Abstract
Introduction:
Barrett’s esophagus (BE) is the precursor to esophageal adenocarcinoma (EAC). Endoscopic eradication therapy (EET) can be effective in eradicating BE and related neoplasia, and has greater risk of harms and resource use than surveillance endoscopy. This clinical practice guideline aims to inform clinicians and patients by providing evidence-based practice recommendations for the use of EET in BE and related neoplasia.
Methods:
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework was used to assess evidence and make recommendations. The panel prioritized clinical questions and outcomes according to their importance for clinicians and patients, conducted an evidence review, and used the Evidence-to-Decision Framework to develop recommendations regarding the use of EET in patient with BE under the following scenarios: presence of 1) high grade dysplasia (HGD), 2) low grade dysplasia (LGD), 3) no dysplasia, or choice of 4) stepwise endoscopic mucosal resection (EMR) vs. focal EMR plus ablation, and 5) endoscopic submucosal dissection (ESD) vs EMR. Clinical recommendations were based on the balance between the desirable and undesirable effects, patient values, costs, and health equity considerations.
Results:
The panel agreed on 5 recommendations for the use of EET in BE and related neoplasia. Based on the available evidence, the panel made a strong recommendation in favor of EET in patients with BE HGD and conditional recommendation against EET in BE without dysplasia. The panel made a conditional recommendation in favor of EET in BE LGD; patients with BE LGD who place a higher value on the potential harms, and lower value on the uncertain benefits regarding reduction of esophageal cancer mortality could reasonably select surveillance endoscopy. In patients with visible lesions, a conditional recommendation was made in favor of focal EMR plus ablation over stepwise EMR. In patients with visible neoplastic lesions undergoing resection, a conditional recommendation was made in favor of EMR over ESD.
Conclusions:
This document provides a comprehensive outline of the indications for EET in the management of BE and related neoplasia. Guidance is also provided regarding the considerations surrounding implementation of EET. Providers should engage in shared decision making based on patient preferences. Limitations and gaps in the evidence are highlighted to guide future research opportunities.
Keywords: Barrett’s esophagus, cryosurgery, endoscopic mucosal resection, esophageal neoplasms, radiofrequency ablation
Executive Summary
The advent of endoscopic eradication therapy (EET) for treatment of dysplasia and early-stage cancer has revolutionized the management of Barrett’s esophagus (BE), reducing the morbidity and mortality related to esophagectomy and ultimately preventing esophageal adenocarcinoma (EAC) mortality. This evidence-based guideline from the American Gastroenterological Association (AGA) aims to provide recommendations for the use of endoscopic eradication therapy in patients with BE and related neoplasia. The panel agreed on 5 recommendations for the use of EET in BE and related neoplasia and provided multiple additional implementation considerations.
How to Read These Guidelines
Table 1 provides an overview of each guideline recommendation along with the associated strength of recommendation and certainty of evidence. Additional information about the background, methods, evidence reviews, and detailed justifications for each recommendation is provided after Table 1 for readers wishing to read the full guideline. Corresponding forest plots for each intervention and evidence profiles provide a synthesis of the evidence as well as Evidence to Decision framework tables that summarize the panel’s detailed judgments supporting each recommendation are provided in the tables. Each recommendation is accompanied by clinical practice considerations (based on the collective experience of the panel members) that are meant to help guideline users implement the recommendations. The term “recommend” was used to indicate strong recommendations, and the term “suggest” was used to indicate conditional recommendations. The interpretation of certainty of evidence and implications of strong and conditional recommendations for healthcare providers, patients, and policymakers are presented in Tables 2 and 3, respectively.
Table 1.
Summary of Recommendations and Implementation Considerations
| Recommendations | Strength of Recommendation | Certainty of Evidence |
|---|---|---|
|
Strong | Moderate |
Implementation Considerations for Recommendation #1:
| ||
|
Conditional | Low |
Implementation Considerations for Recommendation #2:
| ||
|
Conditional | Very Low |
|
Conditional | Very Low |
Implementation Considerations for Recommendation #4:
| ||
|
Conditional | Very Low |
Implementation Considerations for Recommendation #5:
| ||
Table 2.
Interpretation of the Certainty of Effects Using the GRADE Framework
| Certainty of Evidence | Definition |
|---|---|
| High | We are very confident that the true effect lies close to that of the estimate of the effect. |
| Moderate | We are moderately confident that the true effect lies close to that of the estimate of the effect. There is a possibility that it is substantially different. |
| Low | Our confidence that the true effect lies close to that of the estimate of the effect is low. The true effect may be substantially different from the estimate of the effect. |
| Very low | Our confidence that the true effect lies close to that of the estimate of the effect is very low. The true effect is likely substantially different from the estimate of the effect. |
Table 3.
Interpretation of a Strong and Conditional Recommendation
| Implications | Strong Recommendation | Conditional Recommendation |
|---|---|---|
| For Patients | Most individuals in this situation would want the recommended course of action and only a small proportion would not. | The majority of individuals in this situation would want the suggested course of action, but many would not. |
| For Clinicians | Most individuals should receive the intervention. Formal decision aids are not likely to be needed to help individuals make decisions consistent with their values and preferences. | Different choices will be appropriate for individual patients consistent with his or her values and preferences. Use shared decision making. Decision aids may be useful in helping patients make decisions consistent with their individual risks, values, and preferences. |
| For Policy Makers | The recommendation can be adapted as policy or performance measure in most situations. | Policy making will require substantial debate and involvement of various stakeholders. Performance measures should assess whether decision making is appropriate. |
Introduction
Description of the Health Problem
The incidence of EAC rose 5-fold from the 1970s to the 2010s, and adenocarcinoma now represents the most common form of esophageal cancer in the United States.1 Survival from all but the earliest stage of esophageal adenocarcinoma remains poor.2 BE is the only known associated precursor of esophageal adenocarcinoma.3, 4 BE is believed to pass through steps of low grade dysplasia (LGD), then high grade dysplasia (HGD) before developing into adenocarcinoma. The advent of EET for treatment of dysplasia and early-stage cancer has revolutionized the management of BE, reducing the morbidity and mortality related to esophagectomy and ultimately preventing esophageal adenocarcinoma mortality.5-7
Objective of the Review and Guideline
The AGA developed this systematic review and clinical guideline to provide evidence-based recommendations for the EET of BE and related neoplasia. EET includes resection techniques (endoscopic mucosal resection [EMR] and endoscopic submucosal dissection [ESD]) as well as ablation (including radiofrequency ablation [RFA], cryoablation, and other techniques). Future guidelines from the AGA will address screening and surveillance.
Target Audience
The target audience for these guidelines includes primary care, internal medicine, family medicine, gastroenterology, oncology, and surgery healthcare providers; patients; and policymakers. The recommendations in this document are not intended to be used as the standard of care. Instead, they can be used to guide the management of patients with BE and related neoplasia. Although no single recommendation can encompass every individual circumstance and context, it can be used to address the benefits and harms of treatments and support the processes of shared decision making so that patients are treated based on their values and preferences.
Methods
Overview
This document represents the official recommendations of the AGA. These recommendations were developed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach.
Organization and Panel Composition
The guideline panel members were selected based on their clinical and methodological expertise. Each member underwent a vetting process that required disclosing all conflicts of interest. The panel included a total of 14 guideline committee members, either with clinical/research expertise in the content or specialized in methodology. Panel members comprising the evidence review team included gastroenterologists with expertise in Barrett’s esophagus, 1 senior methodologist, and 3 junior methodologists. The senior methodologist supervised the evidence synthesis for all the interventions across the subcommittees. Members of the guideline committee helped review all the synthesized evidence, contributed to discussion, and helped develop the clinical decision support tool. A librarian assisted with designing and executing the relevant literature searches.
Management of Conflict of Interest and Guideline Funding
Panel members disclosed all potential conflicts of interest. Conflicts were managed according to AGA policies, the National Academy of Medicine, and Guidelines International Network standards.8-10 Development of this guideline was wholly funded by the AGA with no support from the industry.
Scope
The guideline panel and evidence review team formulated clinically relevant questions on endoscopic therapies for BE and related neoplasia. The most recent comprehensive position paper by the AGA on BE was published in 2011, and included guidance on screening, surveillance, biomarkers, and endoscopic therapy.11 Since then, the AGA has published Clinical Practice Updates on the management of BE with low-grade dysplasia (LGD),12 ESD (including outside of the setting of BE),13 endoscopic treatment of neoplastic BE,14 and screening and surveillance.15 The current guideline panel undertook a comprehensive review following the GRADE approach, the results of which add to and update the prior documents. Given the breadth of the review, the guideline panel split the publication of the recommendations into this document on endoscopic treatment and forthcoming guidance on screening and surveillance.
Formulation of Clinical Questions and Determining Outcomes of Interest
Through an iterative process, the guideline panel developed focused clinical questions deemed relevant for clinical practice that the guideline would address, related to the endoscopic treatment of BE and related neoplasia. From these focused questions, well-defined statements in terms of patients, intervention, comparator, and outcome (PICO) were defined, and these formed the framework for formulating the study inclusion and exclusion criteria and guided the literature search. The AGA Governing Board approved the final set of questions and statements (Table 4).
Table 4.
PICO Questions
| Focused Question | Patients | Intervention | Comparator | Outcomes |
|---|---|---|---|---|
|
Adult patients with BE and HGD | EET | Endoscopic Surveillance | Benefits:
Harms:
|
|
Adult patients with BE and LGD | EET | Endoscopic Surveillance | Benefits:
Harms:
|
|
Adult patients with NDBE | EET | No EET | Benefits:
Harms:
|
|
Adult patients with dysplastic BE (LGD/HGD) or T1a EAC that are undergoing EET | Endoscopic resection alone (of the entire BE segment) | Endoscopic resection followed by ablation | Benefits:
|
|
Adult patients with neoplastic BE that are undergoing endoscopic resection | ESD | EMR | Benefits:
|
BE: Barrett’s esophagus, EAC: esophageal adenocarcinoma, EMR: endoscopic mucosal resection, ESD: endoscopic submucosal dissection, HGD: high-grade dysplasia, LGD: low-grade dysplasia, ND: non-dysplastic
Search Strategy
A protocol guided the systematic review process. For the first 4 PICO questions we identified recently published systematic reviews and meta-analyses that used a comprehensive search strategy (PubMed, Embase, and Cochrane Library), then updated the search to January 2023, with the help from a medial librarian. Details were included under evidence summaries for each PICO question. For PICO 5 there was no systematic review or meta-analysis meeting our inclusion criteria. Thus, a separate comprehensive search was conducted on the following databases: EMBASE, MEDLINE, Cochrane, and PubMed. The search terms used, and the final strategy can be found in the supplementary material (Supplementary Tables 1-3). References from included references and prior guidelines were searched to identify any missing relevant studies. Furthermore, content experts aided in the identification of any ongoing studies.
Study Selection, Data Collection, and Analysis
Searches from all the databases were combined in Rayyan bibliographic software,16 and duplicates were removed. One content expert and one methodologist screened each title and conducted a full-text review of the eligible studies, and a consensus was reached on inclusion (see Supplementary Figure 1 for the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Flow Diagram).17 In summary, we included randomized controlled trials (RCTs). Where RCT data was not available or sparse, we also considered observational studies, giving preference to observational studies with control arms over un-controlled observations. Any conflicts were resolved with adjudication by the senior methodologist. Data were extracted from each study, including study characteristics, such as year of publication, study site, study population, intervention, comparison group, outcomes and methods for risk-of-bias assessment. Meta-analyses were conducted when more than 1 study contributed data for the same intervention and outcome. We combined the dichotomous outcomes to obtain a relative risk (RR) and 95% confidence interval (CI). For the meta-analyses, we used the generic inverse variance method of weighting and applied the random-effects model, unless 3 or fewer studies were present, we used a fixed-effects model due to the instability of between-study variance. We assessed the statistical heterogeneity by using the I2 index. We used Review Manager RevMan software version 5.3 for the comparative studies (The Nordic Cochrane Centre. Copenhagen, Denmark: The Cochrane Collaboration, 2014), and OpenMeta analyst for statistical analyses of single arm studies (OpenMetaAnalyst: Wallace, Byron C., Issa J. Dahabreh, Thomas A. Trikalinos, Joseph Lau, Paul Trow, and Christopher H). We used the Cochrane Risk of Bias tool to assess the risk of bias in the included studies incorporated in RevMan. This tool assesses the risk of bias in the following domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other biases.
Certainty of the Evidence
We used the GRADE approach to assess the certainty of evidence for the effect of the intervention on each outcome using the software GradePro Guideline Development Tool (https://gradepro.org). The GRADE approach considers factors such as study design, population studied, risk of bias, inconsistency, indirectness, imprecision, and risk of publication bias to rate the certainty of evidence as high, moderate, low, or very low (Table 2).18 The results of certainty assessment are reported in evidence profiles available in tables 5-9 for all the interventions included in this review.
Table 5.
Grading of Recommendations Assessment, Development and Evaluation Evidence Profile for PICO Question 1: Comparing EET with surveillance in individuals with BE and HGD
| Certainty assessment | № of patients* | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies |
Study design |
Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations |
EET | surveillance | Relative (95% CI) |
Absolute (95% CI) |
||
| Progression of HGD to EAC (Evidence from RCTs) follow up time was between 1-4 years | ||||||||||||
| 2 | randomized trials | not serious | not serious | not serious | seriousa | none | 20/210 (9.5%) | 35/124 (28.2%) |
RR 0.40 (0.23 to 0.69) |
169 fewer per 1,000 (from 217 fewer to 88 fewer) |
⨁⨁⨁◯ Moderate |
CRITICAL |
| Progression of HGD to EAC (Evidence from NRS) | ||||||||||||
| 19 | non-randomized studies | seriousb | not serious | not serious | not serious | none | 67/9023 | 69/1241 |
Rate ratio 0.28 (0.22 to 0.32) |
40 fewer per 1000 patient(s) per years (from 43 fewer to 38 fewer)c |
⨁◯◯◯ Very low |
IMPORTANT |
| Serious Adverse events (Evidence from RCT) | ||||||||||||
| 2 | randomized trials | not serious | not serious | not serious | very seriousd | none | 7/222 (3.2%) | 1/113 (0.9%) |
RR 2.56 (0.45 to 14.54) |
14 more per 1,000 (from 5 fewer to 120 more) |
⨁⨁◯◯ Low |
IMPORTANT |
| Stricture, With EMR and RFA (Evidence from NRS) | ||||||||||||
| 40 | non-randomized studies | not serious | not serious | not serious | not serious | strong association | 558/12790 (4.4%) | 1/10000 (0.0%) | not estimable |
56 more per 1,000 (from 46 more to 67 more)e |
⨁⨁⨁◯ Moderate |
CRITICAL |
| Bleeding with EMR + RFA (Evidence from NRS) | ||||||||||||
| 20 | non-randomized studies | not serious | not serious | not serious | not seriousf | strong association | 53/5902 (0.9%) | 1/10000 (0.0%) | not estimable |
6 more per 1,000 (from 4 more to 9 more)g |
⨁⨁⨁◯ Moderate |
CRITICAL |
| Perforation with EMR + RFA (Evidence from NRS) | ||||||||||||
| 28 | non-randomized studies | not serious | not serious | not serious | not seriousf | strong association | 16/5799 (0.3%) | 1/10000 (0.0%) | not estimable |
2 more per 1,000 (from 1 more to 4 more)h |
⨁⨁⨁◯ Moderate |
CRITICAL |
These are not weighted proportions, for weighted proportions please refer to forest plots.
Low event number
All studies are single arm cohort and lacking comparison
Rate ratio calculated between 2 separately pooled incidence rates
very few events
Stricture events in Surveillance group with esophageal biopsy is very low
Although low events were observed, given the extremally low baseline risk and large total number of patients, we did not rate down for imprecision.
Major bleeding events in Surveillance group with esophageal biopsy is very low
Perforation events in Surveillance group with esophageal biopsy is very low in studies usually referenced in-between 1/2500 and 1/11,000
Table 9.
