Abstract
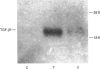
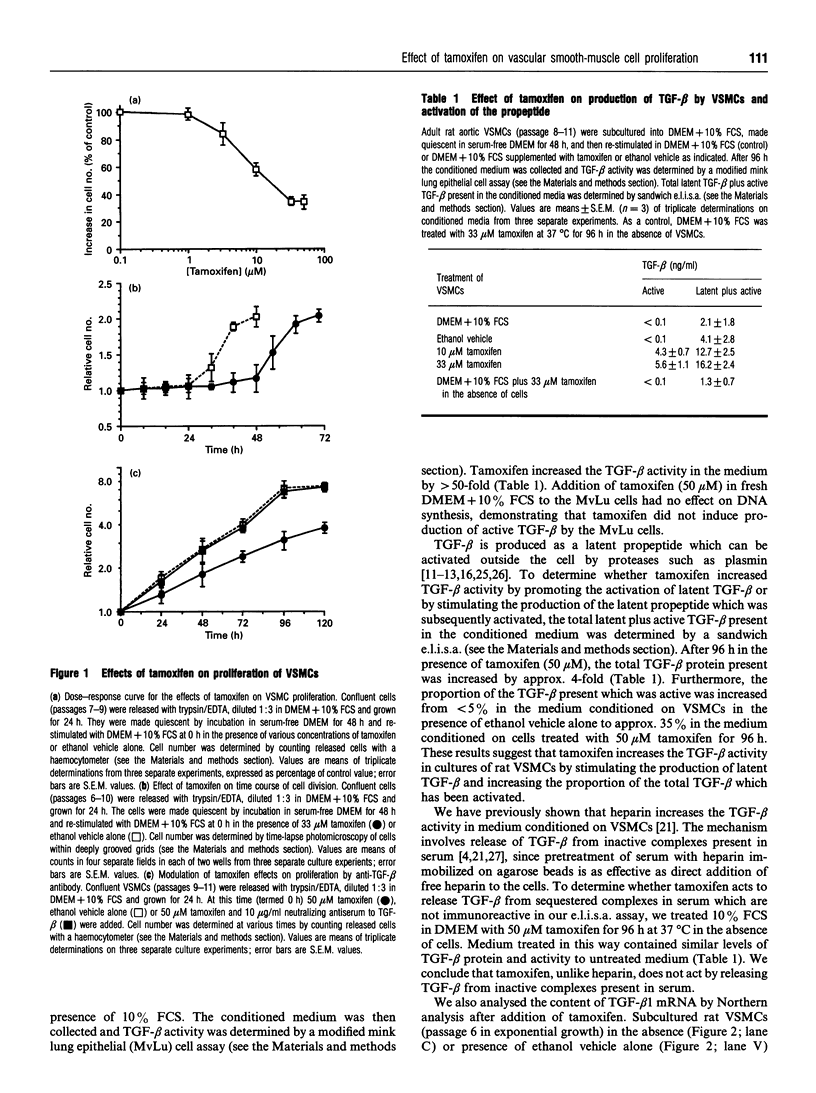
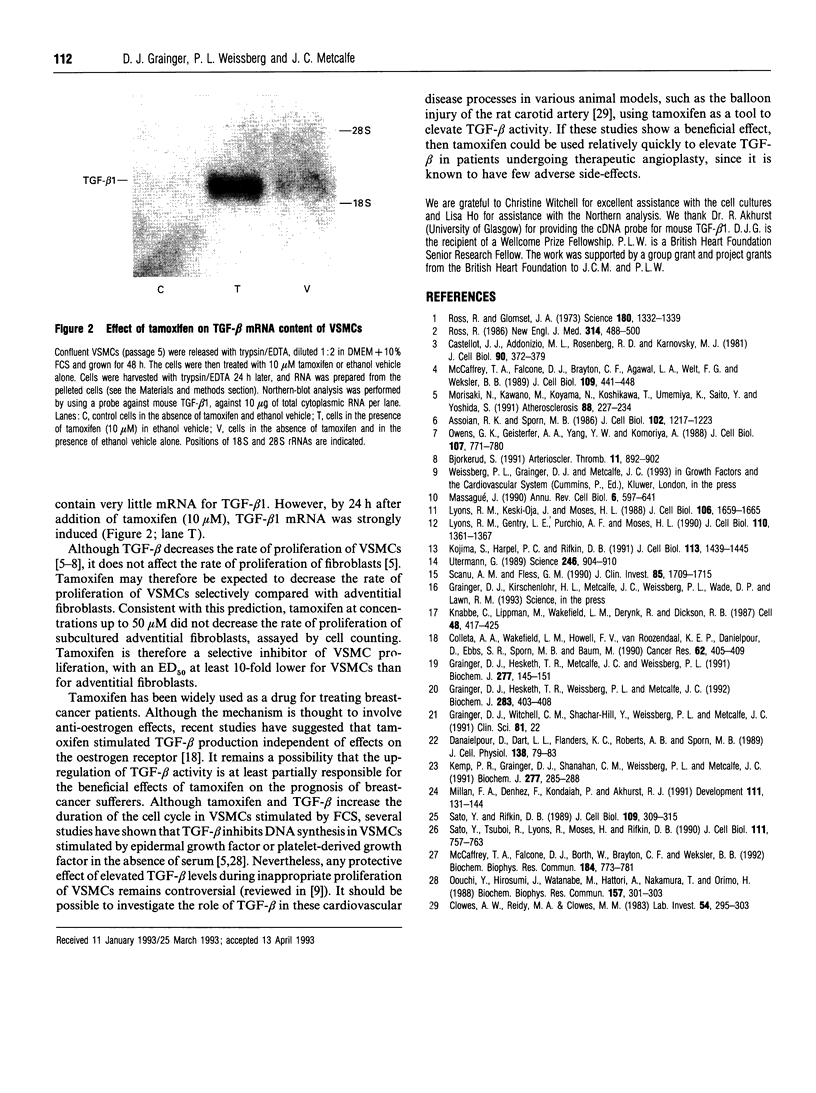
Tamoxifen selectively and reversibly decreased the rate of proliferation of adult rat aortic vascular smooth-muscle cells (VSMCs). Half-maximal inhibition of proliferation occurred at 2-5 microM tamoxifen for VSMCs and at > 50 microM for adventitial fibroblasts. The cell cycle time for all the VSMCs in the population was increased from 35 +/- 2 h to 54 +/- 4 h in the presence of 33 microM tamoxifen. Tamoxifen did not affect the time of entry into DNA synthesis, but delayed arrival at mitosis by > 24 h. It therefore extended the duration of the G2-to-M phase of the cell cycle. However, the rate of proliferation of VSMCs was not decreased by tamoxifen (at concentrations up to 50 microM) in the presence of neutralizing antibody to transforming growth factor beta (TGF-beta). The level of mRNA for TGF-beta 1 in VSMCs was strongly induced by 10 microM tamoxifen, and TGF-beta activity in conditioned medium from tamoxifen-treated cells was more than 50-fold higher than from control cells. Tamoxifen therefore extended the G2-to-M phase of the cell cycle in VSMCs by increasing TGF-beta activity in the culture.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Sporn M. B. Type beta transforming growth factor in human platelets: release during platelet degranulation and action on vascular smooth muscle cells. J Cell Biol. 1986 Apr;102(4):1217–1223. doi: 10.1083/jcb.102.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkerud S. Effects of transforming growth factor-beta 1 on human arterial smooth muscle cells in vitro. Arterioscler Thromb. 1991 Jul-Aug;11(4):892–902. [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M., Reidy M. A. Kinetics of cellular proliferation after arterial injury. III. Endothelial and smooth muscle growth in chronically denuded vessels. Lab Invest. 1986 Mar;54(3):295–303. [PubMed] [Google Scholar]
