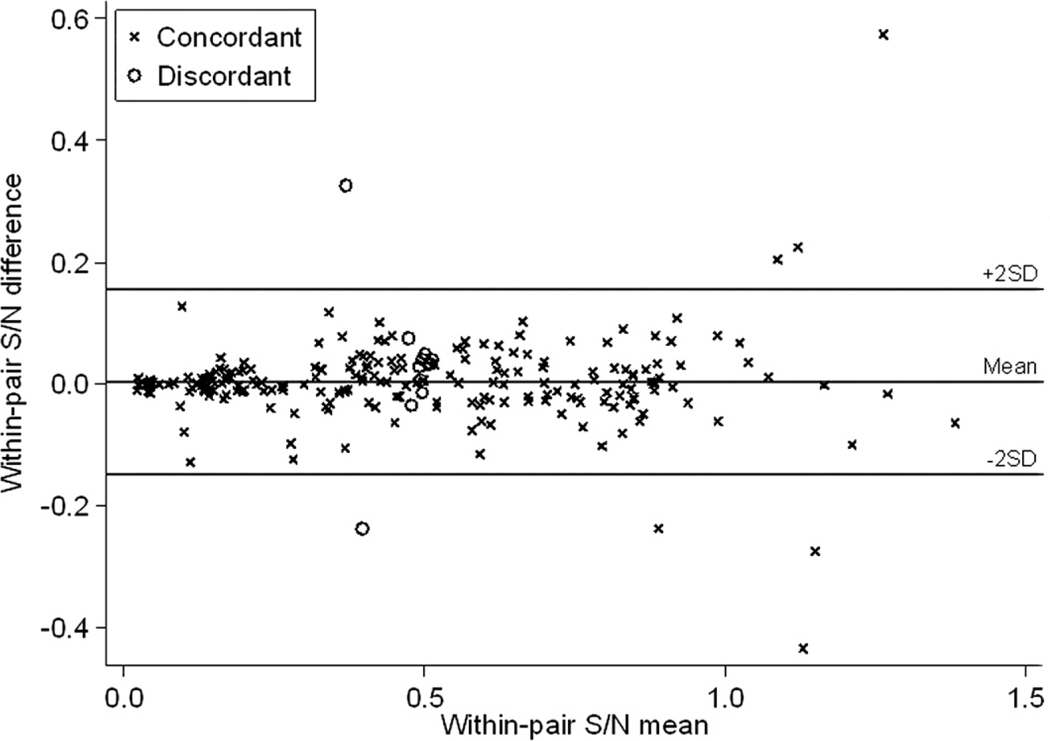
Results of duplicate bELISA testing on paired serum samples from 225 birds are depicted in Figure 1. The mean coefficient of variation for the duplicate testing was 7.3%, and the total percent agreement between duplicates was 94.7% (κ=0.89). Of the 225 samples for which duplicate testing was performed, 12 (5.3%) had an S/N value <0.50 on one test and ≥0.50 on the other test. Among these 12 samples, nine (75%) had S/N values between 0.45 and 0.55 on both tests, illustrating that most of the samples with discordant results were those having S/N values close to the manufacturer’s recommended diagnostic cutoff of 0.50.
Figure 1.
Bland-Altman plot for duplicate blocking enzyme-linked immunosorbent assay testing performed on serum samples collected from 225 wild birds (nine species). Samples having a within-pair serum-sample-to-negative-control (S/N) absorbance value mean <0.50 were considered positive for antibodies to avian influenza virus. Samples marked with an “x” had concordant results on both tests, whereas those marked with an open circle had an S/N value <0.50 on one test and ≥0.50 on the other.
DISCUSSION
In a previous study using samples from multiple bird species experimentally infected with AI viruses, the IDEXX bELISA was significantly more sensitive than the AGID test (Brown et al., 2009). Based on these experimental samples, the diagnostic sensitivity and specificity of the bELISA was 82% (95% CI=75.6, 87.4) and 100% (95% CI=96.5, 100.0), respectively. Similar findings were reported by Spackman et al. (2009), who determined the IDEXX bELISA to be a more sensitive, type-specific serologic assay than the AGID test in domestic ducks experimentally infected with a low pathogenic AI virus. The results of this current study correspond with these experimental data and provide further support for the IDEXX bELISA as a useful species-independent serologic assay. The bELISA is significantly more sensitive than the AGID test, but based on the overall κ of 0.604 (95% CI=0.565, 0.643) and the detection of positive samples in most avian groups, the AGID test could be used as an alternative type-specific serologic assay in wild birds. The poor sensitivity of the AGID test in waterfowl has been reported previously (Slemons and Easterday, 1972) and is presumably due to deficiencies of duck immunoglobulins in properties requiring multivalency, including precipitation or agglutination (Higgins, 1998).
