Abstract
Natural products are important precursors for antibiotic drug design. These chemical scaffolds serve as synthetic inspiration for chemists who leverage their structures to develop novel antibacterials and chemical probes. We have previously studied carolacton, a natural product macrolactone fromSorangium cellulosum, and discovered a simplified derivative, A2, that maintained apparent biofilm inhibitory activity, although the biological target was unknown. Herein, we utilize affinity-based protein profiling (AfBPP) in situ during biofilm formation to identify the protein target using a photoexcitable cross-linking derivative of A2. From these studies, we identified glucan binding protein B (GbpB), a peptidoglycan hydrolase, as the primary target of A2. Further characterization of the interaction between A2 and GbpB, as well as PcsB, a closely related homologue from the more pathogenic S. pneumoniae, revealed binding to the catalytic CHAP (cysteine, histidine, aminopeptidase) domain. To the best of our knowledge, this is the first report of a small-molecule binder of a conserved and essential bacterial CHAP hydrolase, revealing its potential as an antibiotic target. This work also highlights A2 as a useful tool compound for streptococci and as an initial scaffold for the design of more potent CHAP binders.
Introduction
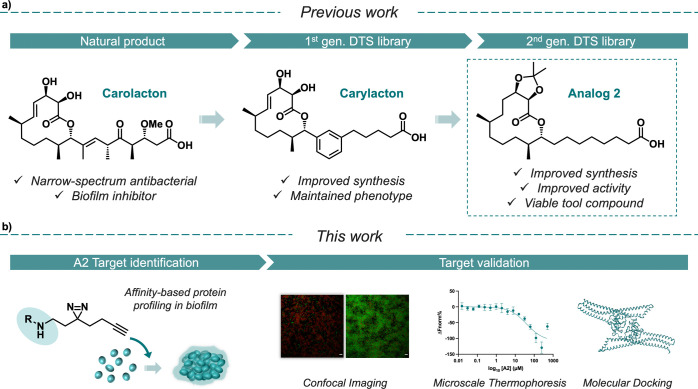
Antibiotic development has been impeded by an increase in multidrug resistant organisms and a decline in investment. Natural products are often considered “privileged scaffolds”, as they have been evolved to impart specific binding with therapeutically relevant targets, evidenced by the fact that 65% of approved antibacterials are derived from natural products.1 Unfortunately, they are limited in their practical use due to their poor therapeutic indices, bioavailability, and synthetic accessibility.2,3 To overcome these challenges, many have been motivated to develop synthetic strategies to leverage their advantages in biological settings through the construction of natural product-inspired libraries. Examples include biology-oriented synthesis (BOS),4,5 diverted total synthesis (DTS),6,7 diversity-oriented synthesis (DOS),8,9 complexity to diversity (CtD),10,11 and natural product simplification (NPS).12−15 Such chemical platforms have enabled the design of chemical probes, the identification of unexplored targets, and the development of antibiotics that circumvent resistance.3,16−23
Carolacton is a natural product biosynthesized by Sorangium cellulosum and has been shown to affect carbon utilization, cell wall biosynthesis, amino acid metabolism, and biofilm formation in Streptococcus mutans.24−30 Demonstration of activity against S. pneumoniae alluded to a conserved streptococcal target. Subsequently, Müller and co-workers reported that carolacton targets folate dehydrogenase (FolD) after selecting for a resistant mutation in this gene using an Escherichia coli efflux knockout (E. coli ΔtolC).31 This finding was further corroborated in an in vitro biochemical assay using repurified FolD. However, to date FolD has not been linked to any cell wall or biofilm processes. To leverage the appealing biological activity of carolacton, while reducing the number of synthetic steps, we previously employed two rounds of DTS (Figure 1a). From these efforts emerged a simplified compound, Analog 2 (A2), that displayed improved growth inhibition, killing, and antibiofilm activity when tested against the oral pathogen Streptococcus mutans.14 Using a genetic knockout screen, we connected A2 to a master regulator in cell division and biofilm formation pathways, ccpA, but the molecular target has remained elusive. Leveraging the simplified scaffold, we synthesized a photoaffinity probe for affinity-based protein profiling (AfBPP) in a biofilm-inducing environment (Figure 1b). The subsequent cross-linking proteomic experiments identified a small subset of potential protein targets, which after validation, uncovered glucan binding protein B (GbpB), a putative peptidoglycan hydrolase, as the target of A2. GbpB plays an essential role in cell wall septal division and is highly conserved across the streptococcus genus, providing a novel strategy to prevent pathogenic streptococcal infections.32,33 We report the discovery of a first-in-class binder of the essential peptidoglycan hydrolase, GbpB/PcsB.
