Abstract
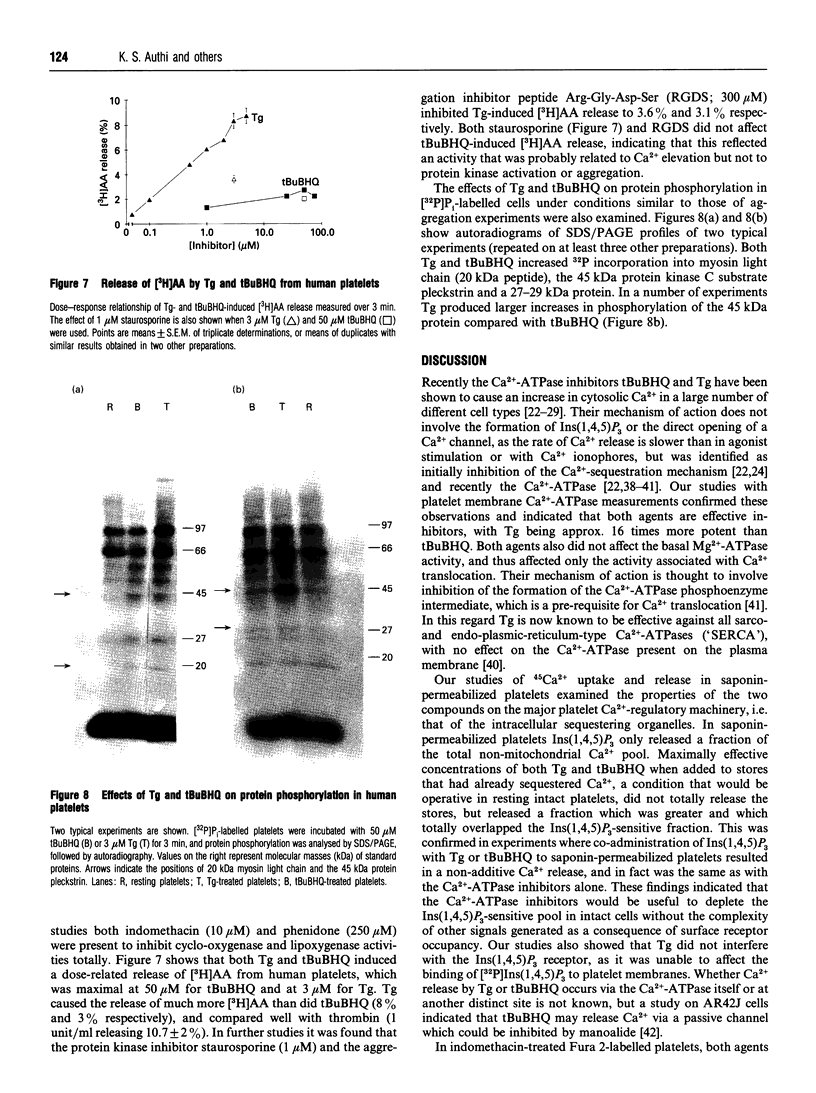
The effects of the Ca(2+)-ATPase inhibitors thapsigargin (Tg) and 2,5-di-(t-butyl)-1,4-benzohydroquinone (tBuBHQ) were examined by using Ca(2+)-regulatory systems of platelet mixed membranes, saponin-permeabilized and intact platelets. Both agents inhibit Ca(2+)-ATPase activities of platelet mixed membranes, without any effect on the basal Mg(2+)-ATPase activity. Tg is more effective (EC50 = 35 nM) than tBuBHQ (EC50 = 580 nM). The effect of the two inhibitors on 45Ca2+ release from saponin-permeabilized platelets has also been characterized. 45Ca2+ uptake into non-mitochondrial intracellular stores occurs via an ATP-dependent mechanism, and if added at equilibrium the second messenger Ins(1,4,5)P3 releases 50% of the accumulated 45Ca2+. Maximally effective concentrations of Tg (1 microM) and tBuBHQ (50 microM) release 77% and 68% of the accumulated 45Ca2+. Addition of Ins(1,4,5)P3 together with either Tg or tBuBHQ resulted in a non-additive release which was the same as with either Tg or tBuBHQ alone, indicating that the Ins(1,4,5)P3-sensitive Ca2+ pool was a subset of the pool that is sensitive to the Ca(2+)-ATPase inhibitors. Release of 45Ca2+ by either Tg or tBuBHQ was not affected by heparin, which totally blocked Ins(1,4,5)P3-induced Ca2+ release, and Tg was found not to affect [32P]Ins(1,4,5)P3 binding to its receptor on mixed membranes. Thus both Tg and tBuBHQ release Ca2+ from a pool that totally overlaps the Ins(1,4,5)P3-sensitive pool without affecting Ins(1,4,5)P3 function. In intact indomethacin-treated Fura 2-loaded platelets, Tg and tBuBHQ cause Ca2+ elevation, arising from release from intracellular stores and influx from the outside. Both Tg and tBuBHQ elevated Ca2+ to similar levels, which were less and slower than those observed with thrombin. Addition of thrombin to cells already treated with Tg or tBuBHQ produced further elevation of Ca2+, indicating agonist utilization of a Ca(2+)-ATPase inhibitor-insensitive pool. In aggregation experiments Tg and tBuBHQ showed different functional effects. In indomethacin-treated cells Tg induces slow aggregation and secretion responses, whereas tBuBHQ only induces shape change. Both agents show synergistic secretory responses with the protein kinase C activator dioctanoylglycerol (DiC8). Tg also showed greater ability than tBuBHQ to release [3H]arachidonic acid (AA) from [3H]AA-labelled platelets. Additionally, in [32P]Pi-labelled platelets both Tg and tBuBHQ induced phosphorylation of myosin light chain, a 27 kDa protein and the 45 kDa protein pleckstrin, but Tg showed a greater ability than tBuBHQ to cause phosphorylation of pleckstrin. These studies indicate that Tg and tBuBHQ are effective in releasing the Ins(1,4,5)P3-sensitive Ca2+ pool in platelets.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adunyah S. E., Dean W. L. Inositol triphosphate-induced Ca2+ release from human platelet membranes. Biochem Biophys Res Commun. 1985 May 16;128(3):1274–1280. doi: 10.1016/0006-291x(85)91078-2. [DOI] [PubMed] [Google Scholar]