Because the previous infection status of birds contributing these field samples was unknown, diagnostic sensitivity or specificity was not calculated. On the level of taxonomic order, however, the serology results from this study are consistent with the existing knowledge of AI epidemiology and host range in wild birds. Species with detectable antibodies in this study were associated with aquatic habitats and generally represent avian taxonomic groups and species that have consistently tested positive by virus isolation or RT-PCR (Stallknecht and Shane, 1988; Krauss et al., 2004; Olsen et al., 2006; Munster et al., 2007; Hanson et al., 2008). The prevalence of antibodies to AI virus were highest in species in the orders Anseriformes and Charadriiformes and low or negative in terrestrial species in the orders Passeriformes (0/234 [0%]), Falconiformes (2/483 [0.4%]), and Columbiformes (0/6 [0%]). Although AI virus isolations have been reported previously from Passeriformes, Falconiformes, and Columbiformes species, the prevalence of infection is very low and they are generally not considered reservoirs for these viruses (Stallknecht et al., 2007). The correspondence between serologic results in this study and previously published virus detection data (virus isolation and RT-PCR) is apparent at the species level within Anseriformes and Charadriiformes. Within the Anseriformes, the highest antibody prevalence was in dabbling ducks of the genus Anas, which is consistent with reports based on virus detection (Olsen et al., 2006). Of the five dabbling duck species included in this study, antibody prevalence was highest in Mallards (Anas platyrhynchos), which are generally considered the most globally important AI reservoir species (Fouchier et al., 2007; Stallknecht et al., 2007).
Currently, there seems to be two groups of birds, Laridae (gulls and terns) and Scolopacidae (shorebirds), within the order Charadriiformes that are important in the epidemiology of AI viruses. The shorebird cycle is characterized by an annual epidemic in Ruddy Turnstones that occurs at Delaware Bay, USA, during May (Stallknecht and Brown, 2007; Hanson et al., 2008). At this time and location, prevalence of infection based on virus isolation has ranged from 8% to 15% in Ruddy Turnstones, but prevalence in all other comingling shorebirds, including Dunlins (Calidris alpine), Red Knots (Calidris canutus), Short-billed Dowitchers (Limnodromus giseus), and Sanderlings has generally been very low (<1%; Hanson et al., 2008). These differences in local infection rates, however, are not consistently reflected in the serologic data for all species. For example, although the reported isolation rates for Dunlins and Red Knots were consistently low at Delaware Bay (<1%), the prevalence of antibodies to AI in these species in the current study were 36% and 54%, respectively. These results indicate that Dunlins and Red Knots are infected with AI viruses at some point and that the inability to isolate these viruses from these species at Delaware Bay may reflect population immunity related to previous infections at another time or location. In situations such as these, the combination of serologic and virologic data helps define and justify additional study of a potentially complicated multihost epidemiology of AI viruses within shorebird populations.
In gulls and terns, antibody prevalences based on the bELISA results were 45.0% and 1.3%, respectively. Most isolations of AI virus from species in the family Laridae have been associated with gulls rather than terns (D. E. Stallknecht, personal communication). The high antibody prevalence in gulls is consistent with the accepted notion that these species represent an important reservoir of AI virus (Stallknecht and Brown, 2007). A diversity of HA subtypes have been isolated from gull species and these birds are considered as the reservoirs for the H13 and H16 subtypes (Munster et al., 2007). Antibodies to AI virus were detected at a moderate (40–45%) to high prevalence (>80%) with the bELISA in all five gull species in this study.