Figure 1.
(a) Natural product inspiration and simplification led to the discovery of A2. (b) The terminal carboxylic acid was used to append the minimalist probe to access a photo-cross-linking probe, A2-PP, which was used for biological target identification.
Results
Unlike Carolacton A2 Does Not Inhibit FolD
Due to the synthetic similarity of carolacton and A2, we wanted to first determine if A2 also inhibited FolD. Bacterial growth assays and confocal laser microscopy were conducted using an S. mutans FolD knockout strain and a WT strain. The effect of A2 on bacterial growth and biofilm morphology was unchanged between the two strains, which likely excludes FolD as the responsible player behind the observed phenotype (Figures S1, S2). A2 may not interact with FolD due to limited access to the cytosol, therefore, we conducted a dehydrogenase assay using purified E. coli FolD as previously described.31 Under these assay conditions, carolacton exhibited an IC50 value of 94 nM, but A2 was inactive up to 10 μM (Figure S3). This evidence demonstrates that the effect against S. mutans biofilms imparted by the simplified scaffold is likely not related to FolD inhibition, and the natural product and A2 have, at least in part, different targets.
Affinity-Based Protein Profiling Identifies GbpB as the Biological Target of A2
We have had previous success using AfBPP to identify the targets of active compounds.34−36 We leveraged the terminal carboxylic acid as a handle to develop a chemical probe for AfBPP experiments (Figure 1b). We synthesized A2-photoprobe (A2-PP) that contained a minimalist photoaffinity handle with a photoexcitable diazirine and an alkyne handle for enrichment and analysis. A2-PP comes directly from A2 in 61% yield using standard amidation conditions (Figure S4). We were interested in exploring AfBPP using both mature and developing biofilms. In the former, cells are harvested prior to dosing in the photoprobe (Figure 2a), whereas in the latter, the photoprobe is dosed at the beginning of growth (Figure 2b). We observed a higher level of protein enrichment when we incubated A2-PP with developing S. mutans biofilms, in line with the observed inhibitory phenotype, thus we optimized based on this workflow. SDS-PAGE analysis revealed a dose-dependent protein cross-linking response, and optimal concentrations were chosen in the range of 1 and 5 μM (Figure S5). After following the workflow illustrated in Figure 2b, the enriched proteins were digested with trypsin and analyzed by LC-MS/MS. Label-free quantification (LFQ) was used to calculate the protein enrichment using A2-PP.37 Proteins were considered targets only if they were enriched at 1 μM A2-PP (Figure 2c) and exhibited a dose-dependent enrichment upon exposure to 5 μM A2-PP (Figures 2d, S6, S7). Our analysis identified seven putative targets (Figure 2c), but the dose-dependent response (Figure 2d) supported only five of the seven as true interactions. DexA and GbpB exhibited the most significant dose-dependent responses, with the most prominent enrichment observed with GbpB (Figure 2d). Interestingly, in other Streptococcus spp., FtsA and FtsX have been shown to participate in late-stage cell division in a complex with PcsB, a GbpB homologue.38−42 This connection has yet to be shown in S. mutans, but our results suggest this relationship is also likely.
Figure 2.
Workflow for affinity-based protein profiling using mature biofilm cells (a) and in situ biofilm affinity-based protein profiling (b). A2-PP is incubated either with mature or growing biofilms, irradiated, and harvested. After cell lysis, click chemistry is performed with rhodamine-N3 or biotin-N3 enabling either direct in-gel fluorescence analysis, or enrichment for MS quantification, respectively. (c) AfBPP experiment using A2-PP in S. mutans biofilm compared to DMSO control. (d) Dose-dependent Label-Free Quantification intensity (two-sided two-sample t test, n = 3 independent experiments per group).
We sought to determine which of the five candidate proteins is the primary target of A2. We attempted to develop A2-resistant strains of S. mutans through serial passage assays, but our efforts were inconclusive. Alternatively, we once again employed a screen utilizing the Quivey S. mutans knockout library43 where strains lacking the target of A2 should be resistant to compound treatment. We grew ΔftsA, ΔdexT, Δsmu.1208c, and ΔftsX with a serial dilution of A2 and compared the results to WT UA159. We found that deletion of ftsA, dexT, or smu.1208c did not rescue bacterial growth or biofilm formation, suggesting that the gene products are not the direct targets of our compound (Figure S8). We specifically had to test the ftsX mutant under biofilm-promoting conditions and found that although A2 still demonstrated activity, it varied at high concentrations (Figure S9). To investigate this further, we analyzed the ΔftsX biofilm after treatment with A2 using LIVE/DEAD stain. The biofilm IC50 was recorded at 44 μM, and we therefore tested ΔftsX at 63 μM to visualize phenotypic changes.14 The untreated ΔftsX biofilm appears robust and healthy. However, when treated with A2, we observed a decrease in biofilm formation/attachment and very few live cells (Figure S9). These data suggest that the absence of FtsX enhances the killing and antibiofilm effects of A2.
The remaining proteomic lead, GbpB, did not have a knockout strain available as it is essential for growth. Instead, we overexpressed GbpB to test whether it conferred resistance to A2 treatment.15 We constructed a pVPT-GbpB plasmid and transformed it into S. mutans UA159 alongside a strain containing an empty vector that was used as a negative control.44 Incubation of the overexpression strain with 150 μM of A2 is sufficient to inhibit growth (Figure 3a). However, gbpB overexpression was protective against compound treatment at 50 μM and 100 μM. Similarly, the strain overexpressing gbpB demonstrated drastically improved biofilm formation compared to WT when treated with 100 μM of A2 (Figure 3b). We then used Microscale Thermophoresis (MST) to evaluate the binding of A2 and GbpB. We purified GbpB with a C-terminal His-tag to facilitate purification and MST analysis and found that A2 exhibited a dissociation constant of 391 ± 128 μM (Figures 3c, S11). We hypothesize that the weak binding is because GbpB typically functions in a complex with FtsX and FtsE. This would explain why we see activities at lower concentrations but only see moderate binding in vitro with purified enzyme. Although further investigation is needed, our AfBPP results suggest that A2 is in proximity to FtsX thus supporting our hypothesis.
Figure 3.
(a) Growth inhibition of A2-treated bacterial cells in gbpB overexpression strain and empty vector strain at 16h. Data represents two biological replicates (+ s.d.). p value: ns = not significant; ** ≤ 0.01, *** < 0.002; two-tailed Student’s t test; (b) Biofilm formation of A2-treated biofilm cultures in gbpB overexpression strain and empty vector strain at 16h. Biofilm formation was normalized to account for differences in bacterial growth by dividing OD562/OD600. Data represents two biological replicates (+ s.d.). p value: ns = not significant; *** ≤ 0.002; two-tailed Student’s t test; (c) Microscale thermophoresis using RED-tris-NTA-labeled protein with A2 or A2-PP. Data represents the average of 3 independent measurements, error bars represent the standard deviation.
A2 Binds the CHAP Domain of GbpB and PcsB
In S. mutans, GbpB has been implicated primarily in biofilm formation, while its role in bacterial growth is poorly understood.32,33,45 Sequence analysis revealed that GbpB is comprised of a leucine zipper, a long linker domain, and a conserved C-terminal CHAP (cysteine, histidine-dependent, amidohydrolase/peptidase) domain. The GbpB CHAP domain shares high sequence similarity with N-acetylmuramoyl-l-alanine amidases/endopeptidases in various Streptococcus spp. (Figure S13) that are responsible for septum cleavage. The best characterized of these homologues is PcsB derived from S. pneumoniae, and it is the only homologue with a crystal structure.38−41 To compare the structural features of GbpB to PcsB, we used AlphaFold2 to predict the structure of smuGbpB, and found that they were highly homologous (Figures 4a, S14–17).
Figure 4.
(a) Overlay of spnPcsB dimer (blue and teal; PDB: 4CGK) with smuGbpB monomer (pink) that was solved using AlphaFold. (b) Computationally predicted docking pose between A2 and spnPcsB CHAP domain. (c) Computationally predicted docking pose between A2 and spnPcsB CHAP domain with cross-linking sites by A2-PP depicted in green. (d) Microscale thermophoresis of RED-tris-NTA-labeled proteins with A2. Data represents the average of 3 independent measurements, error bars represent the standard deviation. (e) Schematic of inactive PcsB dimer in complex with other members of the divisome.
To date, there are no known binders or inhibitors of the CHAP domain. For these reasons, we were interested in further characterizing the binding interaction of A2. We proceeded with PcsB, as we could utilize the structural data from the Protein Data Bank (PDB: 4CGK) to aid our analyses.38 We expressed and purified two spnPcsB constructs, one with the His6-tag at the N-terminus (spnHis-PcsB) and one with the His6-tag at the C-terminus (spnPcsB-His) to use in MST binding experiments. A2 exhibited a Kd of 40 ± 9 μM for PcsB-His (Figure 3c, S18). On the contrary, we tested A2 with His-PcsB and found that the Kd increased to 312 ± 51 μM, demonstrating that the PcsB-His is the better construct for our binding experiments (Figure S19). Additionally, we observed that carolacton does not bind PcsB at concentrations lower than 1 mM, supporting that carolacton and A2 have different targets (Figure S22). Concurrently, we used the Glide docking module of Maestro (Schrödinger suite) to predict the binding mode of A2 and A2-PP. In two of the top-scoring poses with A2-PP, the diazirine is placed near the entrance of the catalytic site (Figure S23). In the next-highest scoring pose, the elongated side chain in A2-PP is placed peripheral to the catalytic site (Figure S23). In the computationally predicted pose, the alkyl chain is pointing into the catalytic pocket, priming the terminal carboxylic acid for hydrogen bonding interactions with Gly310, Glu291, and Cys292 (Figure 4b). In addition, the lactone carbonyl moiety also interacts with Gly365. The interactions observed with essential members of the catalytic triad support a binding model where A2 blocks the catalytic domain. The importance of the carboxylic acid proton in the binding model provides rationale for the enhanced activity against S. mutans and is further supported by our previous DTS campaigns.6,14,29
To corroborate the MST binding data, we also incubated purified spnPcsB-His or spnHis-PcsB with A2-PP. After irradiation and enrichment, intact protein analysis was carried out via ESI-qTOF-MS and a mass increase corresponding to A2-PP cross-linking was observed between spnPcsB-His but not spnHis-PcsB (Figures S24, 25), suggesting a specific binding interaction as seen previously with MST. In both its monomeric and dimeric forms, PcsB exists in an autoinhibitory conformation, wherein the catalytic CHAP domain is tucked into the N-terminus, blocking the active site. Typically, substrate accessibility is facilitated by FtsX. We postulate that when the His-tag is placed at the C-terminus, it can disrupt the stabilizing interactions between the two domains and allows the probe access to the active site. To map the binding site, the cross-linked samples were also subjected to proteolytic digestion with trypsin and analyzed with LC-MS/MS. Analysis of the protein cross-linking sites revealed localization peripheral to the CHAP domain of PcsB (Figure 4c). To directly test the binding model, we purified a mutated version of spnPcsB-His [PcsB(C292A)-His], where the catalytic cysteine is mutated to an alanine, since the docking model suggested important hydrogen bonding interactions with this residue. We found that this mutation of the catalytic cysteine was sufficient to eliminate binding when measured with MST (Figures 4d, S26). Collectively, these data suggest that A2 binds the CHAP domain of PcsB, making it the first small molecule binder of this domain.
A2 Is a Narrow-Spectrum Streptococci-Specific Inhibitor
The essential and conserved nature of the target of A2, GbpB/PcsB, prompted us to explore our compound’s utility as a general streptococci inhibitor. We screened A2 against a panel of pathogenic and commensal Streptococcus species., including S. pneumoniae, Streptococcus pyogenes (Group A Streptococcus, GAS), S. alagactiae (Group B Streptococcus, GBS), Streptococcus gordonii and Streptococcus sanguinis (Figure S28). A2 exhibited an MIC of 125 μM across all strains and an IC50 between 29 and 62 μM, which is in close agreement with the activity against S. mutans.14
Discussion
Herein, we uncover the protein target for the natural product-inspired compound, A2, which was discovered through the synthetic exploration of carolacton. Using a in situ AfBPP approach in biofilm cultures, we successfully identified five putative targets. Gene deletion, overexpression, and binding studies identified GbpB as the molecular target, which is essential and conserved across the Streptococcus genera. It has been implicated in the early steps of biofilm formation and its essentiality is due to its role in cell wall septum cleavage. In the latter role, it is purported to function in a complex that contains two of the other AfBPP hits, FtsA and FtsX (Figure 4e). We used the best characterized homologue of GbpB, PcsB, to determine the binding site of A2. Molecular docking studies identified a binding site in the CHAP domain, the catalytic site of the protein, which was validated using in vitro cross-linking and MST experiments. We then screened a panel of Streptococcus strains and found that activity was retained across the different species. Although we were unable to use the true target, GbpB, for our validation studies, our results show that A2 promotes cell death through its interaction with GbpB/PcsB.
To the best of our knowledge, this is the first example of in situ biofilm AfBPP and the first small molecule binder of a bacterial CHAP domain. As the GbpB/PcsB cell wall complex is further characterized, our understanding of the precise mechanism of A2 will only become clearer, as an inhibition assay of this complex is not yet available. This study lays the foundation for additional rounds of medicinal chemistry to improve potency, chemical probe design, and investigation into this class of essential cell wall CHAP hydrolases. Application of these tools can illuminate the biochemistry underlying cell wall division and biofilm formation in Gram-positive streptococci and facilitate the discovery of methods to circumvent current antibiotic resistance mechanisms.
Acknowledgments
We would like to acknowledge Dr. Robert J. Quivey (University of Rochester), Dr. Bettina Buttaro (Temple University), Dr. Christopher LaRock (Emory University), and Dr. Elizabeth Lindsay-Tinder (St. Jude Children’s Research Hospital) for providing the bacterial strains used in this study and Vadim Baidin for training in protein purification and microscale thermophoresis. Support was provided in part by the Emory University Integrated Cellular Imaging Microscopy Core, the Harvard Center for Mass Spectrometry, and the Harvard Center for Molecular Interactions.
Glossary
Abbreviations
- AfBPP
affinity-based protein profiling
- GbpB
glucan binding protein B
- MST
microscale thermophoresis
- CHAP
cysteine, histidine-dependent, amidohydrolase/peptidase domain
- smu
Streptococcus mutans
- spn
Streptococcus pneumoniae
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.4c06658.
Assay procedures, compound characterization and associated spectral images (PDF)
Author Present Address
# Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, Massachusetts 02138, United States
Author Present Address
∇ Department of Chemistry, The Pennsylvania University, 104 Benkovic Building, University Park, State College, Pennsylvania 16802, United States.
Author Present Address
○ Division of Biomaterial & Biomedical Sciences, School of Dentistry, Oregon Health & Science University, Portland, Oregon 97239, United States.
Author Present Address
◆ Departments of Cell Biology and Biomedical Engineering, Johns Hopkins University, Baltimore, Maryland 21205, United States.
Author Contributions
¶ A.M.S. and A.E.S. contributed equally. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This research was supported by the National Science Foundation (CHE1755698, W.M.W.), the National Institute of Dental and Craniofacial Research (DE025837, W.M.W.; DE022350, H.W.; DE030859, H.W.), the National Institute of General Medical Sciences (GM119426, W.M.W.; T32 GM007753, J.E.P.), and the National Institutes of Allergies and Infectious Diseases (AI149778, S.R.; AI148752, J.E.P.). The NMR instruments used in this work were supported by the National Science Foundation (CHE1531620). S.A.S. was funded by the Merck Future Insight Prize 2020.
The authors declare no competing financial interest.
Supplementary Material
References
- Newman D. J.; Cragg G. M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83 (3), 770–803. 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- Atanasov A. G.; Zotchev S. B.; Dirsch V. M.; Orhan I. E.; Banach M.; Rollinger J. M.; Barreca D.; Weckwerth W.; Bauer R.; Bayer E. A.; Majeed M.; Bishayee A.; Bochkov V.; Bonn G. K.; Braidy N.; Bucar F.; Cifuentes A.; D’Onofrio G.; Bodkin M.; Diederich M.; Dinkova-Kostova A. T.; Efferth T.; El Bairi K.; Arkells N.; Fan T. P.; Fiebich B. L.; Freissmuth M.; Georgiev M. I.; Gibbons S.; Godfrey K. M.; Gruber C. W.; Heer J.; Huber L. A.; Ibanez E.; Kijjoa A.; Kiss A. K.; Lu A.; Macias F. A.; Miller M. J. S.; Mocan A.; Müller R.; Nicoletti F.; Perry G.; Pittalà V.; Rastrelli L.; Ristow M.; Russo G. L.; Silva A. S.; Schuster D.; Sheridan H.; Skalicka-Woźniak K.; Skaltsounis L.; Sobarzo-Sánchez E.; Bredt D. S.; Stuppner H.; Sureda A.; Tzvetkov N. T.; Vacca R. A.; Aggarwal B. B.; Battino M.; Giampieri F.; Wink M.; Wolfender J. L.; Xiao J.; Yeung A. W. K.; Lizard G.; Popp M. A.; Heinrich M.; Berindan-Neagoe I.; Stadler M.; Daglia M.; Verpoorte R.; Supuran C. T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discovery 2021, 20 (3), 200–216. 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter S. E.; Fletcher M. H.; Wuest W. M. Natural Products as Platforms to Overcome Antibiotic Resistance. Chem. Rev. 2017, 117 (19), 12415–12474. 10.1021/acs.chemrev.7b00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel S.; Bon R. S.; Kumar K.; Waldmann H. Biology-Oriented Synthesis. Angew. Chem., Int. Ed. 2011, 50 (46), 10800–10826. 10.1002/anie.201007004. [DOI] [PubMed] [Google Scholar]
- Karageorgis G.; Waldmann H. Guided by Evolution: Biology-Oriented Synthesis of Bioactive Compound Classes. Synthesis 2019, 51 (01), 55–66. 10.1055/s-0037-1610368. [DOI] [Google Scholar]
- Solinski A. E.; B Koval A.; S Brzozowski R.; R Morrison K.; J Fraboni A.; E Carson C.; R Eshraghi A.; Zhou G.; G Quivey R. Jr.; A Voelz V.; A Buttaro B.; M Wuest W. Diverted Total Synthesis of Carolacton-Inspired Analogs Yields Three Distinct Phenotypes in Streptococcus Mutans Biofilms. J. Am. Chem. Soc. 2017, 139 (21), 7188–7191. 10.1021/jacs.7b03879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpilman A. M.; Carreira E. M. Probing the Biology of Natural Products: Molecular Editing by Diverted Total Synthesis. Angew. Chem., Int. Ed. 2010, 49 (50), 9592–9628. 10.1002/anie.200904761. [DOI] [PubMed] [Google Scholar]
- Galloway W. R. J. D.; Isidro-Llobet A.; Spring D. R. Diversity-Oriented Synthesis as a Tool for the Discovery of Novel Biologically Active Small Molecules. Nat. Commun. 2010, 1 (1), 80 10.1038/ncomms1081. [DOI] [PubMed] [Google Scholar]
- O' Connor C. J.; Beckmann H. S. G.; Spring D. R. Diversity-Oriented Synthesis: Producing Chemical Tools for Dissecting Biology. Chem. Soc. Rev. 2012, 41 (12), 4444–4456. 10.1039/c2cs35023h. [DOI] [PubMed] [Google Scholar]
- Yi S.; Varun B. V.; Choi Y.; Park S. B. A Brief Overview of Two Major Strategies in Diversity-Oriented Synthesis: Build/Couple/Pair and Ring-Distortion. Front. Chem. 2018, 6 (OCT), 507 10.3389/fchem.2018.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardiello J. J.; Stewart H. L.; Sore H. F.; Galloway W. R. J. D.; Spring D. R. A Novel Complexity-to-Diversity Strategy for the Diversity-Oriented Synthesis of Structurally Diverse and Complex Macrocycles from Quinine. Bioorg. Med. Chem. 2017, 25 (11), 2825–2843. 10.1016/j.bmc.2017.02.060. [DOI] [PubMed] [Google Scholar]
- Wang S.; Dong G.; Sheng C. Structural Simplification: An Efficient Strategy in Lead Optimization. Acta Pharm. Sin. B 2019, 9 (5), 880–901. 10.1016/j.apsb.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Dong G.; Sheng C. Structural Simplification of Natural Products. Chem. Rev. 2019, 119 (6), 4180–4220. 10.1021/acs.chemrev.8b00504. [DOI] [PubMed] [Google Scholar]
- Solinski A. E.; M Scharnow A.; J Fraboni A.; M Wuest W. Synthetic Simplification of Carolacton Enables Chemical Genetic Studies in Streptococcus Mutans. ACS Infect. Dis. 2019, 5 (8), 1480–1486. 10.1021/acsinfecdis.9b00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani G.; Cavalluzzi M. M.; Solidoro R.; Salvagno L.; Quintieri L.; Di Somma A.; Rosato A.; Corbo F.; Franchini C.; Duilio A.; