Abstract
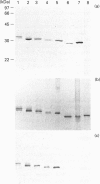
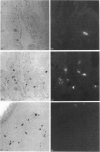
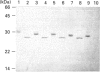
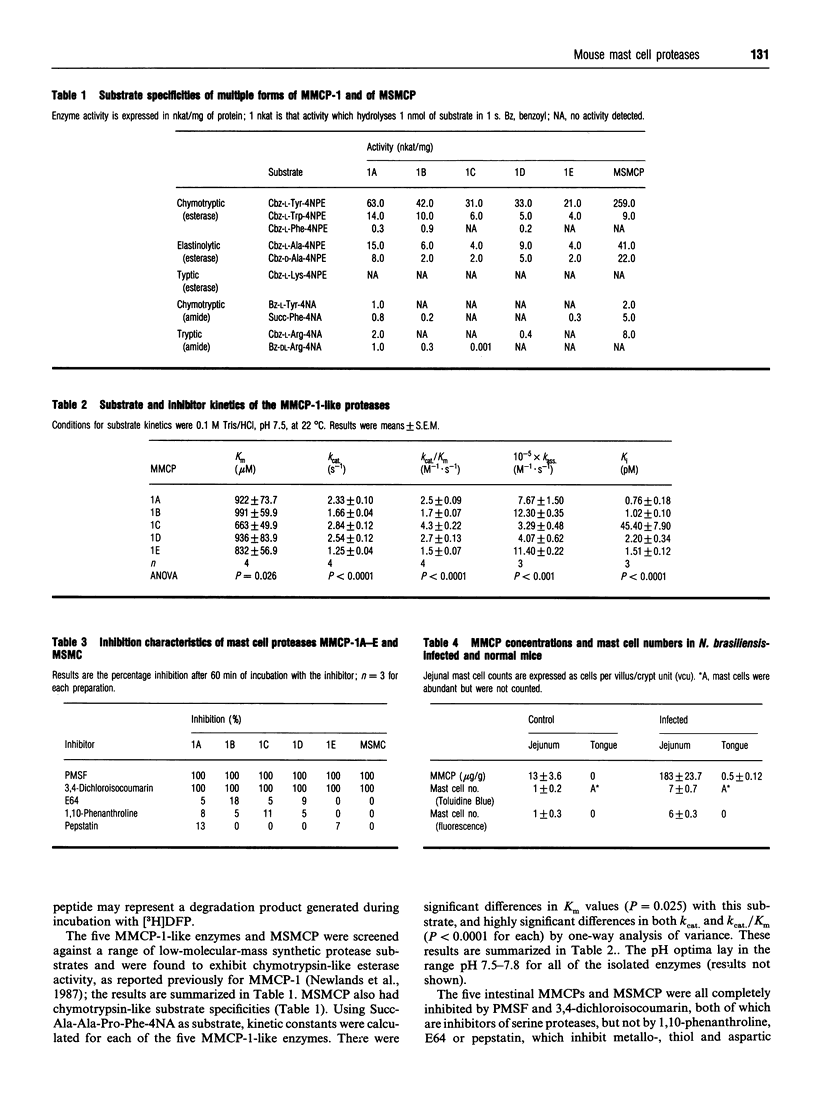
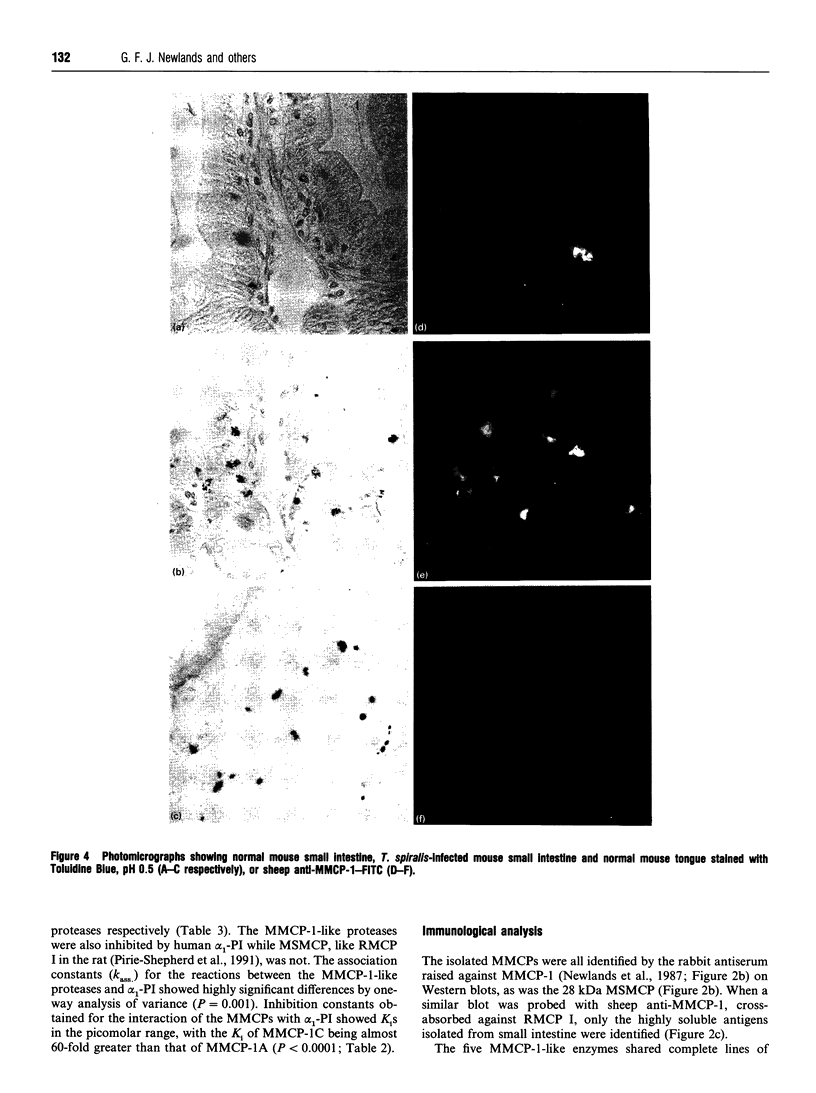
Five highly soluble, chymotrypsin-like, neutral serine proteases, with molecular masses in the range 30-33 kDa, were isolated from Trichinella spiralis-infected mouse small intestine. These enzymes were closely related antigenically on Western blotting and by Ouchterlony double diffusion using a polyclonal, cross-absorbed, sheep antibody raised against mouse mast cell protease-1 (MMCP-1) and on the basis of N-terminal amino acid sequence analysis, were identified as variant forms of MMCP-1. Substrate and inhibitor analysis confirmed that the five variants (MMCP-1 A-E) had similar characteristics, although highly significant (P = 0.025 to P < 0.0001) variations in Km and kcat, were detected. Against human alpha 1-proteinase inhibitor the Ki for MMCP-1C (45 pM) was significantly (P < 0.0001) greater than those for the other proteases (0.76-2.2 pM). The differences in electrophoretic mobility are probably a result of variable glycosylation, since removal of N-linked carbohydrate produced a polypeptide of approx. 28 kDa in each case which was, like the native enzyme, immunoreactive on Western blotting. A much less soluble 28 kDa enzyme was isolated from serosal mast cells and identified as MMCP-4 by N-terminal amino acid sequencing. Like MMCP-1 it has chymotrypsin-like substrate specificities with activity at neutral pH. However, it was antigenically distinct from MMCP-1 and, using sheep anti-MMCP-1, was not detected on Western blotting or by Ouchterlony double diffusion, e.l.i.s.a. or immunohistochemistry. This last technique established that the MMCP-1 variants were uniquely present in enteric mast cells, thereby providing a highly selective means of distinguishing the mucosal and connective tissue mast cell subsets in the mouse.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caughey G. H., Viro N. F., Ramachandran J., Lazarus S. C., Borson D. B., Nadel J. A. Dog mastocytoma tryptase: affinity purification, characterization, and amino-terminal sequence. Arch Biochem Biophys. 1987 Nov 1;258(2):555–563. doi: 10.1016/0003-9861(87)90377-8. [DOI] [PubMed] [Google Scholar]
- Cromlish J. A., Seidah N. G., Marcinkiewicz M., Hamelin J., Johnson D. A., Chrétien M. Human pituitary tryptase: molecular forms, NH2-terminal sequence, immunocytochemical localization, and specificity with prohormone and fluorogenic substrates. J Biol Chem. 1987 Jan 25;262(3):1363–1373. [PubMed] [Google Scholar]
- DuBuske L., Austen K. F., Czop J., Stevens R. L. Granule-associated serine neutral proteases of the mouse bone marrow-derived mast cell that degrade fibronectin: their increase after sodium butyrate treatment of the cells. J Immunol. 1984 Sep;133(3):1535–1541. [PubMed] [Google Scholar]