- Colletta A. A., Wakefield L. M., Howell F. V., van Roozendaal K. E., Danielpour D., Ebbs S. R., Sporn M. B., Baum M. Anti-oestrogens induce the secretion of active transforming growth factor beta from human fetal fibroblasts. Br J Cancer. 1990 Sep;62(3):405–409. doi: 10.1038/bjc.1990.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielpour D., Dart L. L., Flanders K. C., Roberts A. B., Sporn M. B. Immunodetection and quantitation of the two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) secreted by cells in culture. J Cell Physiol. 1989 Jan;138(1):79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
- Grainger D. J., Hesketh T. R., Metcalfe J. C., Weissberg P. L. A large accumulation of non-muscle myosin occurs at first entry into M phase in rat vascular smooth-muscle cells. Biochem J. 1991 Jul 1;277(Pt 1):145–151. doi: 10.1042/bj2770145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger D. J., Hesketh T. R., Weissberg P. L., Metcalfe J. C. Hexamethylenebisacetamide selectively inhibits the proliferation of human and rat vascular smooth-muscle cells. Biochem J. 1992 Apr 15;283(Pt 2):403–408. doi: 10.1042/bj2830403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp P. R., Grainger D. J., Shanahan C. M., Weissberg P. L., Metcalfe J. C. The Id gene is activated by serum but is not required for de-differentiation in rat vascular smooth muscle cells. Biochem J. 1991 Jul 1;277(Pt 1):285–288. doi: 10.1042/bj2770285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knabbe C., Lippman M. E., Wakefield L. M., Flanders K. C., Kasid A., Derynck R., Dickson R. B. Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987 Feb 13;48(3):417–428. doi: 10.1016/0092-8674(87)90193-0. [DOI] [PubMed] [Google Scholar]
- Kojima S., Harpel P. C., Rifkin D. B. Lipoprotein (a) inhibits the generation of transforming growth factor beta: an endogenous inhibitor of smooth muscle cell migration. J Cell Biol. 1991 Jun;113(6):1439–1445. doi: 10.1083/jcb.113.6.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons R. M., Keski-Oja J., Moses H. L. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988 May;106(5):1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- McCaffrey T. A., Falcone D. J., Borth W., Brayton C. F., Weksler B. B. Fucoidan is a non-anticoagulant inhibitor of intimal hyperplasia. Biochem Biophys Res Commun. 1992 Apr 30;184(2):773–781. doi: 10.1016/0006-291x(92)90657-7. [DOI] [PubMed] [Google Scholar]