- Authi K. S., Crawford N. Inositol 1,4,5-trisphosphate-induced release of sequestered Ca2+ from highly purified human platelet intracellular membranes. Biochem J. 1985 Aug 15;230(1):247–253. doi: 10.1042/bj2300247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Authi K. S., Evenden B. J., Crawford N. Metabolic and functional consequences of introducing inositol 1,4,5-trisphosphate into saponin-permeabilized human platelets. Biochem J. 1986 Feb 1;233(3):707–718. doi: 10.1042/bj2330707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Authi K. S., Lagarde M., Crawford N. Diacylglycerol lipase activity in human platelet intracellular and surface membranes. Some kinetic properties and fatty acid specificity. FEBS Lett. 1985 Jan 21;180(1):95–101. doi: 10.1016/0014-5793(85)80239-8. [DOI] [PubMed] [Google Scholar]
- Authi K. S. Localisation of the [32P]IP3 binding site on human platelet intracellular membranes isolated by high-voltage free-flow electrophoresis. FEBS Lett. 1992 Feb 24;298(2-3):173–176. doi: 10.1016/0014-5793(92)80049-m. [DOI] [PubMed] [Google Scholar]
- Authi K. S., Rao G. H., Evenden B. J., Crawford N. Action of guanosine 5'-[beta-thio]diphosphate on thrombin-induced activation and Ca2+ mobilization in saponin-permeabilized and intact human platelets. Biochem J. 1988 Nov 1;255(3):885–893. doi: 10.1042/bj2550885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Cytoplasmic calcium oscillations: a two pool model. Cell Calcium. 1991 Feb-Mar;12(2-3):63–72. doi: 10.1016/0143-4160(91)90009-4. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Brüne B., Ullrich V. Calcium mobilization in human platelets by receptor agonists and calcium-ATPase inhibitors. FEBS Lett. 1991 Jun 17;284(1):1–4. doi: 10.1016/0014-5793(91)80747-q. [DOI] [PubMed] [Google Scholar]
- Brüne B., Ullrich V. Different calcium pools in human platelets and their role in thromboxane A2 formation. J Biol Chem. 1991 Oct 15;266(29):19232–19237. [PubMed] [Google Scholar]
- Burgoyne R. D., Cheek T. R., Morgan A., O'Sullivan A. J., Moreton R. B., Berridge M. J., Mata A. M., Colyer J., Lee A. G., East J. M. Distribution of two distinct Ca2+-ATPase-like proteins and their relationships to the agonist-sensitive calcium store in adrenal chromaffin cells. Nature. 1989 Nov 2;342(6245):72–74. doi: 10.1038/342072a0. [DOI] [PubMed] [Google Scholar]
- Carey F., Menashi S., Crawford N. Localization of cyclo-oxygenase and thromboxane synthetase in human platelet intracellular membranes. Biochem J. 1982 Jun 15;204(3):847–851. doi: 10.1042/bj2040847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain B. K., Volpe P., Fleischer S. Inhibition of calcium-induced calcium release from purified cardiac sarcoplasmic reticulum vesicles. J Biol Chem. 1984 Jun 25;259(12):7547–7553. [PubMed] [Google Scholar]