Grading of Recommendations Assessment, Development and Evaluation Evidence Profile for PICO Question 5: Comparing EMR with ESD in individuals with BE and visible neoplastic lesions
| Certainty assessment | № of patients* | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies |
Study design | Risk of bias |
Inconsistency | Indirectness | Imprecision | Other considerations |
EMR | ESD | Relative (95% CI) |
Absolute (95% CI) |
||
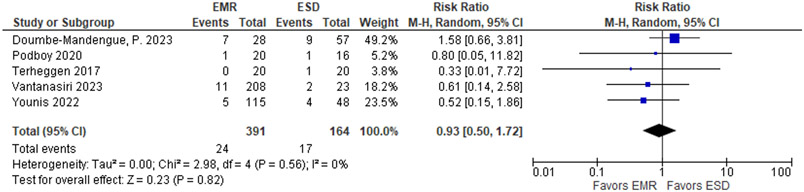
| EAC at 1-2 years of follow-up | ||||||||||||
| 5 | 1 RCT and 4 non-randomized studies | seriousa | not serious | not serious | very seriousb | none | 24 / 391 (6.1%) | 17 / 164 (10.4%) |
RR 0.93 (0.50 to 1.72) |
7 fewer per 1,000 (from 52 fewer to 75 more) |
⨁◯◯◯ Very low |
CRITICAL |
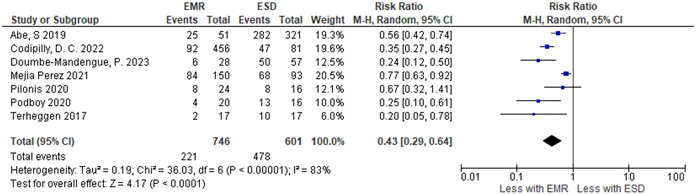
| R0 resection – margins of resection are free of cancer or high-grade dysplasia. | ||||||||||||
| 7 | 1 RCT and 6 non-randomized studies | not serious | seriousc | seriousd | not serious | none | 221 / 746 (29.6%) | 478 / 601 (79.5%) |
RR 0.43 (0.29 to 0.64) |
453 fewer per 1,000 (from 565 fewer to 286 fewer) |
⨁◯◯◯ Very low |
IMPORTANT |
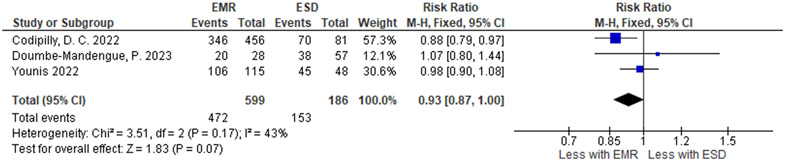
| Complete eradication of neoplasia | ||||||||||||
| 3 | non-randomized studies | not serious | not serious | seriousd | seriousj | none | 472 / 599 (78.8%) | 153 / 186 (82.3%) |
RR 0.93 (0.87 to 1.00) |
58 fewer per 1,000 (from 107 fewer to 0 fewer) |
⨁◯◯◯ Very low |
IMPORTANT |
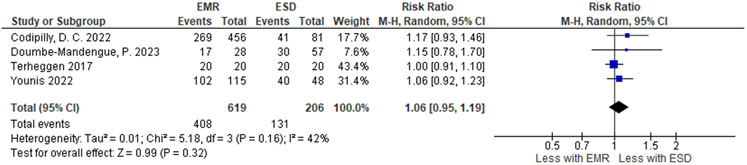
| Complete eradication intestinal metaplasia | ||||||||||||
| 4 | 1 RCT and 3 non-randomized studies | not serious | not serious | seriousd | seriousj | none | 408 / 619 (65.9%) | 131 / 206 (63.6%) |
RR 1.06 (0.95 to 1.19) |
38 more per 1,000 (from 32 fewer to 121 more) |
⨁◯◯◯ Very low |
IMPORTANT |
| Strictures: single proportion comparison: EMR (27 observational studies) vs. ESD (38 observational studies) | ||||||||||||
| 65e | non-randomized studies | seriousf | not serious | not serious | not serious | none | 408 / 3,729 (10.9%) | 361 / 2,731 (13.2%) |
RR 0.83 (0.72 to 0.95) |
22 fewer per 1,000 (from 37 fewer to 7 fewer) |
⨁◯◯◯ Very low |
CRITICAL |
| Bleeding: single proportion comparison: EMR (20 observational studies) vs. ESD (32 observational studies) | ||||||||||||
| 52g | non-randomized studies | seriousf | not serious | not serious | serioush | none | 39 / 2,061 (1.9%) | 64 / 2,589 (2.5%) |
RR 0.77 (0.52 to 1.14) |
6 fewer per 1,000 (from 12 fewer to 3 more) |
⨁◯◯◯ Very low |
CRITICAL |
| Perforation single proportion comparison: EMR (27 observational studies) vs. ESD (33 observational studies) | ||||||||||||
| 60i | non-randomized studies | not seriousf | not serious | not serious | serioush | none | 17 / 2,845 (0.6%) | 46 / 2,693 (1.8%) |
RR 0.34 (0.20 to 0.60) |
11 fewer per 1,000 (from 14 fewer to 7 fewer) |
⨁◯◯◯ Very low |
CRITICAL |
These are not weighted proportions, for weighted proportions please refer to forest plots.
Suspected residual confounding because of lesion size. ESD lesions were usually larger and could have underestimated the results.
Low event number and the effect is from small benefit with EMR to small benefit with ESD, minimal important difference for cancer recurrence is 5%, thus the CI involves 2 thresholds.
I2 83%
Indirect outcome
EMR (27 observational studies) vs. ESD (34 observational studies)
Comparison of independent single arm studies with no time concurrent controls
EMR (20 observational studies) vs. ESD (32 observational studies)
Low event number
Single arm comparison: EMR (27 observational studies) vs. ESD (33 observational studies)
Pooled estimate is imprecise because the confidence intervals involves benefit from both interventions EMR and ESD.
Development of Recommendations
The process of translation of evidence into guideline recommendations followed the GRADE Evidence to decision framework and was achieved by discussion during virtual meetings of the guideline committee.19 The Evidence to decision framework considers the certainty of evidence, balance of benefits and harm, patient values and preferences, feasibility, acceptability, equity, and resource use.19 All evidence to decision tables are presented in tables 5-9. Consensus was reached for all the recommendations among the group. The interpretation of strength of recommendations is summarized in Table 3. In situations where the recommendation is only supported with very low certainty for the benefits and very low certainty for the harm outcomes, the guideline panel put a higher value on risk avoidance.
Document Review
The guideline underwent external peer review and public comments. The guideline document was revised to address pertinent comments.
Recommendations
A summary of all recommendations is provided in Table 1.
General Implementation Considerations:
|
Importance of tobacco cessation and weight loss
Tobacco use and obesity are risks factor for esophageal adenocarcinoma,20-22 and the most common causes of death in patients with BE undergoing EET is cardiovascular disease and other cancers, for which tobacco use and obesity are also major risk factors.23-25 In addition, tobacco use is associated with stricture formation following endoscopic mucosal resection (EMR).26 Therefore, patients with BE who use tobacco or are overweight, and in particular those undergoing EET, should be counseled to abstain from tobacco use and weight loss. The prospect of progression to cancer in patients with dysplastic BE often holds greater valence than prior counseling attempts, and patients may re-commit to such efforts following consultation for EET.
Referral to experts
Patients found to have dysplastic BE should be referred to high volume endoscopists, including in its endoscopic examination and resection, and pathologists with expertise in its interpretation. There is substantial disagreement among pathologists for interpreting dysplastic BE, particularly for LGD.27 Community pathologists tend to be overly sensitive in their interpretation at the detriment of specificity for risk of progression, and expert pathologists may tend to be more specific, but at the detriment to sensitivity.28 In a meta-analysis, expert pathologists downgraded 31% of LGD diagnoses referred from community settings, but also upgraded 10% to HGD or cancer.29 A working definition of an expert pathologist is a provider with a special interest in BE related neoplasia who is recognized as an expert in the field by peers, in part related to sufficient volume of cases.
Up to 63% of patients with dysplastic BE, including 27% in BE LGD, without a documented visible lesion referred from community settings to expert EET endoscopists are in fact found to have a visible lesion by the expert endoscopists, which requires endoscopic resection rather than ablation.29 Endoscopic resection permits more accurate histologic assessment than biopsy. In one expert center, 55% of patients referred for BE with HGD without a visible lesion at the community site were found to have a visible lesion with invasive adenocarcinoma upon endoscopic resection.30 And 26% of patients thought to have BE LGD were upgraded by the expert endoscopist’s tissue sampling, including adenocarcinoma in 7 to 11% and even some with advanced adenocarcinomas not amenable to EET.29-32 EET performed at higher volume centers and by higher volume endoscopists has been associated with favorable outcomes including complete eradication of intestinal metaplasia (CEIM), reduced risk of recurrence, and reduced risk of complications.33-36 However, how to define expert endoscopists is uncertain. For instance, a threshold of 20 radiofrequency ablation (RFA) procedures has been associated with improved CEIM, but at least 40 may be required to minimize recurrence following RFA. The specific number of procedures may also vary by type of EET. A working definition of an expert BE endoscopist is one who is recognized as an expert in the field by peers, in part related to sufficient volume of cases in addition to training in advanced imaging, selection of patients for EET, technical skills to perform both resection and ablation, and management of adverse events.
Reflux management
Patients with BE have greater reflux than other GERD patients, frequently have severe nocturnal reflux which may persist with once daily proton pump inhibitor (PPI), and often asymptomatic reflux events complicating the ability of the provider to manage reflux based on symptoms alone.37-40 In the RCTs of EET for BE, patients were prescribed twice daily PPI. Patients with incomplete response to EET are more likely to have uncontrolled reflux.41, 42 Therefore, patients should be prescribed twice daily dosing of PPI with appropriate timing 30-45 minutes before meals prior to initiating EET. They should also be advised to avoid eating 4 hours before lying down, and to raise the head of their bed to minimize nocturnal reflux.
In patients failing to achieve CEIM, the mainstay of management is centered on adequately controlling reflux. In a single center study, failure to achieve CEIM was most commonly associated with poorly controlled reflux, and 41% of those were due to nonadherence to twice daily PPI dosing with appropriate timing.43 After optimization of reflux control with re-education, change to a more potent PPI, or fundoplication, 94% of those initially failing CEIM ultimately achieved CEIM. Potassium competitive acid blockers (PCABs) might also have a useful role in patients who are not responding adequately to EET and its role needs to be assessed in future studies. Ambulatory reflux monitoring can help guide such decisions, including whether to refer for fundoplication before resuming EET. Similarly, patients who have ulceration found at the time of planned repeat EET should have EET delayed until reflux control is optimized. Whether changing the method of EET (either dosimetry or equipment) adds additional benefit beyond optimization of reflux control is not certain.
Goals of EET
The goal of EET should be CEIM and complete eradication of neoplasia (CEN). Among BE patients with HGD or early cancer who had underwent endoscopic resection of the visible lesion, 40% of patients randomized to surveillance had recurrent HGD or cancer within 3 years compared to 3% among those randomized to ablation of the remaining BE.44 Similarly, in a retrospective observational study of patients undergoing endoscopic resection of HGD or cancer, those who did not undergo ablation after complete resection of the neoplasia had a relative risk of 2.5 for recurrent neoplasia over a median follow-up of 63 months.45 And in the US RFA Registry, patients who achieved CEIM were less likely to progress to death with an odds ratio of 0.4.25 Repeat EET sessions are typically performed every 2 to 3 months to allow adequate healing between sessions. Persistent or recurrent non-dysplastic IM limited to the gastric cardia is common, but typically evanescent, and appears to have a very low risk of neoplastic progression.46, 47 Therefore, while ablation sessions should include treatment of the gastric cardia circumferentially, non-dysplastic IM limited to the gastric cardia found after CEIM of the tubular esophagus does not warrant continued EET. Persistent or recurrent IM in the tubular esophagus without dysplasia can be an indication for repeat EET depending on age, comorbidities, baseline histology, and prior course of EET attempts
Monitoring of Quality Metrics
A number of quality metrics in EET have been proposed, with varying levels of validation and specification.48 Although measurement errors related to small numbers of procedures can limit the accuracy in estimation of rare outcomes among individual practices, and particularly among individual endoscopists, monitoring and reporting key outcome measures can provide assurance to referring providers and patients regarding the quality of the EET provided. Key metrics to report include: proportion achieving CEIM (suggested minimum threshold 70%) and CEN (suggested minimum threshold 80%) 18 months after initiating EET, number of EET procedures required to achieve CEIM and CEN, recurrence of neoplasia following EET, and adverse event rates including bleeding events, perforation, and stricture.
| Recommendation 1: In individuals with Barrett’s esophagus with high grade dysplasia, the AGA recommends endoscopic eradication therapy over surveillance. (strong recommendation, moderate certainty of evidence) Implementation Consideration:
|
Summary of the Evidence
Evidence informing the recommendation for the management of BE with HGD was derived from both RCTs and observational cohort studies. Data from observational cohort studies was used as supplemental evidence for rate of progression to EAC due to limited follow up time from the RCTs. Evidence from a published well done systematic review and a meta-analysis was used.49 To update this systematic review, we identified new studies using similar search terms and a start date of January 1st, 2016 (Supplementary Table 1).
Two RCTs were included in the previous meta-analysis which reported progression from HGD to EAC between EET versus surveillance in patients with HGD.50, 51 No additional RCTs were identified in the updated search. Shaheen et al51 compared RFA to surveillance in BE patients with HGD and Overholt et al50 compared photodynamic therapy to surveillance. The 2 RCTs had similar baseline characteristics: mean age was 66 years and studies included predominantly white men. Mean length of BE was 5.3 cm in Shaheen et al.51 and > 50% of patients had BE > 6 cm in Overholt et al. Shaheen et al followed patients up to 1 year, whereas Overholt et al followed patients up to 3.6 years. Patients in the Overholt et al. trial had a surveillance endoscopy every 3 months until 4 consecutive quarterly biopsies were negative for HGD, then every 6 months thereafter. Patients in the Shaheen et al. trial underwent RFA at 3, 6, 9, and 12 months.
Benefits
The critical outcome for this question was progression rate to cancer among patients with HGD who were treated with EET compared to endoscopic surveillance alone. Pooled analysis of 2 RCTs using fixed-effects models with a total of 180 participants in the EET group vs 91 participants in the endoscopic surveillance group demonstrated decrease in progression to EAC when EET was used compared to surveillance with RR of 0.40 (95% CI, 0.23, 0.69) (Table 5 and Supplementary Figure 2).
These results were supported by the indirect evidence from observational studies that reported disease progression rates in patients treated with EET compared with those undergoing surveillance. The previous meta-analysis was updated with an additional 8 studies.49 A total of 19 studies were included for the indirect comparison including 3,155 patients. A total of 234 patients progressed to EAC over 13,595 person-year. Incidence rate for progression to EAC was pooled using inverse variance. The incidence rate of disease progression in the EET group was 1.9 per 100 person-years (95% CI: 1.1, 2.7) (Supplementary Figure 3). The incidence rate of disease progression in the surveillance group was 6.6 per 100 patient-years (95% CI: 5.0, 8.2).52
Harms
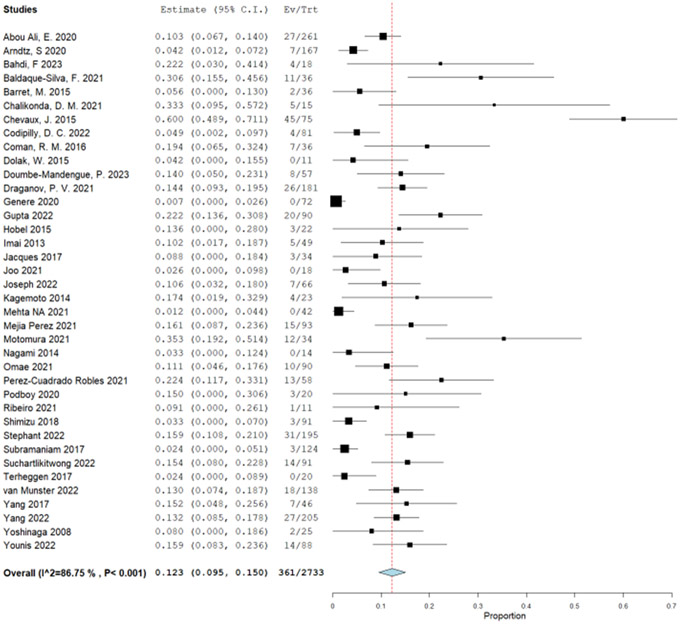
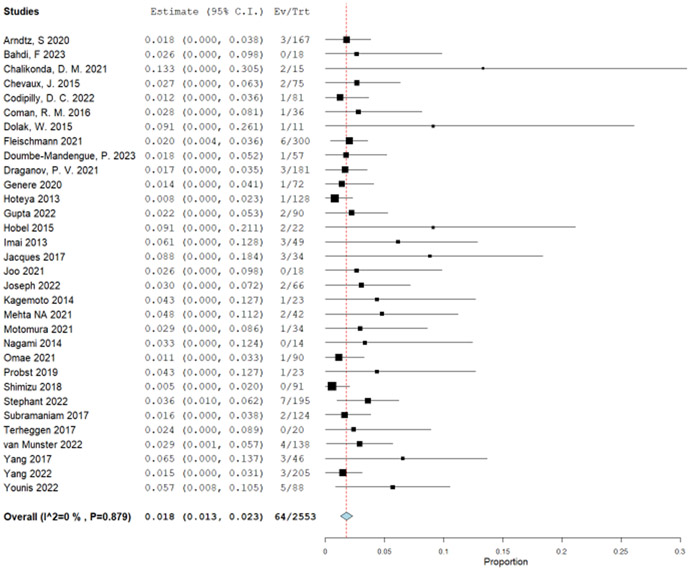
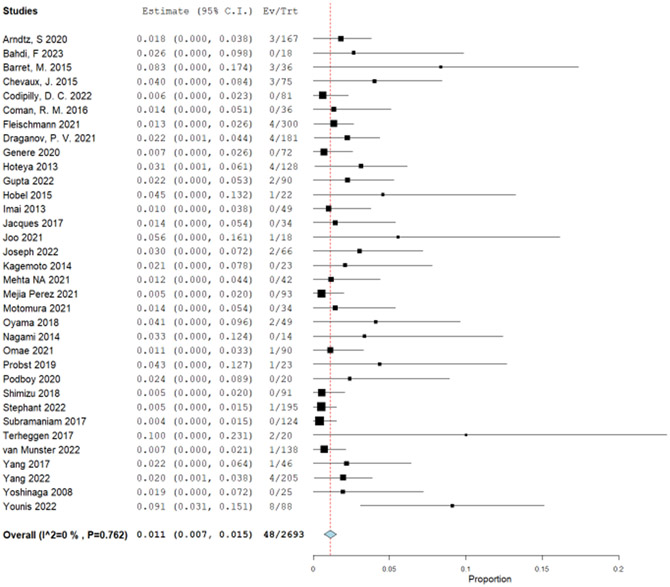
The patient-important outcomes that informed the harms for this PICO question were: (1) strictures, (2) major bleeding, (3) perforation, and (4) serious adverse events. Stricture was defined as any symptomatic dysphagia post treatment that required endoscopic dilation. Bleeding was defined as major bleeding (requiring blood transfusion, repeat EGD or hospitalization). Perforation was defined as any full thickness defect that required endoscopic or surgical intervention. Due to very sparse events occurring in the RCTs (total of 8 events: 7 in the EET and 1 in the surveillance), we used single arm retrospective cohort studies to determine the proportions of patients experiencing strictures, bleeding, and perforation. We used a published systematic review from 2016 and updated it with newly published studies.53 Because of the same treatment approach of EET with RFA with or without EMR, both population groups with BE and LGD and/or HGD were included in the analysis. The original systematic review had 28 published manuscripts and 9 meeting abstracts. In addition to those, we identified 21 studies35, 43, 47, 54-71 including some with the full text of the prior abstracts. The proportion of stricture formation was reported in 40 studies. There were 704 strictures out of 12,790 patients undergoing EET at a pooled proportion of 6.3% (95% CI: 5.0%, 7.6%) (Supplementary Figure 4.1). To calculate difference between EET and surveillance group, a very low event rate was used for stricture formation in the surveillance group with esophageal biopsies (1/10,0000). The absolute effect was calculated to be 56 more strictures per 1,000 patients undergoing RFA with or without EMR, with a 95% CI of 46 more to 67 more strictures per 1,000 (Table 5).