Two major applications of serologic testing for AI virus surveillance in wild birds are to 1) provide a cost-efficient and technically simple means to efficiently screen a wild avian population for previous AI virus infection; and 2) provide an additional perspective to interpret and support epidemiologic information derived from virus detection-based data. In this study, known AI virus reservoir species were clearly identified by both the AGID test and the bELISA. Antibodies were not detected in terrestrial species in the orders Passeriformes, Columbiformes, or Piciformes, nor in aquatic bird species in the orders Gaviiformes, Ciconiiformes, Procellariiformes, or Pelecaniformes. Although the sample sizes were low for many antibody-negative species, the results are consistent with historic low prevalence estimates of AI virus in these birds (Olsen et al., 2006). With regard to using serology to search for potential avian reservoirs, the bELISA is a tool that can easily be applied to difficult field and laboratory conditions associated with both sampling and testing. One requirement of the bELISA that may be prohibitive is the need for an ELISA plate reader. In areas where this equipment is not available, the AGID test remains a viable screening option to detect antibodies to AI virus in wild birds. Although it is significantly less sensitive, the AGID has good specificity and can produce useful data with many of the same applications for wild bird surveillance as described for the bELISA.
The second application or benefit of serology for wild bird AI surveillance relates to maximizing epidemiologic data to better understand these complex systems. We have previously discussed this utility in relation to the shorebird results. Similar discrepancies between the antibody prevalence detected in the present study and published reports of AI infection also were observed with Canada Geese (Branta canadensis) and American Coots (Fulica americana) and may relate to similar unknowns associated with sampling time and location. For Canada Geese, such differences could also reflect limited viral shedding during infection; experimental studies suggest that in Canada Geese infected with AI virus, the duration of viral shedding is brief and at a very low titer (Winkler et al., 1972; Pasick et al., 2007). For serologic testing to have a role in global wild bird surveillance efforts, at least three basic requirements are necessary: there must be a validated assay, the assay must be widely available, and the serologic data must contribute some level of understanding beyond existing diagnostic surveillance strategies. Our evaluation of the AGID test and bELISA on sera from experimentally infected birds (Brown et al. 2009) and the field samples tested in the present study yielded a good correspondence with existing data on AI virus infection in wild birds. Collectively, these data suggest the examined commercial bELISA performs well as a species-independent assay for detection of antibodies to AI virus. Although this tool has obvious applications for understanding the epidemiology of AI viruses in diverse wild bird populations, further evaluation of this approach is warranted, especially related to examining the performance of other bELISA kits, test validation in additional wild avian species, determining the duration of detectable antibodies in wild birds, and the development and testing of subtype-specific species-independent assays.
ACKNOWLEDGMENTS
We thank T. Costa, C. Lebercheron, A. Maxted, S. McGraw, J. Parrish, J. Slusher, J. TeSlaa, S. Nashold, J. Siembieda, J. Burco, and H. Wilson for assistance in collecting and testing serum samples. Funding for this work was provided by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), Department of Health and Human Services, under contract HHSN266200700007C and Agricultural Research Service Specific Cooperative Agreement 58–6612-2–0220 between the Southeast Poultry Research Laboratory and the Southeastern Cooperative Wildlife Disease Study. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. This work was additionally supported by IDEXX, which generously provided the bELISA test kits.
LITERATURE CITED
- BLAND JM, AND ALTMAN DG. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1: 307–310. [PubMed] [Google Scholar]