- Enerbäck L. Mast cells in rat gastrointestinal mucosa. 2. Dye-binding and metachromatic properties. Acta Pathol Microbiol Scand. 1966;66(3):303–312. doi: 10.1111/apm.1966.66.3.303. [DOI] [PubMed] [Google Scholar]
- Gibson S., Miller H. R. Mast cell subsets in the rat distinguished immunohistochemically by their content of serine proteinases. Immunology. 1986 May;58(1):101–104. [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Gurish M. F., Ghildyal N., McNeil H. P., Austen K. F., Gillis S., Stevens R. L. Differential expression of secretory granule proteases in mouse mast cells exposed to interleukin 3 and c-kit ligand. J Exp Med. 1992 Apr 1;175(4):1003–1012. doi: 10.1084/jem.175.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig D. M., McMenamin C., Redmond J., Brown D., Young I. G., Cohen S. D., Hapel A. J. Rat IL-3 stimulates the growth of rat mucosal mast cells in culture. Immunology. 1988 Oct;65(2):205–211. [PMC free article] [PubMed] [Google Scholar]
- Huang R. Y., Blom T., Hellman L. Cloning and structural analysis of MMCP-1, MMCP-4 and MMCP-5, three mouse mast cell-specific serine proteases. Eur J Immunol. 1991 Jul;21(7):1611–1621. doi: 10.1002/eji.1830210706. [DOI] [PubMed] [Google Scholar]
- Huntley J. F., Gooden C., Newlands G. F., Mackellar A., Lammas D. A., Wakelin D., Tuohy M., Woodbury R. G., Miller H. R. Distribution of intestinal mast cell proteinase in blood and tissues of normal and Trichinella-infected mice. Parasite Immunol. 1990 Jan;12(1):85–95. doi: 10.1111/j.1365-3024.1990.tb00938.x. [DOI] [PubMed] [Google Scholar]
- Huntley J. F., Mackellar A., Newlands G. F., Irvine J., Miller H. R. Mapping of the rat mast cell granule proteinases RMCPI and II by enzyme-linked immunosorbent assay and paired immunofluorescence. APMIS. 1990 Oct;98(10):933–944. doi: 10.1111/j.1699-0463.1990.tb05018.x. [DOI] [PubMed] [Google Scholar]
- Jameson G. W., Roberts D. V., Adams R. W., Kyle W. S., Elmore D. T. Determination of the operational molarity of solutions of bovine alpha-chymotrypsin, trypsin, thrombin and factor Xa by spectrofluorimetric titration. Biochem J. 1973 Jan;131(1):107–117. doi: 10.1042/bj1310107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D. P., Gibson S., Huntley J. F. The catalytic properties of a proteinase isolated from sheep abomasal mucosal mast cells. Int J Biochem. 1986;18(10):961–964. doi: 10.1016/0020-711x(86)90079-0. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagunoff D., Pritzl P. Characterization of rat mast cell granule proteins. Arch Biochem Biophys. 1976 Apr;173(2):554–563. doi: 10.1016/0003-9861(76)90292-7. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Le Trong H., Parmelee D. C., Walsh K. A., Neurath H., Woodbury R. G. Amino acid sequence of rat mast cell protease I (chymase). Biochemistry. 1987 Nov 3;26(22):6988–6994. doi: 10.1021/bi00396a020. [DOI] [PubMed] [Google Scholar]