- Cheek T. R., Barry V. A., Berridge M. J., Missiaen L. Bovine adrenal chromaffin cells contain an inositol 1,4,5-trisphosphate-insensitive but caffeine-sensitive Ca2+ store that can be regulated by intraluminal free Ca2+. Biochem J. 1991 May 1;275(Pt 3):697–701. doi: 10.1042/bj2750697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler L., Rodan G., Feinstein M. B. Cytochemical localization of adenylate cyclase and of calcium ion, magnesium ion-activated ATPases in the dense tubular system of human blood platelets. Biochim Biophys Acta. 1978 Sep 6;542(3):357–371. doi: 10.1016/0304-4165(78)90367-7. [DOI] [PubMed] [Google Scholar]
- Ely J. A., Hunyady L., Baukal A. J., Catt K. J. Inositol 1,3,4,5-tetrakisphosphate stimulates calcium release from bovine adrenal microsomes by a mechanism independent of the inositol 1,4,5-trisphosphate receptor. Biochem J. 1990 Jun 1;268(2):333–338. doi: 10.1042/bj2680333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh T. K., Mullaney J. M., Tarazi F. I., Gill D. L. GTP-activated communication between distinct inositol 1,4,5-trisphosphate-sensitive and -insensitive calcium pools. Nature. 1989 Jul 20;340(6230):236–239. doi: 10.1038/340236a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hack N., Authi K. S., Crawford N. Introduction of antibody (PL/IM 430) to a 100 kDa protein into permeabilised platelets inhibits intracellular sequestration of Ca2+. Biosci Rep. 1988 Aug;8(4):379–388. doi: 10.1007/BF01115229. [DOI] [PubMed] [Google Scholar]
- Hack N., Croset M., Crawford N. Studies on the bivalent-cation-activated ATPase activities of highly purified human platelet surface and intracellular membranes. Biochem J. 1986 Feb 1;233(3):661–668. doi: 10.1042/bj2330661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne V., Piiper A., Söling H. D. Inositol 1,4,5-trisphosphate and 5'-GTP induce calcium release from different intracellular pools. FEBS Lett. 1987 Jun 22;218(1):153–158. doi: 10.1016/0014-5793(87)81037-2. [DOI] [PubMed] [Google Scholar]
- Hill T. D., Dean N. M., Boynton A. L. Inositol 1,3,4,5-tetrakisphosphate induces Ca2+ sequestration in rat liver cells. Science. 1988 Nov 25;242(4882):1176–1178. doi: 10.1126/science.2847317. [DOI] [PubMed] [Google Scholar]
- Hornby E. J., Brown S., Wilkinson J. M., Mattock C., Authi K. S. Activation of human platelets by exposure to a monoclonal antibody, PM6/248, to glycoprotein IIb-IIIa. Br J Haematol. 1991 Oct;79(2):277–285. doi: 10.1111/j.1365-2141.1991.tb04533.x. [DOI] [PubMed] [Google Scholar]
- Jackson T. R., Patterson S. I., Thastrup O., Hanley M. R. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem J. 1988 Jul 1;253(1):81–86. doi: 10.1042/bj2530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass G. E., Duddy S. K., Moore G. A., Orrenius S. 2,5-Di-(tert-butyl)-1,4-benzohydroquinone rapidly elevates cytosolic Ca2+ concentration by mobilizing the inositol 1,4,5-trisphosphate-sensitive Ca2+ pool. J Biol Chem. 1989 Sep 15;264(26):15192–15198. [PubMed] [Google Scholar]
- Kijima Y., Ogunbunmi E., Fleischer S. Drug action of thapsigargin on the Ca2+ pump protein of sarcoplasmic reticulum. J Biol Chem. 1991 Dec 5;266(34):22912–22918. [PubMed] [Google Scholar]
- Lapetina E. G., Reep B., Ganong B. R., Bell R. M. Exogenous sn-1,2-diacylglycerols containing saturated fatty acids function as bioregulators of protein kinase C in human platelets. J Biol Chem. 1985 Feb 10;260(3):1358–1361. [PubMed] [Google Scholar]
- Llopis J., Chow S. B., Kass G. E., Gahm A., Orrenius S. Comparison between the effects of the microsomal Ca(2+)-translocase inhibitors thapsigargin and 2,5-di-(t-butyl)-1,4-benzohydroquinone on cellular calcium fluxes. Biochem J. 1991 Jul 15;277(Pt 2):553–556. doi: 10.1042/bj2770553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton J., Westlin M., Hanley M. R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991 Sep 15;266(26):17067–17071. [PubMed] [Google Scholar]
- Menashi S., Authi K. S., Carey F., Crawford N. Characterization of the calcium-sequestering process associated with human platelet intracellular membranes isolated by free-flow electrophoresis. Biochem J. 1984 Sep 1;222(2):413–417. doi: 10.1042/bj2220413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S., Loessberg P., Sachs G., Wheeler L. A. Agonist-sensitive and -insensitive intracellular Ca2+ pools. Separate Ca(2+)-releasing mechanisms revealed by manoalide and benzohydroquinone. Biochem J. 1991 Oct 15;279(Pt 2):367–375. doi: 10.1042/bj2790367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullaney J. M., Yu M., Ghosh T. K., Gill D. L. Calcium entry into the inositol 1,4,5-trisphosphate-releasable calcium pool is mediated by a GTP-regulatory mechanism. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2499–2503. doi: 10.1073/pnas.85.8.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke F. A., Halenda S. P., Zavoico G. B., Feinstein M. B. Inositol 1,4,5-trisphosphate releases Ca2+ from a Ca2+-transporting membrane vesicle fraction derived from human platelets. J Biol Chem. 1985 Jan 25;260(2):956–962. [PubMed] [Google Scholar]
- Ozawa T., Schulz I. H+ uptake increases GTP-induced connection of inositol 1,4,5-trisphosphate- and caffeine-sensitive calcium pools in pancreatic microsomal vesicles. Biochem Biophys Res Commun. 1991 Oct 31;180(2):755–764. doi: 10.1016/s0006-291x(05)81130-1. [DOI] [PubMed] [Google Scholar]
- Papp B., Enyedi A., Kovács T., Sarkadi B., Wuytack F., Thastrup O., Gárdos G., Bredoux R., Levy-Toledano S., Enouf J. Demonstration of two forms of calcium pumps by thapsigargin inhibition and radioimmunoblotting in platelet membrane vesicles. J Biol Chem. 1991 Aug 5;266(22):14593–14596. [PubMed] [Google Scholar]