- BROWN JD, STALLKNECHT DE, BERGHAUS RD, LUTTRELL MP, VELEK K, KISTLER W, COSTA T, YABSLEY MJ, AND SWAYNE DE. 2009. Evaluation of a commercial blocking enzyme-linked immunosorbent assay to detect avian influenza virus antibodies in multiple experimentally infected avian species. Clinical and Vaccine Immunology 16: 824–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATTOLI G, AND CAPUA I. 2007. Diagnosing avian influenza in the framework of wildlife surveillance efforts and environmental samples. Journal of Wildlife Diseases 43 Supplemental: S35–S39. [Google Scholar]
- FOUCHIER RAM, MUNSTER VJ, KEAWCHAROEN J, OSTERHAUS ADME, AND KUIKEN T. 2007. Virology of avian influenza in relation to wild birds. Journal of Wildlife Diseases 43 Supplemental: S7–S14. [Google Scholar]
- HANSON BA, LUTTRELL MP, GOEKJIAN VH, NILES L, SWAYNE DE, SENNE DA, AND STALLKNECHT DE. 2008. Is the occurrence of avian influenza virus in Charadriiformes species and location dependent? Journal of Wildlife Diseases 44: 351–361. [DOI] [PubMed] [Google Scholar]
- HIGGINS DA. 1998. Comparative immunology of avian species. In Poultry immunology. 24th Edition, Davison TF, Morris TR and Payne LN (eds.). Carfax Publishing Company, Abingdon, UK, pp. 149–205. [Google Scholar]
- KRAUSS S, WALKER D, PAUL PRYOR S, NILES L, CHENGHONG L, HINSHAW VS, AND WEBSTER RG. 2004. Influenza A viruses in migrating wild aquatic birds in North America. Vector-Borne and Zoonotic Diseases 4: 177–189. [DOI] [PubMed] [Google Scholar]
- LANDIS JR, AND KOCH GG. 1977. The measurement of observer agreement for categorical data. Biometrics 33: 159–174. [PubMed] [Google Scholar]
- MUNSTER VJ, BAAS C, LEXMOND P, WALDENSTROM J, WALLENSTEN A, FRANSSON T, RIMMELZWAAN GF, BEYER WE, SCHUTTEN M, OLSEN B, OSTERHAUS AD, AND FOUCHIER RA. 2007. Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathogen 3 (5): e61, doi: 10.1371/journal.ppat.0030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLSEN B, MUNSTER VJ, WALLENSTEN A, WALDENSTROM J, OSTERHAUS ADME, AND FOUCHIER RAM. 2006. Global patterns of influenza A virus in wild birds. Science 312: 384–388. [DOI] [PubMed] [Google Scholar]
- PASICK J, BERHANE Y, EMBURY-HYATT C, COPPS J, KEHLER H, HANDEL K, BABIUK S, HOOPER-MCGREVY K, LI Y, MAY LE Q, AND PHUONG SL. 2007. Susceptibility of Canada Geese (Branta canadensis) to highly pathogenic avian influenza virus (H5N1). Emerging Infectious Diseases 13: 1821–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLEMONS RD, AND EASTERDAY BC. 1972. Host response differences among 5 avian species to an influenza virus-A/turkey/Ontario/7732/66 (Hav5N?). Bulletin of the World Health Organization 47: 521–525. [PMC free article] [PubMed] [Google Scholar]
- SNYDER DB, MARQUARDT WW, YANCEY FS, AND SAVAGE PK. 1985. An enzyme-linked immunosorbent assay for the detection of antibody against avian influenza virus. Avian Diseases 29: 136–144. [PubMed] [Google Scholar]
- SPACKMAN E, SUAREZ DL, AND SENNE DA. 2008. Avian influenza diagnostics and surveillance methods. In Avian influenza. Swayne DE (ed.). Blackwell Publishing, Ames, Iowa, pp. 299–308. [Google Scholar]
- SPACKMAN E, PANTIN-JACKWOOD MJ, SWAYNE DE, AND SUAREZ DL. 2009. An evaluation of avian influenza diagnostic methods with domestic duck specimens. Avian Diseases 53: 276–280. [DOI] [PubMed] [Google Scholar]
- STALLKNECHT DE, AND BROWN JD. 2007. Wild birds and the epidemiology of avian influenza. Journal of Wildlife Diseases 43 Supplement: S15–S20. [Google Scholar]
- STALLKNECHT DE, AND SHANE SM. 1988. Host range of avian influenza virus in free-living birds. Veterinary Research Communications 12: 125–141. [DOI] [PubMed] [Google Scholar]
- STALLKNECHT DE, NAGY E, HUNTER DB, AND SLEMONS RD. 2007. Avian Influenza. In Infectious diseases of wild birds. 1st Edition. Thomas NJ, Hunter DB, and Atkinson CT (eds.). Blackwell Publishing, Ames, Iowa, pp. 108–130. [Google Scholar]
- SWAYNE DE, SENNE DA, AND SUAREZ DL. 2008. Influenza. In A laboratory manual for the isolation and identification of avian pathogens. 5th Edition. Dufour-Zavala L, Swayne DE, Glisson JR, Pearson JE, Reed WM, Jackwood MW, and Woolcock PR (eds.). American Association of Avian Pathologists, Kennett Square, Pennsylvania, pp. 128–134. [Google Scholar]
- WEBSTER RG, BEAN WJ, GORMAN OT, CHAMBERS TM, AND KAWAOKA Y. 1992. Evolution and ecology of influenza A viruses. Microbiological Reviews 56: 152–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINKLER G, TRAINER DO, AND EASTERDAY BC. 1972. Influenza in Canada geese. Bulletin of the World Health Organization 47: 507–513. [PMC free article] [PubMed] [Google Scholar]