- McNeil H. P., Austen K. F., Somerville L. L., Gurish M. F., Stevens R. L. Molecular cloning of the mouse mast cell protease-5 gene. A novel secretory granule protease expressed early in the differentiation of serosal mast cells. J Biol Chem. 1991 Oct 25;266(30):20316–20322. [PubMed] [Google Scholar]
- Miller H. R., Huntley J. F., Newlands G. F., Irvine J. Granule chymases and the characterization of mast cell phenotype and function in rat and mouse. Monogr Allergy. 1990;27:1–30. [PubMed] [Google Scholar]
- Miller H. R., Huntley J. F., Newlands G. F., Mackellar A., Lammas D. A., Wakelin D. Granule proteinases define mast cell heterogeneity in the serosa and the gastrointestinal mucosa of the mouse. Immunology. 1988 Dec;65(4):559–566. [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Newlands G. F., Gibson S., Knox D. P., Grencis R., Wakelin D., Miller H. R. Characterization and mast cell origin of a chymotrypsin-like proteinase isolated from intestines of mice infected with Trichinella spiralis. Immunology. 1987 Dec;62(4):629–634. [PMC free article] [PubMed] [Google Scholar]
- Newlands G. F., Lammas D. A., Huntley J. F., MacKellar A., Wakelin D., Miller H. R. Heterogeneity of murine bone marrow-derived mast cells: analysis of their proteinase content. Immunology. 1991 Mar;72(3):434–439. [PMC free article] [PubMed] [Google Scholar]
- Newlands G. F., MacKellar A., Miller H. R. Intestinal mucosal mast cells in Nippostrongylus-infected mice: lack of sensitivity to corticosteroids. Int J Parasitol. 1990 Aug;20(5):669–672. doi: 10.1016/0020-7519(90)90125-7. [DOI] [PubMed] [Google Scholar]
- Pirie-Shepherd S. R., Miller H. R., Ryle A. Differential inhibition of rat mast cell proteinase I and II by members of the alpha-1-proteinase inhibitor family of serine proteinase inhibitors. J Biol Chem. 1991 Sep 15;266(26):17314–17319. [PubMed] [Google Scholar]
- RINDERKNECHT H. Ultra-rapid fluorescent labelling of proteins. Nature. 1962 Jan 13;193:167–168. doi: 10.1038/193167b0. [DOI] [PubMed] [Google Scholar]
- Reynolds D. S., Gurley D. S., Austen K. F., Serafin W. E. Cloning of the cDNA and gene of mouse mast cell protease-6. Transcription by progenitor mast cells and mast cells of the connective tissue subclass. J Biol Chem. 1991 Feb 25;266(6):3847–3853. [PubMed] [Google Scholar]
- Reynolds D. S., Stevens R. L., Lane W. S., Carr M. H., Austen K. F., Serafin W. E. Different mouse mast cell populations express various combinations of at least six distinct mast cell serine proteases. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3230–3234. doi: 10.1073/pnas.87.8.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter N. M., Choi J. K., Slavin D. A., Deresienski D. T., Sayama S., Dong G., Lavker R. M., Proud D., Lazarus G. S. Identification of a chymotrypsin-like proteinase in human mast cells. J Immunol. 1986 Aug 1;137(3):962–970. [PubMed] [Google Scholar]