- Rossier M. F., Bird G. S., Putney J. W., Jr Subcellular distribution of the calcium-storing inositol 1,4,5-trisphosphate-sensitive organelle in rat liver. Possible linkage to the plasma membrane through the actin microfilaments. Biochem J. 1991 Mar 15;274(Pt 3):643–650. doi: 10.1042/bj2740643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier M. F., Capponi A. M., Vallotton M. B. The inositol 1,4,5-trisphosphate-binding site in adrenal cortical cells is distinct from the endoplasmic reticulum. J Biol Chem. 1989 Aug 25;264(24):14078–14084. [PubMed] [Google Scholar]
- Sagara Y., Wade J. B., Inesi G. A conformational mechanism for formation of a dead-end complex by the sarcoplasmic reticulum ATPase with thapsigargin. J Biol Chem. 1992 Jan 15;267(2):1286–1292. [PubMed] [Google Scholar]
- Stauderman K. A., McKinney R. A., Murawsky M. M. The role of caffeine-sensitive Ca2+ stores in agonist- and inositol 1,4,5-trisphosphate-induced Ca2+ release from bovine adrenal chromaffin cells. Biochem J. 1991 Sep 15;278(Pt 3):643–650. doi: 10.1042/bj2780643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Supattapone S., Worley P. F., Baraban J. M., Snyder S. H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988 Jan 25;263(3):1530–1534. [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Thastrup O., Foder B., Scharff O. The calcium mobilizing tumor promoting agent, thapsigargin elevates the platelet cytoplasmic free calcium concentration to a higher steady state level. A possible mechanism of action for the tumor promotion. Biochem Biophys Res Commun. 1987 Feb 13;142(3):654–660. doi: 10.1016/0006-291x(87)91464-1. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Linnebjerg H., Bjerrum P. J., Knudsen J. B., Christensen S. B. The inflammatory and tumor-promoting sesquiterpene lactone, thapsigargin, activates platelets by selective mobilization of calcium as shown by protein phosphorylations. Biochim Biophys Acta. 1987 Jan 19;927(1):65–73. doi: 10.1016/0167-4889(87)90066-8. [DOI] [PubMed] [Google Scholar]
- Thastrup O. Role of Ca2(+)-ATPases in regulation of cellular Ca2+ signalling, as studied with the selective microsomal Ca2(+)-ATPase inhibitor, thapsigargin. Agents Actions. 1990 Jan;29(1-2):8–15. doi: 10.1007/BF01964706. [DOI] [PubMed] [Google Scholar]
- Volpe P., Krause K. H., Hashimoto S., Zorzato F., Pozzan T., Meldolesi J., Lew D. P. "Calciosome," a cytoplasmic organelle: the inositol 1,4,5-trisphosphate-sensitive Ca2+ store of nonmuscle cells? Proc Natl Acad Sci U S A. 1988 Feb;85(4):1091–1095. doi: 10.1073/pnas.85.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vostal J. G., Jackson W. L., Shulman N. R. Cytosolic and stored calcium antagonistically control tyrosine phosphorylation of specific platelet proteins. J Biol Chem. 1991 Sep 5;266(25):16911–16916. [PubMed] [Google Scholar]
- White J. G. Interaction of membrane systems in blood platelets. Am J Pathol. 1972 Feb;66(2):295–312. [PMC free article] [PubMed] [Google Scholar]