- McCaffrey T. A., Falcone D. J., Brayton C. F., Agarwal L. A., Welt F. G., Weksler B. B. Transforming growth factor-beta activity is potentiated by heparin via dissociation of the transforming growth factor-beta/alpha 2-macroglobulin inactive complex. J Cell Biol. 1989 Jul;109(1):441–448. doi: 10.1083/jcb.109.1.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan F. A., Denhez F., Kondaiah P., Akhurst R. J. Embryonic gene expression patterns of TGF beta 1, beta 2 and beta 3 suggest different developmental functions in vivo. Development. 1991 Jan;111(1):131–143. doi: 10.1242/dev.111.1.131. [DOI] [PubMed] [Google Scholar]
- Morisaki N., Kawano M., Koyama N., Koshikawa T., Umemiya K., Saito Y., Yoshida S. Effects of transforming growth factor-beta 1 on growth of aortic smooth muscle cells. Influences of interaction with growth factors, cell state, cell phenotype, and cell cycle. Atherosclerosis. 1991 Jun;88(2-3):227–234. doi: 10.1016/0021-9150(91)90085-h. [DOI] [PubMed] [Google Scholar]
- Ouchi Y., Hirosumi J., Watanabe M., Hattori A., Nakamura T., Orimo H. Inhibitory effect of transforming growth factor-beta on epidermal growth factor-induced proliferation of cultured rat aortic smooth muscle cells. Biochem Biophys Res Commun. 1988 Nov 30;157(1):301–307. doi: 10.1016/s0006-291x(88)80047-0. [DOI] [PubMed] [Google Scholar]
- Owens G. K., Geisterfer A. A., Yang Y. W., Komoriya A. Transforming growth factor-beta-induced growth inhibition and cellular hypertrophy in cultured vascular smooth muscle cells. J Cell Biol. 1988 Aug;107(2):771–780. doi: 10.1083/jcb.107.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973 Jun 29;180(4093):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989 Jul;109(1):309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Tsuboi R., Lyons R., Moses H., Rifkin D. B. Characterization of the activation of latent TGF-beta by co-cultures of endothelial cells and pericytes or smooth muscle cells: a self-regulating system. J Cell Biol. 1990 Aug;111(2):757–763. doi: 10.1083/jcb.111.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanu A. M., Fless G. M. Lipoprotein (a). Heterogeneity and biological relevance. J Clin Invest. 1990 Jun;85(6):1709–1715. doi: 10.1172/JCI114625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utermann G. The mysteries of lipoprotein(a). Science. 1989 Nov 17;246(4932):904–910. doi: 10.1126/science.2530631. [DOI] [PubMed] [Google Scholar]