Major bleeding events were reported in total of 20 studies. Fifty-three events out of 5,902 patients were identified for a pooled proportion of 0.6% (95% CI: 0.4%, 0.9%) (Supplementary Figure 4.2). Similar to using the stricture outcome to calculate the difference between EET and surveillance group, a very low event rate was used for the major bleeding in the surveillance group with esophageal biopsies (1/10,000). The absolute effect was calculated to be 6 more major bleeding events per 1,000 patients undergoing RFA with or without EMR, with a 95% confidence limit (CL) of 4 more to 9 more bleeding events per 1,000 (Table 5). Lastly, for the outcome of perforation, we used 28 studies and there were total of 16 perforations reported in 5,799 patients for a pool proportion of 0.2% (95% CI: 0.1%, 0.4%) (Supplementary Figure 4.3). As for the other harms, perforations in the surveillance group with esophageal biopsy is very low, and is usually referenced between 1/2,500 and 1/11,000.72 Thus, the difference between groups in absolute effect were 2 more perforations per 1,000 patients undergoing EET, from 1 more to 4 more per 1,000 (Table 5).
Certainty in Evidence of Effects
The overall certainty in the evidence across the critical outcomes and considering both benefits and harms was moderate. See Table 5 for the full evidence profile. Our certainty in the critical desirable outcomes of disease progression to EAC was moderate. The major concern regarding the effect of EET on progression to EAC was imprecision given the low number of events. Data on benefits from non-randomized studies was used to complement the RCT data and those outcomes were considered important, although very low in certainty. The observational data is at serious risk of bias due to comparison of independent single arm studies without time concurrent controls; however, this did not impact the overall quality of evidence because the baseline stricture event number is extremely low. For the outcome of adverse events, the quality of evidence was moderate. Stricture formation was considered as the most common harm. Despite no studies with concurrent controls, we are certain that the baseline stricture rate in surveillance upper endoscopies with biopsy is very rare. Additionally, we rated up given the large difference between groups, thus the certainty in harms was judged to be moderate.
Discussion
In the setting of BE with HGD, EET results in a large decrease in progression to cancer with moderate certainty of evidence. The harms associated with EET were considered small, though not trivial. Bleeding and perforation are rare. Strictures are not uncommon, but usually easily treatable with appropriate acid suppression and endoscopic dilation. Patients frequently have chest pain following EET,61, 73-75 but this is a short-term effect. The costs of EET were considered moderate, and cost-effectiveness analyses favor EET over surveillance.76 There is probably no important uncertainty or variability in how much patients value the main benefits and harms unless they have life-threatening comorbidities. Patients generally find EET for HGD acceptable and implementing it has been largely feasible except for challenges related to less access to EET among rural residents. Finally, given the relatively small number of individuals with HGD and the large impact on cancer progression, a strategy of EET in this setting probably does not have a substantial negative impact on equity. On balance, the authors believed that EET is favored over surveillance for BE with HGD.
Implementation Considerations
Following completion of EET, there is a risk of recurrent neoplasia and intestinal metaplasia, though typically at the same degree or less severe than at initiation of EET.77, 78 EET performed at higher volume centers is associated with lower risk of early recurrence, suggesting that recurrences may actually be progression of prevalent microscopic foci of persistent BE to macroscopic lesions rather than de novo development of new BE.35 In the US national registry of RFA for BE with HGD or early adenocarcinoma, the cumulative incidence of adenocarcinoma was 6.3% at 5 years following CEIM.79 In some studies, the risk appeared greatest within the first year following completion of EET, but cancers continue to be identified long after that. Based on the registry data, a suggestion has been made of performing surveillance at 3, 6, and 12 months following CEIM for HGD or T1a adenocarcinoma, then annually, which seems reasonable until more definitive studies are conducted accounting for the risks and benefits of continued surveillance and repeated EET.77 Surveillance should continue until patients have life-limiting comorbidities or wish to discontinue surveillance based on their values and preferences.
When performing surveillance post-EET, the esophagus and cardia should be examined under white light and virtual chromoendoscopy with near focus, particularly using a clear cap. Targeted tissue sampling should be performed of visible lesions including islands or tongues of columnar mucosa, nodules (including subsquamous), altered crypt pattern, or erosions. Nodules, including subsquamous nodules, are best assessed by endoscopic resection. A majority but not all neoplastic recurrences are found at the esophagogastric junction.47, 80, 81 Among expert endoscopists, fewer than 1% of patients will be found to have dysplasia in biopsies from normal appearing squamous mucosa.80, 81 And the vast majority of those are found within the 2cm proximal to the esophagogastric junction, though this may be a function of the small prevalence of very long BE segments undergoing EET. In contrast, up to 50% of dysplastic recurrences in the gastric cardia are found only on random biopsies of normal appearing columnar mucosa; the absolute yield is still low, albeit greater than in normal appearing squamous mucosa.47, 80, 81 Therefore, during surveillance, random biopsies should be obtained from the gastric cardia immediately distal to the squamocolumnar junction, and of the distal 2cm of the neosquamous epithelium in the tubular esophagus. Recurrent lesions are typically small and treatable with repeat EET, but prior scarring may make endoscopic resection more challenging. Additional research is warranted to make more firm recommendations on biopsy protocols during surveillance.
| Recommendation 2: In individuals with Barrett’s esophagus with low grade dysplasia, the AGA suggests endoscopic eradication therapy over surveillance. (conditional recommendation, low certainty) Comment: Patients who place a higher value on the well-defined harms, and lower value on the uncertain benefits regarding reduction of esophageal cancer mortality would reasonably select surveillance. Implementation Consideration:
|
Summary of the evidence
Evidence informing the recommendation for the management of BE with LGD was derived from both RCTs and observational cohort studies. Data from observational cohort studies were explored to supplement the evidence for progression to EAC due to limited follow up in the RCTs. There was a previously published well done systematic review that assessed the risk of progression to EAC among patients with BE with LGD treated with RFA.82 The authors analyzed data from 2 RCTs51, 83 and 3 observational cohort studies;25, 84, 85 their systematic search ended on December 31st, 2015. To update this systematic review, we identified new studies using similar search criteria using a start date of January 1st, 2016. An additional 1 RCT86 and 9 observational cohort studies57, 68, 70, 86-92 were identified and analyzed together with the studies from the existing systematic review.86 The historical incidence rate for natural progression of BE with LGD from a previously published in systematic review was used.93 The 3 RCTs had similar demographic and baseline characteristics of the population.51, 83, 86 Mean age ranged between 63 and 67 years and the populations were predominantly male and white. Mean length of Barrett’s esophagus was similar between the studies and ranged from 2-4 cm circumferential and 5-7 cm in the longest extent. The follow up period was 3 years for 2 RCTs and 1 year for one RCT. All patients in the ablation group had surveillance endoscopy 6 months after treatment was completed, then annually. Patients in the surveillance group had follow up endoscopy every 12 months. The 2 largest cohort studies were conducted using national registries.25, 70 One was from the United Kingdom with 10 year follow-up, and another was from the US with 2.4 years of follow-up.25, 70 The other 11 studies were either multi- or single center retrospective single arm cohort studies with a follow-up period between 1 and 6 years. These had very similar demographics compared to the RCTs, with mean age between 60 and 70 years, mostly males and whites, with BE length of 4-6 cm.
Benefits
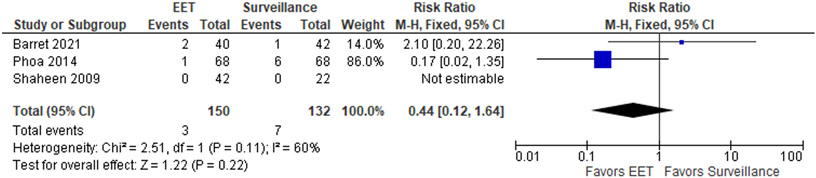
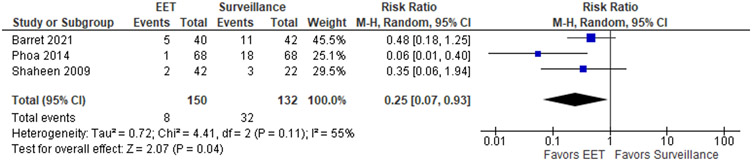
The patient-important outcomes that informed the benefits for this PICO question were: (1) progression to cancer, (2) disease progression defined as a composite outcome of progression to HGD and/or EAC and (3) progression to advanced cancer requiring esophagectomy and/or radiation/chemotherapy. The pooled analysis of 3 RCTs with a total of 150 participants in the EET group vs 132 participants in the endoscopic surveillance group demonstrated no significant difference in progression to EAC when EET was compared to surveillance, with RR of 0.44 (95% CI: 0.12, 1.64) (Figure 1.1), with absolute decrease of 30 cancers per 1,000 patients (95% CI: 47 fewer to 34 more). For the combined outcome of HGD/EAC, EET was associated with a reduced risk of progression compared to surveillance (RR 0.25; 95% CI: 0.07, 0.93) (Figure 1.2) and absolute decrease of 182 per 1,000 patients (95% CI: 225 fewer to 17 fewer).
Figure 1.1.
Forest plot RCTs comparing progression to EAC among patients with LGD who were treated with EET compared to endoscopic surveillance alone
Figure 1.2.
Forest plot of RCTs comparing progression to HGD or EAC among patients with LGD who were treated with EET compared to endoscopic surveillance alone
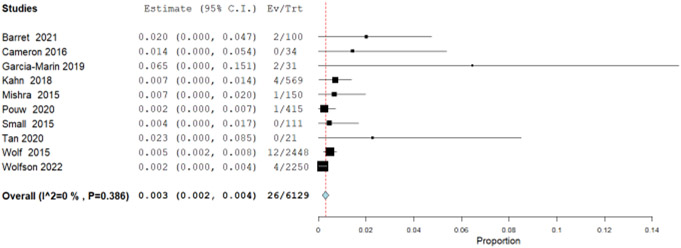
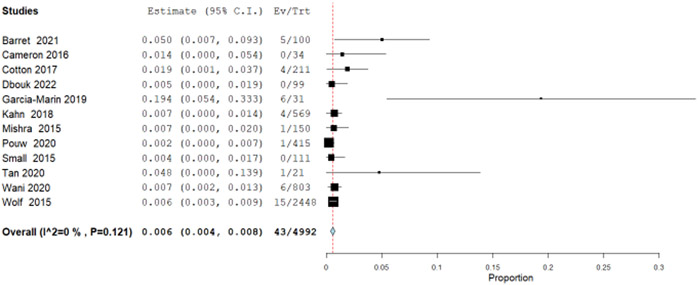
Additionally, we explored observational data from 10 single arm studies that retrospectively analyzed patients with BE and LGD treated with RFA. The incidence rate for progression to EAC was 0.3 per 100 patient-years (95% CI: 0.2, 0.4) (Figure 1.3) calculated by pooling using inverse variance from 10 studies with a total of 26 EAC outcomes in 6129 patient-years. In a previously published systematic review and meta-analysis, the pooled annual rate of progression from LGD to EAC was reported to be 0.54 per 100 patient-years (95% CI: 0.33, 0.76).93 The rate ratio for RFA compared to surveillance in these observational studies showed a decrease in progression to EAC of 0.55 (95% CI: 0.52, 0.61). For the composite outcome of disease progression to HGD and/or EAC, similarly we pooled rates from 12 single arm cohort studies with 43 events in total of 4,992 patient years, for an incidence rate of 0.6 per 100 patient years (95% CI: 0.3, 0.8) (Figure 1.4). The previously reported natural disease progression from LGD to HGD and/or EAC was reported to be 1.7 per 100 patient years (95% CI: 1.0, 2.5).94 The calculated rate ratio for RFA compared to surveillance in these observational studies for progression to HGD and/or EAC was 0.34 (95% CI: 0.24, 0.40) (Table 6).
Figure 1.3.
Forest plot from observational studies of pooled incidence rate of progression to EAC per patient-year among patients with LGD treated with EET.
Figure 1.4.
Forest plot from observational studies of pooled incidence rate of progression to EAC or HGD per patient-year among patients with LGD treated with EET.
Table 6.
Grading of Recommendations Assessment, Development and Evaluation Evidence Profile for PICO Question 2: Comparing endoscopic EET with surveillance in individuals with BE and LGD
| Certainty assessment | № of patients* | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies |
Study design |
Risk of bias |
Inconsistency | Indirectness | Imprecision | Other considerations |
EET | surveillance | Relative (95% CI) |
Absolute (95% CI) |
||
| Progression to HGD and/or EAC - RCT follow up time between 1-3 years | ||||||||||||
| 3 | randomized trials | not serious | seriousa | not seriousa | seriousb | none | 8/150 (5.3%) | 32/132 (24.2%) |
RR 0.25 (0.07 to 0.93) |
182 fewer per 1,000 (from 225 fewer to 17 fewer) |
⨁⨁◯◯ Low | CRITICAL |
| Progression to EAC (evidence from RCT) follow up time between 1-3 years | ||||||||||||
| 3 | randomized trials | not serious | not seriousc | not serious | very seriousc,d | none | 3/150 (2.0%) | 7/132 (5.3%) |
RR 0.44 (0.12 to 1.64) |
30 fewer per 1,000 (from 47 fewer to 34 more) |
⨁⨁◯◯ Low |
IMPORTANT |
| Progression to EAC (evidence from NRS) | ||||||||||||
| 10 | non-randomized studies | seriouse | not serious | not serious | not serious | none | 26/6139 | 119/16672 |
Rate ratio 0.55 0.52 to 0.61) |
3 fewer per 1000 patient(s) per years (from 4 fewer to 2 fewer)f |
⨁◯◯◯ Very low |
IMPORTANT |
| Progression to HGD and/or EAC (evidence from NRS) | ||||||||||||
| 12 | non-randomized studies | seriouse | not serious | not serious | not serious | none | 43/5001 | 1.7% |
Rate ratio 0.34 (0.24 to 0.40) |
11 fewer per 1000 patient(s) per years (from 13 fewer to 10 fewer) |
⨁◯◯◯ Very low |
IMPORTANT |
| Stricture, With EMR and RFA (Evidence from NRS) | ||||||||||||
| 40 | non-randomized studies | not serious | not serious | not serious | not serious | strong association | 558/12790 (4.4%) | 1/10000 (0.0%) | not estimable |
56 more per 1,000 (from 46 more to 67 more)g |
⨁⨁⨁◯ Moderate |
CRITICAL |
| Bleeding with EMR + RFA (Evidence from NRS) | ||||||||||||
| 20 | non-randomized studies | not serious | not serious | not serious | not serioush | strong association | 53/5902 (0.9%) | 1/10000 (0.0%) | not estimable |
6 more per 1,000 (from 4 more to 9 more)i |
⨁⨁⨁◯ Moderate |
CRITICAL |
| Perforation with EMR + RFA (Evidence from NRS) | ||||||||||||
| 28 | non-randomized studies | not serious | not serious | not serious | not serioush | strong association | 15/5799 (0.3%) | 1/10000 (0.0%) | not estimable |
2 more per 1,000 (from 1 more to 4 more)j |
⨁⨁⨁◯ Moderate |
CRITICAL |
These are not weighted proportions, for weighted proportions please refer to forest plots.
Although there is some inconsistency in between the studies with I2 of 55%, it was felt to be due to indirectness of outcome since this is a composite outcome of HGD and EAC; thus, because of the correlation between inconsistency and indirectness we decided to rate down once only .
There were few events
Although there is some inconsistency in between the studies with I2 of 60%, it was felt to be due to very serious imprecision; thus, we decided to rate down twice for imprecision and not for inconsistency.
very few events
All studies are single arm cohort and no comparison group
Rate ratio was used between 2 separately pooled incidence rates
Stricture events in Surveillance group with esophageal biopsy is very low
Although low events were observed, given the extremally low baseline risk and large total number of patients we did not rate down for imprecision.
Major bleeding events in Surveillance group with esophageal biopsy is very low.
Perforation events in Surveillance group with esophageal biopsy is very low in studies, usually referenced in-between 1/2500 and 1/11,000
When assessing for progression to advanced cancer requiring esophagectomy and/or radiation/ chemotherapy in the 3 RCTs 51, 83, 86 we identified only one event of esophagectomy in the surveillance group,83 with all other reported cancers amendable to EET. There was no cancer related mortality reported. Observational studies were lacking robust data on advanced cancer and mortality specific for the LGD population. In the US registry there were no deaths nor advanced cancers in the LGD group 25. Similarly, no advanced cancer requiring surgery nor increased cancer mortality was reported in 2 other studies.68, 92
Harms
The patient-important outcomes that informed the harms for this PICO question were: (1) strictures, (2) major bleeding either requiring blood transfusion, intervention, or hospitalization, (3) perforation, and (4) serious adverse events. In the 3 RCTs51, 83, 86 there were only 7 such serious adverse events, all in the EET groups, and none reported in the surveillance groups. Due to sparse events, the aforementioned systematic review of observational studies was used to estimate the risk of adverse events in LGD as both LGD and HGD used similar treatment approaches with EET (See the harm section under HGD, Supplementary Figures 4.1-4.3).
Certainty of the Evidence
The overall certainty in the evidence across the critical outcomes with consideration of both benefits and harms was low. See Table 6 for the full evidence profile. Our certainty in the critical desirable outcomes such as progression to EAC and progression to the composite outcome of HGD and/or EAC from RCTs was low. The major concern for progression to EAC was imprecision as there were very few events. Additionally, there was some inconsistency between the studies with I2 of 60%, which was felt to be due to imprecision in the individual studies, so the certainty of evidence was rated down twice for imprecision rather than for heterogeneity of results. Similarly, for the composite outcome of HGD and/or EAC there was concern for serious imprecision due to low events for which we rated down once. Also, there was a concern for inconsistency between the studies with I2 of 55%, but it was felt to be due to indirectness of outcome since this is a composite outcome of HGD and/or EAC; thus, because of the correlation between inconsistency and indirectness we decided to rate down once only. Data from non-randomized studies were very low in certainty due to serious risk of bias resulting from comparison of independent single arm studies without concurrent controls. The overall certainty for harms was moderate. Stricture formation was the most common adverse event. Despite no concurrent controls, we are certain that the baseline stricture rate in surveillance upper endoscopies with biopsy is very low. We rated up for certainty, given the large difference between groups. However, due to low certainty in benefits the overall certainty across all outcomes was low.
Discussion
For the critical outcome of HGD and combined outcomes of HGD and/or EAC, there were only 3 RCTs;51, 83, 86 that showed a substantial magnitude of benefit, but with inconsistent and imprecise estimates. The guideline authors had spirited conversations whether progression to EAC alone (not as a combined outcome with HGD) should be included as a critical outcome or just an important outcome, settling on important. Arguing against it being included as a critical outcome is that HGD is a finding that should be an actionable event, triggering EET. Furthermore, conducting prospective studies of EET in LGD aimed at a primary outcome of progression to EAC not amenable to EET would be largely infeasible due to the extremely large number of subjects that would be required. Arguing in favor of using EAC alone as the critical outcome is the fact that individuals do not die from HGD but rather advanced cancer; if prospective studies assessing the outcome of cancer are impractical because surveillance of LGD successfully identifies HGD prompting EET and thereby preventing cancer, then that same success indicates that surveillance could be preferred in clinical practice over EET for LGD. The summary estimate from the 3 RCTs did not demonstrate a statistically significant decrease in EAC burden for EET compared to surveillance, but with very imprecise estimates that could range to as many as 47 fewer EACs per 1,000 patients with LGD undergoing EET. Observational studies suggested EET was associated with a significant decrease in EAC, but with a much smaller absolute magnitude of benefit (4 fewer EACs per 1,000 patients) than in the RCTs. This may be due to the lower progression rates of LGD without EET reported in the observational studies (0.54% per year) as compared to patients enrolled in surveillance in the RCTs with central pathology review, highlighting the importance of expert pathology review before considering EET. The life-time cumulative incidence for a patient with BE to be diagnosed with LGD is substantial. Cost-effectiveness analyses have indicated that if EET were performed for all LGD diagnoses, 64% of BE patients would eventually undergo EET.95 Those analyses found that EET is only cost-effective if LGD is confirmed on repeat EGD, which would decrease the proportion of BE patients eventually undergoing EET to 36%. Overall, the benefits of EET in LGD were considered small to moderate. The harms were judged to be similar to that of EET for HGD (small). Patients without HGD and/or EAC are less likely to undergo concomitant EMR, and so the stricture rate could conceivably be lower, but there were only 3 small studies assessing strictures in patients without HGD and/or EAC undergoing ablation. The costs were expected to be similar to that of EET for HGD (moderate). Cost-effectiveness analyses suggest EET is probably favored over surveillance for BE with LGD only if LGD is confirmed with repeat endoscopy.76 A strategy of EET for LGD is largely feasible, but since LGD is commonly found in BE and the benefits of EET are diminished compared to EET for HGD, widespread EET for LGD probably reduces overall health equity. EET for LGD is probably acceptable to most patients, but there is possible important uncertainty and variability in how people value the main outcomes as discussed above. Overall, the guideline authors felt that the balance of benefits to harms probably favors EET, but the importance of shared decision making with patients with LGD is emphasized. The risks, expected discomfort, need for multiple EET sessions, and need for continued surveillance after completion of EET should be discussed in detail in addition to detailing the benefits in terms of reduction in progression to HGD and the uncertainty around the potential benefits of prevention of EAC and mortality to help patients decide their preferences.
Implementation Considerations
LGD, even when confirmed by expert pathologists, will regress to non-dysplastic Barrett’s esophagus (NDBE) during surveillance in 28% to 66% of patients.83, 96, 97 This could be due to multiple reasons, including sampling error during follow-up, false positive interpretation of LGD, or true regression. One of the reasons for the substantial interobserver variability in the histologic interpretation of LGD is that regenerative changes seen in the esophageal mucosa secondary to inflammatory injury related to uncontrolled reflux can share some of the same histologic features as dysplasia.98 Assessment with ambulatory reflux monitoring has demonstrated that regression of ostensible LGD is associated with more effective suppression of esophageal reflux, and fundoplication is more closely associated with regression than PPI.99, 100 And as discussed above in the general implementation considerations, the most common cause for failure to achieve CEIM is poorly controlled reflux; furthermore, among the 3 RCTs of EET for LGD, the one with the worst rate of CEIM and CEN was the one that did not include a specific PPI regimen in the protocol for patients undergoing EET.86 Therefore, the concept of optimizing reflux control is particularly emphasized in the management of LGD.
In patients with LGD undergoing EET, the goal should be similar to that in HGD. However, if CEIM is not achieved with the initial set of EET sessions, or if NDBE recurs, the balance of potential benefits to harms of continued EET is attenuated compared to the balance in patients with HGD. As a result, patients might reasonably elect to pursue surveillance of the remaining NDBE and only re-initiate EET if dysplasia is encountered during surveillance.
In the US national registry of RFA for BE with LGD, the cumulative incidence of adenocarcinoma following CEIM was 1.3% at 5 years.79 Based on the registry data, a suggestion has been made of performing surveillance at 1 and 3 years after CEIM.77 An initial surveillance at one and three years seems appropriate, but since the observed incidence of adenocarcinoma appears similar to that observed in patients with NDBE without EET, surveillance intervals following CEIM of LGD might justifiably be even less frequent than every 2 years after that, and can revert to the same intervals used in NDBE undergoing surveillance without any prior EET. Surveillance examinations and tissue sampling should be performed in the same manner as following EET for HGD.
| Recommendation 3: In individuals with non-dysplastic Barrett’s esophagus, the AGA suggests against the routine use of endoscopic eradication therapy (conditional recommendation, very low certainty). |
Summary of the evidence
We identified a published systematic review and meta-analysis that used a comprehensive search strategy (PubMed, and Embase) from inception to August 24, 2012, including EET in non-dysplastic BE (NDBE).101 We updated the systematic review with a search that ended on January 1, 2023 (Supplementary Table 2). Seven studies entered qualitative analysis to inform the benefits. Although the specific PICO was on NDBE, the evidence of harms in this histology group was very sparse. Therefore, we explored evidence on treatment not only in NDBE but in populations with dysplasia.
Benefits
No comparative evidence from RCT or cohort studies was found regarding EET of NDBE with outcomes of progression to EAC or esophageal cancer related mortality. A previously published systematic review and meta-analysis evaluating the natural history of BE included 57 studies and 11,434 patients with histologically confirmed NDBE for a total of 58,547 patient-years of follow-up.101 This systematic review identified 186 incident cases of EAC and calculated a pool incidence of 3.3 per 1,000 person-years (95% CI: 2.8, 3.8).101 Population-based studies from large BE RFA registries and single-arm EET cohort studies with consecutive patients were used for comparison. The US RFA Patient Registry was utilized to collect information on progression of NDBE to EAC post EET. The incidence of EAC in patients with NDBE following EET was 0.47 per 1,000 patient years; 2 out of 668 and 5 out of 668 patients developed HGD and LGD over 2.4 years follow up respectively.25 However, a large database study utilizing the TriNetX research network reported an incidence of EAC following EET of NDBE that was 3.34 per 1,000 person-years (95% CI: 0.75, 7.04), which is numerically similar to the incidence found in the systematic review of natural history of NDBE.101, 102 A small cohort study reported results of 53 patients followed at least a decade post RFA of NDBE. Only one patient developed neoplasia (LGD).103 Similarly, a cohort study with 123 patients followed for 7 years, reported 1 patient progressing to HGD and 3 to LGD. 104 Lastly, a single-arm cohort study followed 61 patients who were treated with RFA and achieved complete eradication of their NDBE. After 3.3 years, 12 out of 61 had recurrence of intestinal metaplasia, but none progressed to HGD or EAC. 92
Harms
The patient-important outcomes that informed the harms for this PICO question were: (1) strictures, (2) major bleeding either requiring blood transfusion, intervention, or hospitalization and (3) perforation and (4) post- procedure pain. For these outcomes, we used the same previously published systematic review that informed the decision regarding HGD and LGD.53 However, endoscopic resection would be unlikely to be needed in NDBE, so we focused on analyses restricted to the use of RFA, although those studies did include patients with dysplasia. A total of 10 studies (3 from the published systematic review and 7 that we identified) were used to inform the harm outcomes. Stricture formation was reported in all 10 studies. There were 75 strictures out of 1,489 patients undergoing RFA at a pooled proportion of 3.8% (95% CI: 2.8%, 4.8%) (Table 7, Supplementary Figure 5.1). To calculate the difference between EET and the surveillance group, a very low event rate was used for the stricture formation in the surveillance group with esophageal biopsies (1/10,000). Major bleeding events were reported in a total of 9 studies with 12 events from a total of 1,439 patients for a pooled proportion of 0.9% (95% CI: 0.4%, 1.4%) (Table 7, Supplementary Figure 5.2). Eight studies reported on perforations, and there were no perforations in 541 patients (Supplementary Figure 5.3). Additionally, as an important outcome we evaluated for post procedural pain. Pain was reported in 5 studies including a total of 370 patients. The pooled proportion of pain was 2.1% (95% CI: 0.1%, 4.2%) (Supplementary Figure 5.4).
Table 7.
Grading of Recommendations Assessment, Development and Evaluation Evidence Profile for PICO Question 3: Comparing EET with surveillance in individuals with NDBE
| Certainty assessment | № of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design |
Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations |
EET | no treatment |
Relative (95% CI) |
Absolute (95% CI) |
||
| Disease progression and mortality | ||||||||||||
| 6 | non-randomized studies | seriousa | not serious | not serious | seriousb | none | No comparative evidence from RCT or cohort studies. Population-based studies and single-arm cohort studies with consecutive patients: Progression to EAC (incidence): - The US RFA Patient Registry [Wolf 2015]: incidence of EAC in patients with NDBE was 0.47 per 1000PY; - USA large database -TriNetX [Smith 2023]: incidence of EAC was 3.34 per 1000 PY (95% CI: 0.75, 7.04); Progression to LGD and HGD (incidence): - US RFA Registry [Pasricha 2014] in cadence of LGD and HGD was 1.2 per 1000PY and 3.12 per 1000PY respectively. - Small cohort study [Corbett 2022]: 53 patients followed for 11.5 years post RFA of NDBE for incidence of LGD of 1.64 pre 1000PY. - Single arm cohort study [Wang 2022]: 123 patients followed for 7 years, incidence of LGD and HGD 3.48 and 1.16 per 1000 PY respectively - Single-arm cohort study [Wani 2020]: 61 patients 3.3 years of follow up no progression to progress to HGD or adenocarcinoma. |
⨁◯◯◯ Very low | CRITICAL | |||
| Stricture | ||||||||||||
| 10 | non-randomized studies | not serious | not serious | not serious | seriousc | strong association | 75/1489 (5.0%) | 1/10000 (0.0%) | not estimable |
38 more per 1,000 (from 28 more to 48 more)d |
⨁⨁◯◯ Low |
CRITICAL |
| Bleeding | ||||||||||||
| 9 | non-randomized studies | not serious | not serious | not serious | very seriousb | none | 12/1439 (0.8%) | 1/10000 (0.0%) | not estimable |
9 more per 1,000 (from 4 more to 14 more)e |
⨁◯◯◯ Very low |
CRITICAL |
| Pein | ||||||||||||
| 8 | non-randomized studies | not serious | not serious | not serious | very seriousb | none | 11/370 (3.0%) | 1/10000 (0.0%) | not estimable |
21 more per 1,000 (from 1 more to 42 more)f |
⨁◯◯◯ Very low |
IMPORTANT |
No comparison group, poorly defined intervention (mostly combining 2 different endoscopic methods). Also some studies limited the cohort to responders to endoscopic treatment only. Furthermore, major confounders such as PPI use and smoking were not adjusted for in most of the studies.
Very low event numbers
low events were observed
Stricture events in Surveillance group with esophageal biopsy is very low
Major bleeding events in Surveillance group with esophageal biopsy is very low
Severe pain post upper endoscopy with biopsy is very rare
Certainty in the evidence of effects
The certainty of evidence was very low across all outcomes, including benefits and harms (Table 7). The key concerns across the outcomes were: use of single-arm cohort studies (serious risk of bias due to lack of a comparator), poorly defined interventions (most combining 2 different endoscopic methods), and some studies limiting the cohort to responders to EET only. Also, major confounders such as PPI use and smoking were not adjusted for in most of the studies. Furthermore, there was serious imprecision for the outcome of progression to HGD and/or EAC because the data was very sparse. Most studies did not document how pain was assessed, and many of those that were documented were restricted to emergency department visits or hospitalizations for pain.
Discussion
The maximum potential benefit of EET in the setting of NDBE is bound by the small incidence of progression to invasive cancer without EET, which is likely approximately 0.6% per year averaged over 20 years of follow-up, and even smaller for shorter durations of follow-up.105 The vast majority of patients with NDBE ultimately die from causes other than EAC.23, 24 Therefore, even if large, high quality RCTs with long-term follow-up were available, the potential magnitude of benefit from EET in the setting of NDBE would be trivial at best.
In the setting of such small potential benefit, the expected harms from EET become relatively magnified. The harms of complications from EET including bleeding and perforation are rare but present and greater than with surveillance endoscopy. Strictures are not uncommon but relatively easy to manage. Importantly, patients undergoing EET experience the inconvenience of the potential need for multiple EET sessions with associated loss of work, changes in diet, time away from other pursuits, and burden for both the patient and their chaperone. Though the evidence review found pain was rare, this seems to be under-assessed in those studies, relying on emergency department or hospitalizations for ascertainment. In other studies where pain symptoms were actively collected, patients nearly universally experience considerable chest pain for days to weeks following EET, particularly with RFA for which there is the highest quality data on effectiveness.61, 73, 74 In one multi-center study published since completion of the systematic review, 95% of patients undergoing RFA experienced chest pain, including 65% with major chest pain.75 In individuals with NDBE, the harms may outweigh any small benefit. Finally, there is moderate cost associated with EET, particular as patients continue to undergo surveillance following EET. Compared to strategies of surveillance of NDBE followed by EET for dysplasia, cost-effectiveness analyses indicate that EET for NDBE followed by surveillance for recurrence would either be more expensive than the commonly accepted willingness-to-pay threshold in the US, or even dominated (meaning EET is both more expensive and lead to fewer quality-adjusted life-years).76, 106-108 There is limited data regarding patient preferences for or against EET in the setting of NDBE.109 While EET for NDBE is probably feasible from a health system standpoint, and may be acceptable to patients, it would likely also reduce equity since those diagnosed with NDBE are ipso facto those with access to expensive healthcare resources and undergoing EET would further direct resources away from other individuals. Balancing these potential benefits and harms, the data probably supports against EET for NDBE.
There might be specific populations with NDBE in whom the benefits of EET outweigh the harms. The risk of progression stratified by variables such as first degree relative with esophageal cancer, young onset at age of BE, length of BE is not well known. In patients with potentially increased risk for EAC based on these variables, decision to perform EET should be made considering the net benefit for the patient and their values and preferences. Further research is needed to determine the place of such risk factors in guiding EET, but using the Progression in Barrett’s Esophagus Score,110 which relies on length, sex, smoking status, and LGD, even the highest risk group only had an annual incidence of combined HGD or cancer of 1.5% in a large validation cohort,111 which is approximately only one-eighth that found in patients with confirmed LGD assigned to surveillance in the RCTs of EET for LGD (Table 6). This indicates that it may be difficult to find clinical risk factors beyond confirmed LGD that raise the risk of cancer enough to warrant EET in NDBE. While some patients with NDBE may express severe anxiety about the risk of neoplastic progression and initially state a preference for EET over surveillance, they should be counseled regarding the considerations outlined above, and the typical practice of continued surveillance even after successful EET. Thus, EET might only lead to temporary and incomplete decrease in the associated anxiety. The authors acknowledge that individuals who may be at increased risk of progression to cancer might be identified by tissue based biomarkers, particularly aberrant p53 or Tissue Systems Pathology Test-9 alone or in combination with clinical and endoscopic characteristics.28, 112-117 Whether such biomarkers should be routinely used in patients with NDBE, and how those results should be used is a topic that is deferred to the forthcoming AGA guideline on surveillance in BE.
| Recommendation 4: In patients undergoing EET, the AGA suggests resection of visible lesions followed by ablation of the remaining BE segment over resection of the entire BE segment. (conditional recommendation, very low-quality evidence) Implementation Consideration:
|
Summary of the evidence
Evidence informing this PICO question comes from a previously published systematic review of single arm observational cohort studies.118 In this systematic review, data from 20 studies were analyzed. There was only 1 randomized controlled trial directly comparing these 2 strategies.119 The RCT had enrolled 47 patients and showed no difference in the CEN, but the stenosis rate was significantly higher in stepwise or complete EMR (sEMR) (88%) versus focal EMR (fEMR) + RFA (14%). However, because of the limited sample size, it is not possible to extrapolate these findings on a larger scale; thus, the authors of the systematic review analyzed the results of the RCT with the observational studies. Nine single arm cohort studies reported on fEMR + RFA and 11 single arm cohort studies reported on sEMR; both are established strategies for eradication of BE-related HGD and/or EAC. In addition, we identified one larger study from the national Dutch database with long-term follow-up reporting on EET for BE neoplasia with fEMR + RFA.47 We also updated the systematic review for the harms. Thirty-one additional single arm studies were used to update the harms for fEMR + RFA and 2 studies for the s-EMR.
Demographics between the studies and the 2 interventions were similar. The follow-up period ranged from 12 to 61 months in the fEMR + RFA group and 15 to 54.7 months in the sEMR group. Reported BE length was 2 to 8 cm in the fEMR + RFA group and 2 to 5.5 cm in the s-EMR group. The fEMR + RFA intervention strategy was the same throughout the studies: all studies had initial focal EMR for a visible lesion followed by RFA. Serial RFA was done every 3 months until CEN and/or CEIM was achieved. For the s-EMR strategy, the protocols were different among the individual studies in terms of resections per session and the timing between the repeat endoscopies.
Benefits
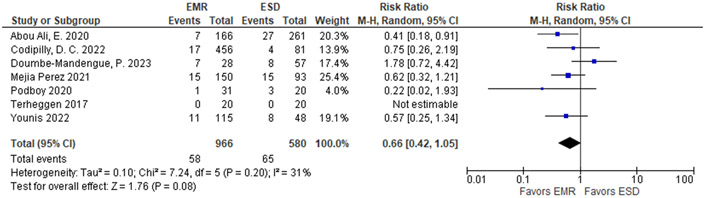
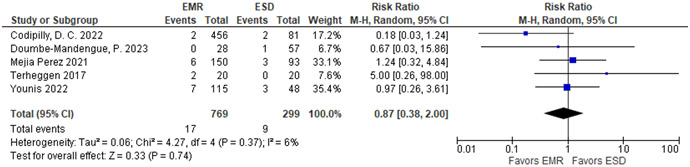
We considered 2 outcomes informing the benefits: (1) EAC at 1 to 2 year follow-up as a critical outcome, and (2) CEN as an important outcome. In the prior meta-analysis, a total of 701 patients in the sEMR vs 702 patients in the fEMR + RFA group showed no substantial difference in regard to EAC outcomes with a pooled estimate of 0.7% (95% CI: 0.1%, 1.4%), and 1.4% (95% CI: 0.2%, 2.7%), respectively, for a RR of 0.83 (95% CI: 0.36, 1.92) (Table 8).118 Similarly, there was no substantial difference in the pooled estimate for CEN, with 94.9% (95% CI: 92.2%, 97.5%) for sEMR compared to 93.4% (95% CI: 90.8%, 96.1%) for fEMR+RFA with RR of 1.01 (95% CI: 0.98, 1.04). The proportion achieving CEIM in the fEMR + RFA group was 73.1% (95% CI: 63.0%, 83.1%) and in the sEMR group was 79.6% (95% CI: 75.2%, 84.1%). Similar rates for recurrence of EAC were observed in the newer long-term follow up study for fEMR+ RFA: a total of 1,386 patients were followed over 43 months with 22 having progression or recurrence of EAC (1.6%; 95% CI: 1.1%, 2.4%).47
Table 8.
Grading of Recommendations Assessment, Development and Evaluation Evidence Profile for PICO Question 4: Comparing resection of visible lesions followed by ablation of the remaining BE segment with resection of the entire BE segment.
| Certainty assessment | № of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies |
Study design | Risk of bias |
Inconsistency | Indirectness | Imprecision | Other considerations |
sEMR | fEMR+RFA | Relative (95% CI) |
Absolute (95% CI) |
||
| EAC at 1-2 yrs (only 3 studies had follow up of more than 3 yrs); single arm comparison: sEMR (11 observational studies) vs. fEMR + RFA (9 observational studies) | ||||||||||||
| 20a | observational studies | seriousb | not serious | not serious | very seriousc | publication bias strongly suspectedd | 10/701 (1.4%) | 12/702 (1.7%) |
RR 0.83 (0.36 to 1.92) |
3 fewer per 1,000 (from 11 fewer to 16 more) |
⨁◯◯◯ Very low |
CRITICAL |
| Complete eradication of neoplasia at 1-2 yrs (only 3 studies had follow up of more than 3 yrs) indirect comparison: sEMR (11 observational studies) vs. fEMR + RFA (9 observational studies) | ||||||||||||
| 20a | observational studies | seriousb | not serious | seriouse | not serious | publication bias strongly suspectedd | 717/774 (92.6%) | 699/747 (93.6%) |
OR 1.33 (0.56 to 3.15) |
15 more per 1,000 (from 45 fewer to 43 more) |
⨁◯◯◯ Very low |
CRITICAL |
| Stricture | ||||||||||||
| 52f | observational studies | seriousg | not serioush | not serious | not serious | none | 269/840 (32.0%) | 585/13882 (4.2%) |
RR 7.33 (6.46 to 8.31) |
267 more per 1,000 (from 230 more to 308 more) |
⨁◯◯◯ Very low |
CRITICAL |
| Bleeding | ||||||||||||
| 32i | observational studies | seriousb | not serioush | not serious | seriousj | none | 59/840 (7.0%) | 53/5902 (0.9%) |
RR 7.82 (5.44 to 11.25) |
61 more per 1,000 (from 40 more to 92 more) |
⨁◯◯◯ Very low |
CRITICAL |
| Perforation | ||||||||||||
| 40k | observational studies | seriousb | not serious | not serious | seriousj | none | 13/840 (1.5%) | 16/5799 (0.3%) |
RR 5.62 (2.72 to 11.65) |
13 more per 1,000 (from 5 more to 29 more) |
⨁◯◯◯ Very low |
CRITICAL |
sEMR (11 observational studies) vs. fEMR + RFA (9 observational studies)
Comparison of independent single arm studies with no time concurrent controls
Very small event number in both treatment groups
Publication bias was noted by Desai et al using Eggers regression test for the sEMR studies
Indirectness suspected since the outcome is eradication of dysplasia and not recurrence of cancer or mortality from cancer
sEMR (12 observational studies) vs. fEMR + RFA (40 observational studies)
Comparison of independent single arm studies with no time concurrent controls. Additionally the sEMR intervention was not standardized and differed between studies; some studies had less resections per procedure and used steroid, while other studies had more resections per procedure.
Cannot assess for inconsistency because 2 treatment interventions are pooled from a single arm studies, but there was heterogeneity observed when the pooled estimate was calculate for each intervention separately.
sEMR (12 observational studies) vs. fEMR + RFA (20 observational studies)
low event number
sEMR (12 observational studies) vs. fEMR + RFA (28 observational studies)
Harms
Three critical outcomes were considered to inform harms: (1) stricture formation, (2) major bleeding, and (3) perforation. A total of 52 studies informed the outcome of stricture formation. There were 269 strictures in 840 patients undergoing sEMR and 585 strictures in 13,382 patients in the fEMR +RFA group for a pooled estimate of 30.4% (95% CI: 17.2%, 43.6%) vs 6.3% (95% CI: 5.0%, 7.6%), respectively (Supplementary Figures 4.1 and 6.1). When compared there was a substantial difference with sEMR more likely to cause a stricture (RR = 7.33; 95% CI 6.46, 8.31). Furthermore, the pooled estimate for major bleed events in the sEMR were 6.5% (95% CI: 3.5%, 9.4%), and in the fEMR +RFA were 0.6% (95% CI: 0.4%, 0.9%) (Supplement Figures 4.2 and 6.2), for a RR of 7.82 (95% CI: 5.44, 11.25). Lastly, there were 13 perforations out of 840 patients for a pooled estimate of 1.2% (95% CI: 0.5%, 2.0%) in the sEMR group, and 16 out of 5,799 patients for a pooled estimate of 0.2% (95% CI: 0.1%, 0.4%) in the fEMR + RFA group with RR of 5.62 (95% CI: 2.72, 11.65) (Supplement Figures 4.3 and 6.3).
Certainty of the Evidence
Across all the critical outcomes, the overall certainty was very low (Table 8). For the outcome of EAC there were multiple concerns regarding the certainty of evidence: (1) only a single RCT exists, (2) serious risk of bias because the comparison was of independent single arm studies with no concurrent controls, (3) very serious imprecision because of few events in both treatment groups, and (4) publication bias as noted by Desai et al,118 suggesting overestimation of CEN in the published sEMR studies. Furthermore, for the CEN outcome, there was indirectness since the outcome is eradication of neoplasia and not specifically cancer or mortality from cancer. Finally, for the harms, in addition to the imprecision due to low events, serious risk of bias was detected because the sEMR intervention was not standardized and differed between studies in terms of number of resections per procedure and whether prophylactic corticosteroids were used.
Discussion
Compared to fEMR followed by ablation, the effect of sEMR on the critical benefits were trivial to small and the effect on harms were moderate, both with very low certainty of evidence. There is probably no uncertainty or variability in how much patients value the main benefits and harms. Either form of EET is probably feasible and accessible, though some endoscopists who perform fEMR may not be adept at sEMR. There may be a moderate increase in resource utilization with sEMR due to the need for additional procedures for dilation of strictures, but there is very low certainty regarding this. The choice of one form of EET over another is unlikely to impact equity. Finally, there were no cost-effectiveness analyses available to guide the recommendation. On balance, the authors believed that focal resection of visible lesions followed by ablation of remaining BE is favored over sEMR, largely due to the likely greater risk of harms with sEMR.
Implementation Considerations
Regardless of the extent of nodularity, all nodularity should be resected rather than ablated. There was large heterogeneity in stricture rates following sEMR, which might be related to differences in techniques or patient populations. There was agreement that in settings of only a small area of remaining BE beyond the visible lesion resected, completion EMR requiring only one or a few additional resections in the same procedure is acceptable and may be preferred over repeating the procedure to perform ablation later, particularly if the additional resections are longitudinally adjacent to the prior resection bed rather than circumferentially.
Multiple ablation techniques exist, including RFA, cryoablation (including multiple different vendors, cryogenic gases, and tools), hybrid-argon plasma coagulation, and multi-polar electrocoagulation. The comparison and specific dosimetries of these techniques are beyond the scope of this guideline. However, RFA has the highest quality and most extensive evidence available from RCTs supporting its use for reaching the critical benefits of interest, while the other modalities have primarily been studied in case series or RCTs with only the important outcomes of CEIM and CEN, and often included mixed populations of EET-naïve and those with prior RFA.51, 83, 86 Therefore, RFA is the preferred ablative modality. Nonetheless, chest pain appears to be of shorter duration and less severity with cryoablation.73 Likewise, which specific technique of EMR used is beyond the scope of this document. Randomized controlled trials comparing various ablation techniques to each other and additional trials comparing EMR techniques to each other are needed.
| Recommendation 5: In individuals with BE with visible neoplastic lesions that are undergoing endoscopic resection, the AGA suggests the use of either EMR or ESD based on lesion characteristics (conditional recommendation, very-low quality evidence) Implementation Consideration:
|
Summary of the Evidence
Evidence informing the recommendation for endoscopic submucosal dissection (ESD) vs endoscopic mucosal resection (EMR) was derived from one RCT and observational cohort studies. No prior systematic review or meta-analysis was identified in our systematic search to answer this question. Thus, we conducted a new systematic review and a meta-analysis. We selected studies that included patients who underwent EET for a visible lesion with ESD or EMR followed by ablative therapy if needed. Once they achieved CEN or CEIM, patients were enrolled in surveillance. Studies that classified outcomes of patients prior to completion of sEMR or did not provide granular data were excluded. R0 resection was defined as absence of the highest-grade histology (HGD or EAC) at the lateral and deep margin on the initial procedure.
One RCT120 and 4 comparative observational cohort studies121-124 were included in the meta-analysis comparing EMR to ESD. The RCT120 included a total of 40 patients randomized to either ESD (20 patients) or EMR (20 patients). The mean age was 64.5 years and male predominant. The mean size of the ESD lesion was significantly larger than the EMR (29 mm vs 18 mm). The mean follow-up was 1.9 years. The 4 observational studies were 2 manuscripts121, 122 and 2 conference abstracts from 2022 −2023.123, 124 We contacted the authors of one of the abstracts to obtain more robust data. The mean age was 68-69 years with male predominance in 85-87%. The initial pathology varied between studies including only EAC (1 study),122 HGD and EAC (2 studies),123, 124 and all degrees of dysplasia (1 study).121 Younis et al. had significantly more EAC in the ESD group 85.2% compared to the EMR group (57.4%).124 Follow-up in these studies ranged between 2.3 years and 3.7 years. The EMR group follow-up was 2.8-3.7 years, whereas the ESD group follow-up was 1.4-2.3 years.
One RCT120 and 6 observational comparative cohort studies121, 122, 124-127 were included in the direct comparison for harm. Given the low number of events and very serious imprecision, we decided to explore data from single arm studies. A total of 42 ESD studies120-122, 124-161 and 32 EMR studies were included in the analysis for harm.
Benefits
The critical outcome for this question was EAC at 1 to 2 years after EET. The pooled analysis of 1 RCT120 and 4 observational comparative studies121-124 using random-effects models with a total of 391 participants in the EMR group vs 164 participants in the ESD group demonstrated no difference in EAC with RR of 0.93 (95% CI: 0.50, 1.72) (Figure 2.1).
Figure 2.1.
Forest plot of comparative studies comparing EAC at 1-2 years follow-up for patients treated with EMR vs ESD.
R0 resection was considered an important but not a critical outcome. Seven studies120-122, 125, 127, 162, 163 (1 RCT and 6 observational studies) were included in the direct comparison. 221/ 746 achieved R0 resection in the EMR group compared 478/601 in the ESD group with RR: 0.43 (95% CI: 0.29, 0.78) (Figure 2.2). For the outcome of CEN, three comparative cohort studies report on CEN, with a total of 472 subjects achieving CEN out of 599 subjects in the EMR group and 153 out of 186 subjects in the ESD group, resulting in a pooled RR of 0.93 (95% CI: 0.87, 1.00) (Figure 2.3). For the outcome of CEIM, 1 RCT and 3 comparative cohort studies were identified with a total of 408 out of 619 subjects in the EMR group achieving CEIM compared to 131 out of 206 subjects in the ESD group, RR = 1.06 (95% CI: 0.87, 1.00) (Figure 2.4).
Figure 2.2.
Forest plot of comparative studies comparing R0 resection among patients treated with EMR vs ESD.
Figure 2.3.
Forest plot of comparative studies comparing CEN among patients treated with EMR vs ESD.
Figure 2.4.
Forest plot of comparative studies comparing CEIM among patients treated with EMR vs ESD.
Harms
The patient-important outcomes that informed the harms for this PICO question were: (1) strictures, (2) major bleeding either requiring blood transfusion, intervention, or hospitalization and (3) perforation. A systematic review and meta-analyses were performed to estimate the risk of adverse events for ESD and EMR.
We included 38 studies in the meta-analysis reporting stricture formation following ESD. 361 out of 2,731 patients developed a stricture post esophageal ESD. The pooled proportion of stricture formation with ESD from single arm studies was 12.4% (95% CI: 9.6%, 15.2%) (Figure 3.1). Twenty-seven studies were included in the EMR single arm analysis. Out of 3,729 patients, 408 developed stricture post EMR. The pooled proportion of stricture formation with EMR was 9.1% (95% CI: 6.4%, 11.7%) (Supplementary Figure 7.1). In the indirect comparison, EMR was associated with fewer strictures compared to ESD (RR 0.83; 95% CI: 0.72, 0.95). Furthermore, there were 1 RCT120 and 6 observational comparative studies121, 122, 124-127 in the direct comparison of stricture formation after ESD and EMR. Fifty-eight of 966 developed stricture post EMR compared to 65 of 580 in the post ESD group with RR 0.66 (95% CI: 0.42, 1.05) (Figure 3.2).
Figure 3.1.
Forest plot of pooled proportion of stricture formation among patients with BE and visible lesion treated with ESD.
Figure 3.2.
Forest plot of direct comparative studies comparing stricture formation in patients with BE and visible lesion treated with EMR vs ESD.
We included 32 studies in the single arm analysis of significant bleeding post ESD. Significant bleeding was found in 64 of 2,589 patients after ESD with pooled proportion of 1.8% (95% CI: 1.3%, 2.3%) (Figure 4.1). We included 20 studies in the single arm analysis of significant bleeding post EMR. Significant bleeding was found in 39 of 2,061 patients after EMR with pooled proportion of 1.5% (95% CI: 0.8%, 2.1%) (Supplementary Figure 7.2). In the indirect comparison, there was no significant difference in bleeding events (EMR vs ESD RR = 0.77; 95% CI: 0.52, 1.14). There were 5 studies in the direct comparison (1 RCT120 and 4 observational studies 122, 124, 125, 127); there was no significant difference in bleeding with 17 of 769 in the EMR group and 9 of 299 in the ESD (RR = 0.87; 95% CI: 0.38, 2.00) (Figure 4.2). We included 33 single arm studies assessing perforation post ESD: 46 out of 2,644 patients developed perforation post ESD with pooled proportion of 1.1% (95% CI: 0.7%, 1.5%) (Figure 5.1). We included 27 single arm studies assessing perforation after EMR: 16 out of 5,799 patients developed perforation post EMR (pooled proportion = 0.34%; 95% CI: 0.19%, 0.58%) (Supplementary Figure 7.3). In the indirect comparison, EMR was associated with fewer perforations than ESD (RR = 0.34; 95% CI: 0.20, 0.60). Additionally, there were 1 RCT120 and 5 observational121, 122, 124, 125, 127 studies in the direct comparison analysis. Seven out of 800 patients developed perforation with EMR compared to 5 of 319 patients in the ESD with RR of 0.93 (95% CI: 0.16, 5.41) (Figure 5.2).
Figure 4.1.
Forest plot of pooled proportion of major bleeding events among patients with BE and visible lesion treated with ESD.
Figure 4.2.
Forest plot of direct comparative studies comparing major bleeding in patients with BE and visible lesion treated with EMR vs ESD.
Figure 5.1.
Forest plot of pooled proportion of perforation among patients with BE and visible lesion treated with ESD.
Figure 5.2.
Forest plot of direct comparative studies comparing perforation in patients with BE and visible lesion treated with EMR vs ESD.
Certainty in Evidence of Effects
The overall certainty in the evidence across the critical outcomes with consideration of both benefits and harms was very low (Table 9). Our certainty in the critical outcome of EAC was very low. The major concern for the EAC outcome when treated with EET was imprecision given the low number of events. There was also concern about the risk of bias in observational studies. For the outcome of adverse events, the quality of evidence was also very low. Data from non-randomized studies was very low in certainty due to serious risk of bias in observational studies due to a comparison of independent single arm studies with no time concurrent controls. Stricture formation was considered the most common harm. There was significant heterogeneity in reported stricture formation in the single arm studies (I2: 86.57% in the ESD studies and 88.25% in the EMR studies).
Discussion
There was considerable discussion among the panel regarding the evidence to support this recommendation. For the critical outcome of EAC, there was no difference. For the important outcome of CEN, only observational studies were available. The results of the meta-analysis were heavily influenced by the largest study, which was a single-center retrospective study reporting overall greater CEN in ESD than in EMR, but also reported improvement in CEN with EMR over time, and those authors found no difference in CEN in sub-analyses comparing ESD to EMR performed during the later time period.125 The other 2 studies in the CEN meta-analysis had point estimates near the null. For the important outcome of CEIM, there was no difference between EMR and ESD, including in the one available RCT. R0 resection was achieved to a greater degree with ESD when compared to EMR. Harms were deemed moderately greater with ESD compared to EMR. There is possibly important variability in how patients may value the relative importance of the various outcomes. ESD is expected to be associated with increased resource utilization since many patients currently are hospitalized following ESD. ESD also requires longer procedure duration and utilization of more devices than EMR. To our knowledge, there are no cost-effectiveness analyses comparing these strategies. Since few providers are trained to competently perform ESD and the learning curve is steep, particularly in the esophagus, and potentially appropriate cases relatively uncommon, widespread implementation of ESD faces substantial barriers to being feasible164 and would likely exacerbate healthcare inequalities. Although ESD is probably acceptable to patients, the relative acceptability compared to EMR has not been well studied. Similar to the comparison of sEMR to fEMR followed by ablation, the authors believed that EMR can be utilized in the vast majority of patients with visible neoplastic lesions and decision to perform ESD may be made based on characteristics of visible neoplastic lesions.
Implementation Considerations
Patients found to have T1 EAC, particularly T1a, may be successfully cured with endoscopic resection if EET is completed to CEN/CEIM and endoscopic surveillance continues. Of note, endoscopic ultrasound is inaccurate for distinguishing T1a from T1b, and to a lesser degree from T2 cancers, so patients suspected endoscopically of T1 cancer should undergo endoscopic resection for tumor depth staging.165 Obstructive or ulcerated lesions are unlikely to represent T1 disease and can forego endoscopic resection. Factors on endoscopic resection associated with favorable prognosis with EET alone include negative deep margin, T1a depth, moderate or well differentiation, and lack of lymphovascular invasion.166-169 Patients not meeting any of those criteria, or those with endosonographic or cross-sectional imaging evidence suggestive of metastases should undergo multi-disciplinary consultation to consider esophagectomy, chemotherapy and/or radiation depending on stage and functional status.
There was substantial uncertainty with regards to the potential benefit of ESD compared to EMR. Guidelines from some other societies have suggested or recommended ESD, particularly in individuals with large lesions.170 The studies assessing EAC outcomes after EET had relatively short durations of follow-up. The patients undergoing ESD in the observational studies tended to have larger lesions, which may have greater risk of technical failure with EMR or progression, but there was insufficient data to perform analyses stratified by lesion size. Since the large difference in R0 rates did not translate to improvements in CEIM or EAC after EET, the R0 resection appears to be of minor importance. Patients undergoing EMR requiring more than one resection during the procedure can have technically successful resections of overlapping, contiguous pieces. Histologically, this will necessarily result in a positive lateral margin on each neighboring piece. Much more important is the deep margin. ESD may hold advantages over EMR for ensuring negative deep margins, but the studies included did not address this. EMR should be sufficient for T1a lesions, and ESD may be more effective for T1b lesions, but one does not know the depth of penetration until after the resection. Certain endoscopic features, including larger size, but more importantly depressed lesions (Paris IIc or IIa+c), may be suggestive of a more deeply invasive lesion, and hence might be preferentially referred for ESD.171 Some prior guidelines have suggested a size threshold above which ESD should be favored over EMR, but this appears to be based on the technical limits of R0 lateral margins with EMR rather than for longitudinal cancer control outcomes. The guideline authors attempted to perform analyses stratified by size of lesions, but the available published data was not presented in a manner that allowed for such analyses. In addition, bulky sessile lesions, even if T1a, may be technically difficult to resect with EMR due to limitations of the cap size. Future randomized controlled studies are needed to demonstrate whether ESD has improved outcomes in such populations (aside from R0 resection) that is worth the added harms and barriers to implementation.131
Knowledge Gaps
These evidence reviews identified several important knowledge gaps that future research should address. These are detailed in the discussions of the individual recommendations. In summary, regarding patient selection for EET, further research is needed to understand the balance of risks and benefits in patients with BE and LGD and identifying if there are populations with NDBE whose risks of EAC warrant EET. Randomized controlled trials are needed comparing ESD and EMR in higher risk populations assessing outcomes of critical importance including long-term cancer control. For management of patients during EET, research is needed to identify optimal control of post-EET pain, stricture prevention, management of resistant/recurrent disease beyond reflux control. For management of patients following EET, better data is needed to identify optimal surveillance intervals and biopsy protocols, and under what circumstances to discontinue endoscopic surveillance following completion of EET, which would likely depend on index histology, age, and comorbidities.
Plans for Updating
Considerable resources are expended for the development of guidelines, and keeping guidelines up to date is a challenging process. Future update of this guideline will depend on the availability of new evidence on the existing interventions and new intervention. We hope to incorporate the advances in the technological platforms and models of guideline development in the future updates without duplication or reproduction of the current guideline document.
Supplementary Material
Acknowledgement:
We would like to thank Ms. Tran Nguyen, Western University for designing and conducting the literature searches.
Funding:
JHR’s effort was funded in part by the National Institutes of Health (U54CA163059, R01CA238433, U01DK129191), Department of Defense (W81XWH2010898), and Veterans Administration Health Services and Research Division (RVR 19-470). SE’s effort was funded in part by the National Institutes of Health (K23DK12338). SW was supported by the National Institute of Health (1U01DK129191, 5U01CA271867-02) and the Katy O and Paul M Rady Endowed Chair in Esophageal Cancer Research
Abbreviations:
- AGA
American Gastroenterological Association
- BE
Barrett’s esophagus
- CEIM
complete eradication of intestinal metaplasia
- CEN
complete eradication of neoplasia
- EAC
esophageal adenocarcinoma
- EET
endoscopic eradication therapy
- EMR
endoscopic mucosal resection
- ESD
endoscopic submucosal dissection
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- HGD
high-grade dysplasia
- LGD
low-grade dysplasia
- ND
non-dysplastic
- PICO
Population, Intervention, Comparison, Outcome
- RCT
randomized controlled trial
- RFA
radiofrequency ablation
Footnotes
Potential Conflicts of Interest:
JHR has received research funding from Lucid Diagnostics. DK has received funding for consultation for Medtronic. Shailendra Singh has received funding for consultation from Boston Scientific and Fujifilm Endoscopy. SW has received funding for consultation to Exact Sciences, Lucid, Enterotracker and research support from Lucid, CDx Diagnostics and Exact Sciences. Siddharth Singh has received research funding from Pfizer. SS, AKC, AP, TS, APT, SE, RP, PD, and JMI had no conflicts of interest.
REFERENCES
- 1.Rodriguez GM, DePuy D, Aljehani M, et al. Trends in Epidemiology of Esophageal Cancer in the US, 1975-2018. JAMA Network Open 2023;6:e2329497–e2329497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Cancer Institute. Surveillance, Epidemiology and End Results (SEER) Program. Cancer Statistics Explorer Network: Adenocarcinoma of the Esophagus Survival: https://seer.cancer.gov/statistics-network/explorer/application.html?site=600&data_type=4&graph_type=5&compareBy=age_range&chk_age_range_1=1&series=9&sex=1&race=1&stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0, accessed January 16, 2023. [Google Scholar]
- 3.Curtius K, Rubenstein JH, Chak A, et al. Computational modelling suggests that Barrett’s oesophagus may be the precursor of all oesophageal adenocarcinomas. Gut 2020;70:1435–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black EL, Ococks E, Devonshire G, et al. Understanding the malignant potential of gastric metaplasia of the oesophagus and its relevance to Barrett's oesophagus surveillance: individual-level data analysis. Gut 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pech O, Bollschweiler E, Manner H, et al. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett's esophagus at two high-volume centers. Ann Surg 2011;254:67–72. [DOI] [PubMed] [Google Scholar]
- 6.Schembre DB, Huang JL, Lin OS, et al. Treatment of Barrett's esophagus with early neoplasia: a comparison of endoscopic therapy and esophagectomy. Gastrointest Endosc 2008;67:595–601. [DOI] [PubMed] [Google Scholar]
- 7.Zehetner J, DeMeester SR, Hagen JA, et al. Endoscopic resection and ablation versus esophagectomy for high-grade dysplasia and intramucosal adenocarcinoma. J Thorac Cardiovasc Surg 2011;141:39–47. [DOI] [PubMed] [Google Scholar]
- 8.Graham R, Mancher M, Wolman DM, et al. Institute of Medicine Committee on Standards for Developing Trustworthy Clinical Practice Guidelines: Clinical Practice Guidelines We Can Trust: National Academies Press, 2011. [PubMed] [Google Scholar]
- 9.Schünemann HJ, Al-Ansary LA, Forland F, et al. Guidelines International Network: Principles for Disclosure of Interests and Management of Conflicts in Guidelines. Ann Intern Med 2015;163:548–53. [DOI] [PubMed] [Google Scholar]
- 10.Schünemann HJ, Wiercioch W, Etxeandia I, et al. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. Canadian Medical Association Journal 2014;186:E123–E142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology 2011;140:1084–91. [DOI] [PubMed] [Google Scholar]
- 12.Wani S, Rubenstein JH, Vieth M, et al. Diagnosis and Management of Low-Grade Dysplasia in Barrett's Esophagus: Expert Review From the Clinical Practice Updates Committee of the American Gastroenterological Association. Gastroenterology 2016;151:822–835. [DOI] [PubMed] [Google Scholar]
- 13.Draganov PV, Wang AY, Othman MO, et al. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin Gastroenterol Hepatol 2019;17:16–25.e1. [DOI] [PubMed] [Google Scholar]
- 14.Sharma P, Shaheen NJ, Katzka D, et al. AGA Clinical Practice Update on Endoscopic Treatment of Barrett’s Esophagus With Dysplasia and/or Early Cancer: Expert Review. Gastroenterology 2020;158:760–769. [DOI] [PubMed] [Google Scholar]
- 15.Muthusamy VR, Wani S, Gyawali CP, et al. AGA Clinical Practice Update on New Technology and Innovation for Surveillance and Screening in Barrett's Esophagus: Expert Review. Clin Gastroenterol Hepatol 2022;20:2696–2706.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan—a web and mobile app for systematic reviews. Systematic Reviews 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64:383–394. [DOI] [PubMed] [Google Scholar]
- 19.Alonso-Coello P, Schünemann HJ, Moberg J, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ 2016;353:i2016. [DOI] [PubMed] [Google Scholar]
- 20.Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. Journal of the National Cancer Institute 2010;102:1344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thrift AP, Shaheen NJ, Gammon MD, et al. Obesity and Risk of Esophageal Adenocarcinoma and Barrett’s Esophagus: A Mendelian Randomization Study. JNCI Journal of the National Cancer Institute 2014;106:dju252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian J, Zuo C, Liu G, et al. Cumulative evidence for the relationship between body mass index and the risk of esophageal cancer: An updated meta-analysis with evidence from 25 observational studies. Journal of Gastroenterology & Hepatology 2020;35:730–743. [DOI] [PubMed] [Google Scholar]
- 23.Solaymani-Dodaran M, Card TR, West J. Cause-specific mortality of people with Barrett's esophagus compared with the general population: a population-based cohort study. Gastroenterology 2013;144:1375–83, 1383.e1. [DOI] [PubMed] [Google Scholar]
- 24.Erichsen R, Horvath-Puho E, Lund JL, et al. Mortality and cardiovascular diseases risk in patients with Barrett's oesophagus: a population-based nationwide cohort study. Alimentary Pharmacology & Therapeutics 2017;45:973–982. [DOI] [PubMed] [Google Scholar]
- 25.Wolf WA, Pasricha S, Cotton C, et al. Incidence of Esophageal Adenocarcinoma and Causes of Mortality After Radiofrequency Ablation of Barrett's Esophagus. Gastroenterology 2015;149:1752–1761.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis JJ, Rubenstein JH, Singal AG, et al. Factors associated with esophageal stricture formation after endoscopic mucosal resection for neoplastic Barrett's esophagus. Gastrointest Endosc 2011;74:753–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vennalaganti P, Kanakadandi V, Goldblum JR, et al. Discordance Among Pathologists in the United States and Europe in Diagnosis of Low-Grade Dysplasia for Patients With Barrett's Esophagus. Gastroenterology 2017;152:564–570.e4. [DOI] [PubMed] [Google Scholar]
- 28.Khoshiwal AM, Frei NF, Pouw RE, et al. The Tissue Systems Pathology Test Outperforms Pathology Review in Risk Stratifying Patients With Low-Grade Dysplasia. Gastroenterology 2023. [DOI] [PubMed] [Google Scholar]
- 29.Davis C, Fuller A, Katzka D, et al. High Proportions of Newly Detected Visible Lesions and Pathology Grade Change Among Patients with Barrett's Esophagus Referred to Expert Centers. Dig Dis Sci 2023;68:3584–3595. [DOI] [PubMed] [Google Scholar]
- 30.Noordzij IC, Van Loon van de Ende MCM, Curvers WL, et al. Dysplasia in Random Biopsies from Barrett's Surveillance Is an Important Marker for More Severe Pathology. Dig Dis Sci 2021;66:1957–1964. [DOI] [PubMed] [Google Scholar]
- 31.Nieuwenhuis EA, van Munster SN, Curvers WL, et al. Impact of expert center endoscopic assessment of confirmed low grade dysplasia in Barrett's esophagus diagnosed in community hospitals. Endoscopy 2022;54:936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsoi EH, Mahindra P, Cameron G, et al. Barrett's esophagus with low-grade dysplasia: high rate of upstaging at Barrett's esophagus referral units suggests progression rates may be overestimated. Gastrointest Endosc 2021;94:902–908. [DOI] [PubMed] [Google Scholar]
- 33.Pasricha S, Cotton C, Hathorn KE, et al. Effects of the Learning Curve on Efficacy of Radiofrequency Ablation for Barrett’s Esophagus. Gastroenterology 2015;149:890–896.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fudman DI, Lightdale CJ, Poneros JM, et al. Positive correlation between endoscopist radiofrequency ablation volume and response rates in Barrett's esophagus. Gastrointestinal Endoscopy;80:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan MC, Kanthasamy KA, Yeh AG, et al. Factors Associated With Recurrence of Barrett’s Esophagus After Radiofrequency Ablation. Clinical Gastroenterology and Hepatology 2019;17:65–72.e5. [DOI] [PubMed] [Google Scholar]
- 36.Markar SR, Mackenzie H, Ni M, et al. The influence of procedural volume and proficiency gain on mortality from upper GI endoscopic mucosal resection. Gut 2018;67:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savarino E, Zentilin P, Frazzoni M, et al. Characteristics of gastro-esophageal reflux episodes in Barrett's esophagus, erosive esophagitis and healthy volunteers. Neurogastroenterology & Motility;22:1061–e280. [DOI] [PubMed] [Google Scholar]
- 38.Gutschow CA, Bludau M, Vallböhmer D, et al. NERD, GERD, and Barrett’s Esophagus: Role of Acid and Non-acid Reflux Revisited with Combined pH-Impedance Monitoring. Digestive Diseases and Sciences 2008;53:3076–3081. [DOI] [PubMed] [Google Scholar]
- 39.Dvorak K, Fass R, Dekel R, et al. Esophageal acid exposure at pH < or = 2 is more common in Barrett's esophagus patients and is associated with oxidative stress. Diseases of the Esophagus;19:366–72. [DOI] [PubMed] [Google Scholar]
- 40.Brandt MG, Darling GE, Miller L. Symptoms, acid exposure and motility in patients with Barrett's esophagus. Can J Surg 2004;47:47–51. [PMC free article] [PubMed] [Google Scholar]
- 41.Krishnan K, Pandolfino JE, Kahrilas PJ, et al. Increased risk for persistent intestinal metaplasia in patients with Barrett's esophagus and uncontrolled reflux exposure before radiofrequency ablation. Gastroenterology 2012;143:576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akiyama J, Marcus SN, Triadafilopoulos G. Effective intra-esophageal acid control is associated with improved radiofrequency ablation outcomes in Barrett's esophagus. Dig Dis Sci 2012;57:2625–32. [DOI] [PubMed] [Google Scholar]
- 43.Komanduri S, Kahrilas PJ, Krishnan K, et al. Recurrence of Barrett's Esophagus is Rare Following Endoscopic Eradication Therapy Coupled With Effective Reflux Control. Am J Gastroenterol 2017;112:556–566. [DOI] [PubMed] [Google Scholar]
- 44.Manner H, Rabenstein T, Pech O, et al. Ablation of residual Barrett's epithelium after endoscopic resection: a randomized long-term follow-up study of argon plasma coagulation vs. surveillance (APE study). Endoscopy 2014;46:6–12. [DOI] [PubMed] [Google Scholar]
- 45.Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut 2008;57:1200–6. [DOI] [PubMed] [Google Scholar]
- 46.Eluri S, Earasi AG, Moist SE, et al. Prevalence and Incidence of Intestinal Metaplasia and Dysplasia of Gastric Cardia in Patients With Barrett's Esophagus After Endoscopic Therapy. Clin Gastroenterol Hepatol 2020;18:82–88.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Munster S, Nieuwenhuis E, Weusten B, et al. Long-term outcomes after endoscopic treatment for Barrett's neoplasia with radiofrequency ablation ± endoscopic resection: results from the national Dutch database in a 10-year period. Gut 2022;71:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wani S, Muthusamy VR, Shaheen NJ, et al. Development of Quality Indicators for Endoscopic Eradication Therapies in Barrett's Esophagus: The TREAT-BE (Treatment With Resection and Endoscopic Ablation Techniques for Barrett's Esophagus) Consortium. Am J Gastroenterol 2017;112:1032–1048. [DOI] [PubMed] [Google Scholar]
- 49.Standards of Practice C, Wani S, Qumseya B, et al. Endoscopic eradication therapy for patients with Barrett's esophagus-associated dysplasia and intramucosal cancer. Gastrointest Endosc 2018;87:907–931 e9. [DOI] [PubMed] [Google Scholar]
- 50.Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett's esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc 2005;62:488–98. [DOI] [PubMed] [Google Scholar]
- 51.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency Ablation in Barrett's Esophagus with Dysplasia. N Engl J Med 2009;360:2277–2288. [DOI] [PubMed] [Google Scholar]
- 52.Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett's esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc 2008;67:394–8. [DOI] [PubMed] [Google Scholar]
- 53.Qumseya BJ, Wani S, Desai M, et al. Adverse Events After Radiofrequency Ablation in Patients With Barrett's Esophagus: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2016;14:1086–1095 e6. [DOI] [PubMed] [Google Scholar]
- 54.Agarwal S, Alshelleh M, Scott J, et al. Comparative outcomes of radiofrequency ablation and cryoballoon ablation in dysplastic Barrett's esophagus: a propensity score-matched cohort study. Gastrointest Endosc 2022;95:422–431 e2. [DOI] [PubMed] [Google Scholar]
- 55.Bahin FF, Jayanna M, Hourigan LF, et al. Long-term outcomes of a primary complete endoscopic resection strategy for short-segment Barrett's esophagus with high-grade dysplasia and/or early esophageal adenocarcinoma. Gastrointest Endosc 2016;83:68–77. [DOI] [PubMed] [Google Scholar]
- 56.Belghazi K, van Vilsteren FGI, Weusten B, et al. Long-term follow-up results of stepwise radical endoscopic resection for Barrett's esophagus with early neoplasia. Gastrointest Endosc 2018;87:77–84. [DOI] [PubMed] [Google Scholar]
- 57.Cameron GR, Desmond PV, Jayasekera CS, et al. Recurrent intestinal metaplasia at the gastroesophageal junction following endoscopic eradication of dysplastic Barrett's esophagus may not be benign. Endosc Int Open 2016;4:E849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Canto MI, Trindade AJ, Abrams J, et al. Multifocal Cryoballoon Ablation for Eradication of Barrett's Esophagus-Related Neoplasia: A Prospective Multicenter Clinical Trial. Am J Gastroenterol 2020;115:1879–1890. [DOI] [PubMed] [Google Scholar]
- 59.Kahn A, Priyan H, Dierkhising RA, et al. Outcomes of radiofrequency ablation by manual versus self-sizing circumferential balloon catheters for the treatment of dysplastic Barrett's esophagus: a multicenter comparative cohort study. Gastrointest Endosc 2021;93:880–887 e1. [DOI] [PubMed] [Google Scholar]
- 60.Kaul V, Bittner K, Ullah A, et al. Liquid nitrogen spray cryotherapy-based multimodal endoscopic management of dysplastic Barrett's esophagus and early esophageal neoplasia: retrospective review and long-term follow-up at an academic tertiary care referral center. Dis Esophagus 2020;33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knabe M, Wetzka J, Welsch L, et al. Radiofrequency ablation versus hybrid argon plasma coagulation in Barrett’s esophagus: a prospective randomised trial. Surgical Endoscopy 2023;37:7803–7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kobayashi R, Calo NC, Marcon N, et al. Predictors of recurrence of dysplasia or cancer in patients with dysplastic Barrett's esophagus following complete eradication of dysplasia: a single-center retrospective cohort study. Surg Endosc 2022;36:5041–5048. [DOI] [PubMed] [Google Scholar]
- 63.Koutsoumpas A, Wang LM, Bailey AA, et al. Non-radical, stepwise complete endoscopic resection of Barrett's epithelium in short segment Barrett's esophagus has a low stricture rate. Endosc Int Open 2016;4:E1292–E1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krajciova J, Janicko M, Falt P, et al. Radiofrequency Ablation in Patients with Barrett's Esophagus- related Neoplasia - Long-Term Outcomes in the Czech National Database. J Gastrointestin Liver Dis 2019;28:149–155. [DOI] [PubMed] [Google Scholar]
- 65.Li N, Pasricha S, Bulsiewicz WJ, et al. Effects of preceding endoscopic mucosal resection on the efficacy and safety of radiofrequency ablation for treatment of Barrett's esophagus: results from the United States Radiofrequency Ablation Registry. Dis Esophagus 2016;29:537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Phoa KN, Pouw RE, Bisschops R, et al. Multimodality endoscopic eradication for neoplastic Barrett oesophagus: results of an European multicentre study (EURO-II). Gut 2016;65:555–62. [DOI] [PubMed] [Google Scholar]
- 67.Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett's esophagus with early neoplasia. Clin Gastroenterol Hepatol 2010;8:23–9. [DOI] [PubMed] [Google Scholar]
- 68.Tan WK, Ragunath K, White JR, et al. Standard versus simplified radiofrequency ablation protocol for Barrett's esophagus: comparative analysis of the whole treatment pathway. Endosc Int Open 2020;8:E189–E195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.White JR, Ortiz-Fernandez-Sordo J, Santiago-Garcia J, et al. Endoscopic management of Barrett's dysplasia and early neoplasia: efficacy, safety and long-term outcomes in a UK tertiary centre. Eur J Gastroenterol Hepatol 2021;33:e413–e422. [DOI] [PubMed] [Google Scholar]
- 70.Wolfson P, Ho KMA, Wilson A, et al. Endoscopic eradication therapy for Barrett's esophagus-related neoplasia: a final 10-year report from the UK National HALO Radiofrequency Ablation Registry. Gastrointest Endosc 2022;96:223–233. [DOI] [PubMed] [Google Scholar]
- 71.Vliebergh JH, Deprez PH, de Looze D, et al. Efficacy and safety of radiofrequency ablation of Barrett's esophagus in the absence of reimbursement: a multicenter prospective Belgian registry. Endoscopy 2019;51:317–325. [DOI] [PubMed] [Google Scholar]
- 72.Quine MA, Bell GD, McCloy RF, et al. Prospective audit of perforation rates following upper gastrointestinal endoscopy in two regions of England. Br J Surg 1995;82:530–3. [DOI] [PubMed] [Google Scholar]
- 73.van Munster SN, Overwater A, Haidry R, et al. Focal cryoballoon versus radiofrequency ablation of dysplastic Barrett’s esophagus: impact on treatment response and postprocedural pain. Gastrointestinal Endoscopy 2018;88:795–803.e2. [DOI] [PubMed] [Google Scholar]
- 74.Solomon SS, Kothari S, Smallfield GB, et al. Liquid Nitrogen Spray Cryotherapy is Associated With Less Postprocedural Pain Than Radiofrequency Ablation in Barrett's Esophagus: A Multicenter Prospective Study. Journal of Clinical Gastroenterology 2019;53:e84–e90. [DOI] [PubMed] [Google Scholar]
- 75.Overwater A, Elias SG, Schoon EJ, et al. The course of pain and dysphagia after radiofrequency ablation for Barrett's esophagus-related neoplasia. Endoscopy 2023;55:255–260. [DOI] [PubMed] [Google Scholar]
- 76.Rubenstein JH, Inadomi JM. Cost-Effectiveness of Screening, Surveillance, and Endoscopic Eradication Therapies for Managing the Burden of Esophageal Adenocarcinoma. Gastrointest Endosc Clin N Am 2021;31:77–90. [DOI] [PubMed] [Google Scholar]
- 77.Cotton CC, Haidry R, Thrift AP, et al. Development of Evidence-Based Surveillance Intervals After Radiofrequency Ablation of Barrett's Esophagus. Gastroenterology 2018;155:316–326.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gupta M, Iyer PG, Lutzke L, et al. Recurrence of Esophageal Intestinal Metaplasia After Endoscopic Mucosal Resection and Radiofrequency Ablation of Barrett's Esophagus: Results From a US Multicenter Consortium. Gastroenterology;145:79–86.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cotton CC, Shaheen NJ, Thrift AP. Surveillance After Treatment of Barrett's Esophagus Benefits Those With High-Grade Dysplasia or Intramucosal Cancer Most. Am J Gastroenterol 2022;117:1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Omar M, Thaker AM, Wani S, et al. Anatomic location of Barrett’s esophagus recurrence after endoscopic eradication therapy: development of a simplified surveillance biopsy strategy. Gastrointestinal Endoscopy 2019;90:395–403. [DOI] [PubMed] [Google Scholar]
- 81.Sami SS, Ravindran A, Kahn A, et al. Timeline and location of recurrence following successful ablation in Barrett’s oesophagus: an international multicentre study. Gut 2019;68:1379–1385. [DOI] [PubMed] [Google Scholar]
- 82.Qumseya BJ, Wani S, Gendy S, et al. Disease Progression in Barrett's Low-Grade Dysplasia With Radiofrequency Ablation Compared With Surveillance: Systematic Review and Meta-Analysis. Am J Gastroenterol 2017;112:849–865. [DOI] [PubMed] [Google Scholar]
- 83.Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014;311:1209–17. [DOI] [PubMed] [Google Scholar]
- 84.Mishra A CW, Morales SJ et al. A Tertiary Care Center's Experience With Radiofrequency Ablation in Esophageal Low-Grade Dysplasia. Gastroenterology 2015;148. [Google Scholar]
- 85.Small AJ, Araujo JL, Leggett CL, et al. Radiofrequency Ablation Is Associated With Decreased Neoplastic Progression in Patients With Barrett's Esophagus and Confirmed Low-Grade Dysplasia. Gastroenterology 2015;149:567–76 e3; quiz e13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barret M, Pioche M, Terris B, et al. Endoscopic radiofrequency ablation or surveillance in patients with Barrett's oesophagus with confirmed low-grade dysplasia: a multicentre randomised trial. Gut 2021;70:1014–1022. [DOI] [PubMed] [Google Scholar]
- 87.Cotton CC, Wolf WA, Overholt BF, et al. Late Recurrence of Barrett's Esophagus After Complete Eradication of Intestinal Metaplasia is Rare: Final Report From Ablation in Intestinal Metaplasia Containing Dysplasia Trial. Gastroenterology 2017;153:681–688 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dbouk M SM, Wang B, et al. Durability of Cryoballoon Ablation in Neoplastic Barrett's Esophagus. Techniques and Innovations in Gastrointestinal Endoscopy 2022;24:136–144. [Google Scholar]
- 89.García-Marín AV, Ortiz-Fernández-Sordo J, White J, et al. PTU-049 RFA for barrett’s low grade dysplasia: results of the 1st cohort treated in east midlands. Gut 2019;68. [Google Scholar]
- 90.Kahn A, Al-Qaisi M, Kommineni VT, et al. Longitudinal outcomes of radiofrequency ablation versus surveillance endoscopy for Barrett's esophagus with low-grade dysplasia. Dis Esophagus 2018;31. [DOI] [PubMed] [Google Scholar]
- 91.Pouw RE, Klaver E, Phoa KN, et al. Radiofrequency ablation for low-grade dysplasia in Barrett's esophagus: long-term outcome of a randomized trial. Gastrointest Endosc 2020;92:569–574. [DOI] [PubMed] [Google Scholar]
- 92.Wani S, Han S, Kushnir V, et al. Recurrence Is Rare Following Complete Eradication of Intestinal Metaplasia in Patients With Barrett's Esophagus and Peaks at 18 Months. Clin Gastroenterol Hepatol 2020;18:2609–2617 e2. [DOI] [PubMed] [Google Scholar]
- 93.Singh S, Manickam P, Amin AV, et al. Incidence of esophageal adenocarcinoma in Barrett's esophagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointestinal Endoscopy 2014;79:897–909.e4. [DOI] [PubMed] [Google Scholar]
- 94.Singh S, Manickam P, Amin AV, et al. Incidence of esophageal adenocarcinoma in Barrett's esophagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointest Endosc 2014;79:897–909 e4; quiz 983 e1, 983 e3. [DOI] [PubMed] [Google Scholar]
- 95.Omidvari A-H, Ali A, Hazelton WD, et al. Optimizing Management of Patients With Barrett’s Esophagus and Low-Grade or No Dysplasia Based on Comparative Modeling. Clinical Gastroenterology and Hepatology 2020;18:1961–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sharma P, Falk GW, Weston AP, et al. Dysplasia and cancer in a large multicenter cohort of patients with Barrett's esophagus. Clin Gastroenterol Hepatol 2006;4:566–72. [DOI] [PubMed] [Google Scholar]
- 97.Thota PN, Lee H-J, Goldblum JR, et al. Risk Stratification of Patients With Barrett's Esophagus and Low-grade Dysplasia or Indefinite for Dysplasia. Clinical Gastroenterology and Hepatology 2015;13:459–465.e1. [DOI] [PubMed] [Google Scholar]