- Schwartz L. B., Lewis R. A., Austen K. F. Tryptase from human pulmonary mast cells. Purification and characterization. J Biol Chem. 1981 Nov 25;256(22):11939–11943. [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Serafin W. E., Reynolds D. S., Rogelj S., Lane W. S., Conder G. A., Johnson S. S., Austen K. F., Stevens R. L. Identification and molecular cloning of a novel mouse mucosal mast cell serine protease. J Biol Chem. 1990 Jan 5;265(1):423–429. [PubMed] [Google Scholar]
- Serafin W. E., Sullivan T. P., Conder G. A., Ebrahimi A., Marcham P., Johnson S. S., Austen K. F., Reynolds D. S. Cloning of the cDNA and gene for mouse mast cell protease 4. Demonstration of its late transcription in mast cell subclasses and analysis of its homology to subclass-specific neutral proteases of the mouse and rat. J Biol Chem. 1991 Jan 25;266(3):1934–1941. [PubMed] [Google Scholar]
- Sredni B., Friedman M. M., Bland C. E., Metcalfe D. D. Ultrastructural, biochemical, and functional characteristics of histamine-containing cells cloned from mouse bone marrow: tentative identification as mucosal mast cells. J Immunol. 1983 Aug;131(2):915–922. [PubMed] [Google Scholar]
- Trong H. L., Newlands G. F., Miller H. R., Charbonneau H., Neurath H., Woodbury R. G. Amino acid sequence of a mouse mucosal mast cell protease. Biochemistry. 1989 Jan 10;28(1):391–395. doi: 10.1021/bi00427a054. [DOI] [PubMed] [Google Scholar]
- Vanderslice P., Ballinger S. M., Tam E. K., Goldstein S. M., Craik C. S., Caughey G. H. Human mast cell tryptase: multiple cDNAs and genes reveal a multigene serine protease family. Proc Natl Acad Sci U S A. 1990 May;87(10):3811–3815. doi: 10.1073/pnas.87.10.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelin D., Lloyd M. Immunity to primary and challenge infections of Trichinella spiralis in mice: a re-examination of conventional parameters. Parasitology. 1976 Apr;72(2):173–182. doi: 10.1017/s0031182000048472. [DOI] [PubMed] [Google Scholar]
- Wittwer A. J., Howard S. C., Carr L. S., Harakas N. K., Feder J., Parekh R. B., Rudd P. M., Dwek R. A., Rademacher T. W. Effects of N-glycosylation on in vitro activity of Bowes melanoma and human colon fibroblast derived tissue plasminogen activator. Biochemistry. 1989 Sep 19;28(19):7662–7669. doi: 10.1021/bi00445a022. [DOI] [PubMed] [Google Scholar]
- Wittwer A. J., Howard S. C. Glycosylation at Asn-184 inhibits the conversion of single-chain to two-chain tissue-type plasminogen activator by plasmin. Biochemistry. 1990 May 1;29(17):4175–4180. doi: 10.1021/bi00469a021. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Gruzenski G. M., Lagunoff D. Immunofluorescent localization of a serine protease in rat small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2785–2789. doi: 10.1073/pnas.75.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]