- 98.Bellizzi AM, Odze RD. Histopathology of Barrett's esophagus: A review for the practicing gastroenterologist. Techniques in Gastrointestinal Endoscopy 2010;12:69–81. [Google Scholar]
- 99.Tolone S, Limongelli P, Romano M, et al. The patterns of reflux can affect regression of non-dysplastic and low-grade dysplastic Barrett's esophagus after medical and surgical treatment: a prospective case-control study. Surg Endosc 2015;29:648–57. [DOI] [PubMed] [Google Scholar]
- 100.Rossi M, Barreca M, de Bortoli N, et al. Efficacy of Nissen fundoplication versus medical therapy in the regression of low-grade dysplasia in patients with Barrett esophagus: a prospective study. Ann Surg 2006;243:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Desai TK, Krishnan K, Samala N, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett’s oesophagus: a meta-analysis. Gut 2012;61:970–6. [DOI] [PubMed] [Google Scholar]
- 102.Smith ZL, Thorgerson AM, Dawson AZ, et al. Incidence of Esophageal Adenocarcinoma, Mortality, and Esophagectomy in Barrett’s Esophagus Patients Undergoing Endoscopic Eradication Therapy. Digestive Diseases and Sciences 2023;68:4439–4448. [DOI] [PubMed] [Google Scholar]
- 103.Corbett FS. Long Term Follow-up after Radiofrequency Ablation (RFA) of Barrett's Esophagus in a Relatively Low Risk Cohort. Gastrointestinal Endoscopy 2022;95. [Google Scholar]
- 104.Wang FB M; Ramirez FC et al. Radiofrequency Ablation in Barrett's Esophagus without Dysplasia: The Decision Making, Endoscopic Burden, and the Outcomes. Gastrointestinal Endoscopy 2022;95. [Google Scholar]
- 105.Kroep S, Lansdorp-Vogelaar I, Rubenstein JH, et al. An Accurate Cancer Incidence in Barrett's Esophagus: A Best Estimate Using Published Data and Modeling. Gastroenterology 2015;149:577–85.e4; quiz e14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kroep S, Heberle CR, Curtius K, et al. Radiofrequency Ablation of Barrett's Esophagus Reduces Esophageal Adenocarcinoma Incidence and Mortality in a Comparative Modeling Analysis. Clin Gastroenterol Hepatol 2017;15:1471–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hur C, Choi SE, Rubenstein JH, et al. The cost effectiveness of radiofrequency ablation for Barrett's esophagus. Gastroenterology 2012;143:567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Inadomi JM, Somsouk M, Madanick RD, et al. A cost-utility analysis of ablative therapy for Barrett's esophagus. Gastroenterology 2009;136:2101–2114.e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yachimski P, Wani S, Givens T, et al. Preference of endoscopic ablation over medical prevention of esophageal adenocarcinoma by patients with Barrett's esophagus. Clin Gastroenterol Hepatol 2015;13:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Parasa S, Vennalaganti S, Gaddam S, et al. Development and Validation of a Model to Determine Risk of Progression of Barrett's Esophagus to Neoplasia. Gastroenterology 2018;154:1282–1289.e2. [DOI] [PubMed] [Google Scholar]
- 111.Kunzmann AT, Thrift AP, Johnston BT, et al. External validation of a model to determine risk of progression of Barrett’s oesophagus to neoplasia. Alimentary Pharmacology & Therapeutics 2019;49:1274–1281. [DOI] [PubMed] [Google Scholar]
- 112.Redston M, Noffsinger A, Kim A, et al. Abnormal TP53 Predicts Risk of Progression in Patients With Barrett’s Esophagus Regardless of a Diagnosis of Dysplasia. Gastroenterology 2022;162:468–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kastelein F, Biermann K, Steyerberg EW, et al. Aberrant p53 protein expression is associated with an increased risk of neoplastic progression in patients with Barrett's oesophagus. Gut 2013;62:1676–83. [DOI] [PubMed] [Google Scholar]
- 114.Iyer PG, Codipilly DC, Chandar AK, et al. Prediction of Progression in Barrett's Esophagus Using a Tissue Systems Pathology Test: A Pooled Analysis of International Multicenter Studies. Clin Gastroenterol Hepatol 2022;20:2772–2779.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Davison JM, Goldblum J, Grewal US, et al. Independent Blinded Validation of a Tissue Systems Pathology Test to Predict Progression in Patients With Barrett's Esophagus. Am J Gastroenterol 2020;115:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Frei NF, Konte K, Bossart EA, et al. Independent Validation of a Tissue Systems Pathology Assay to Predict Future Progression in Nondysplastic Barrett's Esophagus: A Spatial-Temporal Analysis. Clinical and Translational Gastroenterology 2020;11:e00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Roumans CAM, Spaander MCW, Lansdorp-Vogelaar I, et al. A personalized and dynamic risk estimation model: The new paradigm in Barrett's esophagus surveillance. PLoS One 2022;17:e0267503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Desai M, Saligram S, Gupta N, et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett's esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc 2017;85:482–495 e4. [DOI] [PubMed] [Google Scholar]
- 119.van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett's oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut 2011;60:765–73. [DOI] [PubMed] [Google Scholar]
- 120.Terheggen G, Horn EM, Vieth M, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett's neoplasia. Gut 2017;66:783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Podboy A, Kolahi KS, Friedland S, et al. Endoscopic submucosal dissection is associated with less pathologic uncertainty than endoscopic mucosal resection in diagnosing and staging Barrett's-related neoplasia. Dig Endosc 2020;32:346–354. [DOI] [PubMed] [Google Scholar]
- 122.Doumbe-Mandengue P, Pellat A, Belle A, et al. Endoscopic submucosal dissection versus endoscopic mucosal resection for early esophageal adenocarcinoma. Clin Res Hepatol Gastroenterol 2023;47:102138. [DOI] [PubMed] [Google Scholar]
- 123.Vantanasiri KJ A; Sachdeva K et al. Rates of Recurrent Intestinal Metaplasia and Dysplasia After Successful Endoscopic Therapy of Barrett’s Neoplasia by Cap Assisted EMR Vs ESD and Ablation: Results From a Large North American Multicenter Cohort Gastrointestinal Endoscopy 2023;97. [DOI] [PubMed] [Google Scholar]
- 124.Younis F AM, Rosch T, et al. Clinical Stratification for Endoscopic Submucosal Dissection Versus Endoscopic Mucosal Resection For Barrett’s Associated Neoplasia: Long-Term Follow-Up. United European Gastroenterology Journal 2022;10:161. [Google Scholar]
- 125.Codipilly DC, Dhaliwal L, Oberoi M, et al. Comparative Outcomes of Cap Assisted Endoscopic Resection and Endoscopic Submucosal Dissection in Dysplastic Barrett's Esophagus. Clin Gastroenterol Hepatol 2022;20:65–73 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.al A AE B A H Re. Management of strictures after endoscopic resection for early esophageal neoplasia. United European Gastroenterology Journal 2020;8:723. [Google Scholar]
- 127.Mejia Perez LK, Yang D, Draganov PV, et al. Endoscopic submucosal dissection vs. endoscopic mucosal resection for early Barrett's neoplasia in the West: a retrospective study. Endoscopy 2022;54:439–446. [DOI] [PubMed] [Google Scholar]
- 128.Arndtz SM R; Subramaniam S, et al. Post oesophageal endoscopic submucosal dissection strictures-is risk related to pathology? a European comparison of barrett's versus squamous neoplasia. United European Gastroenterology Journal 2020;8:712. [Google Scholar]
- 129.Bahdi F, Katti CC, Mansour N, et al. Outcomes of endoscopic submucosal dissection (ESD) plus radiofrequency ablation (RFA) for nodular Barrett's esophagus. Scand J Gastroenterol 2023;58:123–132. [DOI] [PubMed] [Google Scholar]
- 130.Baldaque-Silva FW N; Omae M; et al. Steroid lifting method: A new strategy for the prevention of strictures after esophageal endoscopic submucosal dissection. United European Gastroenterology Journal 2021;9:791. [Google Scholar]
- 131.Barret M, Cao DT, Beuvon F, et al. Endoscopic submucosal dissection for early Barrett's neoplasia. United European Gastroenterol J 2016;4:207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chalikonda DM; Ho J; Kazan A ea. Indications and Outcomes With Endoscopic Submucosal Dissection for High-Grade Barrett’s Esophagus. American Journal of Gastroenterology 2021;116:S206–S207. [Google Scholar]
- 133.Chevaux JB, Piessevaux H, Jouret-Mourin A, et al. Clinical outcome in patients treated with endoscopic submucosal dissection for superficial Barrett's neoplasia. Endoscopy 2015;47:103–12. [DOI] [PubMed] [Google Scholar]
- 134.Coman RM, Gotoda T, Forsmark CE, et al. Prospective evaluation of the clinical utility of endoscopic submucosal dissection (ESD) in patients with Barrett's esophagus: a Western center experience. Endosc Int Open 2016;4:E715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dolak W, Mesteri I, Asari R, et al. A pilot study of the endomicroscopic assessment of tumor extension in Barrett's esophagus-associated neoplasia before endoscopic resection. Endosc Int Open 2015;3:E19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Draganov PV, Aihara H, Karasik MS, et al. Endoscopic Submucosal Dissection in North America: A Large Prospective Multicenter Study. Gastroenterology 2021;160:2317–2327 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Genere JR, Priyan H, Sawas T, et al. Safety and histologic outcomes of endoscopic submucosal dissection with a novel articulating knife for esophageal neoplasia. Gastrointest Endosc 2020;91:797–805. [DOI] [PubMed] [Google Scholar]
- 138.Gupta SW H; McKay O et al. Outcomes Of Endoscopic Submucosal Dissection (ESD) For Barret’s Neoplasia - A Single Center Retrospective Study United European Gastroenterology Journal 2022;10:161–162. [Google Scholar]
- 139.Hobel S, Dautel P, Baumbach R, et al. Single center experience of endoscopic submucosal dissection (ESD) in early Barrett's adenocarcinoma. Surg Endosc 2015;29:1591–7. [DOI] [PubMed] [Google Scholar]
- 140.Imai K, Kakushima N, Tanaka M, et al. Validation of the application of the Japanese curative criteria for superficial adenocarcinoma at the esophagogastric junction treated by endoscopic submucosal dissection: a long-term analysis. Surgical Endoscopy 2013;27:2436–2445. [DOI] [PubMed] [Google Scholar]
- 141.Jacques J, Legros R, Rivory J, et al. The "tunnel + clip" strategy standardised and facilitates oesophageal ESD procedures: a prospective, consecutive bi-centric study. Surg Endosc 2017;31:4838–4847. [DOI] [PubMed] [Google Scholar]
- 142.Joo DC, Kim GH, Lee BE, et al. Endoscopic Submucosal Dissection for Superficial Barrett's Neoplasia in Korea: a Single-Center Experience. J Gastric Cancer 2021;21:426–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Joseph A, Draganov PV, Maluf-Filho F, et al. Outcomes for endoscopic submucosal dissection of pathologically staged T1b esophageal cancer: a multicenter study. Gastrointest Endosc 2022;96:445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kagemoto K, Oka S, Tanaka S, et al. Clinical outcomes of endoscopic submucosal dissection for superficial Barrett's adenocarcinoma. Gastrointest Endosc 2014;80:239–45. [DOI] [PubMed] [Google Scholar]
- 145.Mehta NA SA, Vargo JJ, et al. Insulated-tip Knife Tunneling and C-shaped Incision for Esophageal Endoscopic Submucosal Dissection: An Initial Western Experience. Techniques and Innovations in Gastrointestinal Endoscopy 2021;23:152–158. [Google Scholar]
- 146.Motomura D, Bechara R. Single operator experience of endoscopic submucosal dissection for Barrett's neoplasia in a North American academic center. DEN Open 2022;2:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nagami Y, Machida H, Shiba M, et al. Clinical Efficacy of Endoscopic Submucosal Dissection for Adenocarcinomas of the Esophagogastric Junction. Endosc Int Open 2014;2:E15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Omae M, Hagstrom H, Ndegwa N, et al. Wide-field endoscopic submucosal dissection for the treatment of Barrett's esophagus neoplasia. Endosc Int Open 2021;9:E727–E734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Perez-Cuadrado Robles E, Moreels TG, Piessevaux H, et al. Risk factors of refractory post-endoscopic submucosal dissection esophageal strictures. Rev Esp Enferm Dig 2021;113:813–819. [DOI] [PubMed] [Google Scholar]
- 150.Ribeiro TML, Arantes VN, Ramos JA, et al. Endoscopic Submucosal Dissection with Circumferential Incision Versus Tunneling Method for Treatment of Superficial Esophageal Cancer. Arq Gastroenterol 2021;58:195–201. [DOI] [PubMed] [Google Scholar]
- 151.Shimizu T, Fujisaki J, Omae M, et al. Treatment Outcomes of Endoscopic Submucosal Dissection for Adenocarcinoma Originating from Long-Segment Barrett's Esophagus versus Short-Segment Barrett's Esophagus. Digestion 2018;97:316–323. [DOI] [PubMed] [Google Scholar]
- 152.Stephant S, Jacques J, Brochard C, et al. High proficiency of esophageal endoscopic submucosal dissection with a "tunnel + clip traction" strategy: a large French multicentric study. Surg Endosc 2023;37:2359–2366. [DOI] [PubMed] [Google Scholar]
- 153.Subramaniam S, Chedgy F, Longcroft-Wheaton G, et al. Complex early Barrett's neoplasia at 3 Western centers: European Barrett's Endoscopic Submucosal Dissection Trial (E-BEST). Gastrointest Endosc 2017;86:608–618. [DOI] [PubMed] [Google Scholar]
- 154.Suchartlikitwong SMA PA; Haddad N et al. Utility of Endoscopic Submucosal Dissection For Treatment of Esophageal Neoplasia: An Experience From a Single Tertiary Referral Center Gastrointestinal Endoscopy 2022;95:AB402–AB403. [Google Scholar]
- 155.van Munster SN, Verheij EPD, Nieuwenhuis EA, et al. Extending treatment criteria for Barrett's neoplasia: results of a nationwide cohort of 138 endoscopic submucosal dissection procedures. Endoscopy 2022;54:531–541. [DOI] [PubMed] [Google Scholar]
- 156.Yang D, Coman RM, Kahaleh M, et al. Endoscopic submucosal dissection for Barrett's early neoplasia: a multicenter study in the United States. Gastrointest Endosc 2017;86:600–607. [DOI] [PubMed] [Google Scholar]
- 157.Yang D, King W, Aihara H, et al. Effect of endoscopic submucosal dissection on histologic diagnosis in Barrett's esophagus visible neoplasia. Gastrointest Endosc 2022;95:626–633. [DOI] [PubMed] [Google Scholar]
- 158.Yoshinaga S, Gotoda T, Kusano C, et al. Clinical impact of endoscopic submucosal dissection for superficial adenocarcinoma located at the esophagogastric junction. Gastrointest Endosc 2008;67:202–9. [DOI] [PubMed] [Google Scholar]
- 159.Fleischmann CP A; Ebigbo A et al. Endoscopic submucosal dissection and Barrett's esophagus-results of the german esdregistry. Endoscopy 2021;53. [Google Scholar]
- 160.Hoteya S, Matsui A, Iizuka T, et al. Comparison of the clinicopathological characteristics and results of endoscopic submucosal dissection for esophagogastric junction and non-junctional cancers. Digestion 2013;87:29–33. [DOI] [PubMed] [Google Scholar]
- 161.Probst A, Ebigbo A, Markl B, et al. Stricture Prevention after Extensive Endoscopic Submucosal Dissection of Neoplastic Barrett's Esophagus: Individualized Oral Steroid Prophylaxis. Gastroenterol Res Pract 2019;2019:2075256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pilonis NJ W; Kaminski MF Defining the optimal treatment approach for early neoplasia of the gastroesophageal junction: A multi-center retrospective study. United European Gastroenterology Journal 2020;8:728. [Google Scholar]
- 163.Abe S, Ishihara R, Takahashi H, et al. Long-term outcomes of endoscopic resection and metachronous cancer after endoscopic resection for adenocarcinoma of the esophagogastric junction in Japan. Gastrointest Endosc 2019;89:1120–1128. [DOI] [PubMed] [Google Scholar]
- 164.Draganov PV, Wang AY, Othman MO, et al. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin Gastroenterol Hepatol 2019;17:16–25 e1. [DOI] [PubMed] [Google Scholar]
- 165.Qumseya B, Sultan S, Bain P, et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointestinal Endoscopy 2019;90:335–359.e2. [DOI] [PubMed] [Google Scholar]
- 166.Manner H, Pech O, Heldmann Y, et al. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2013;11:630–5; quiz e45. [DOI] [PubMed] [Google Scholar]
- 167.Dunbar KB, Spechler SJ. The risk of lymph-node metastases in patients with high-grade dysplasia or intramucosal carcinoma in Barrett's esophagus: a systematic review. The American Journal of Gastroenterology 2012;107:850–62; quiz 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ancona E, Rampado S, Cassaro M, et al. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol 2008;15:3278–88. [DOI] [PubMed] [Google Scholar]
- 169.Buskens CJ, Westerterp M, Lagarde SM, et al. Prediction of appropriateness of local endoscopic treatment for high-grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc 2004;60:703–10. [DOI] [PubMed] [Google Scholar]
- 170.Al-Haddad MA, Elhanafi SE, Forbes N, et al. American Society for Gastrointestinal Endoscopy guideline on endoscopic submucosal dissection for the management of early esophageal and gastric cancers: methodology and review of evidence. Gastrointest Endosc 2023;98:285–305 e38. [DOI] [PubMed] [Google Scholar]
- 171.Kawashima K, Abe S, Koga M, et al. Optimal selection of endoscopic resection in patients with esophageal squamous cell carcinoma: endoscopic mucosal resection versus endoscopic submucosal dissection according to lesion size. Dis Esophagus 2021;34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.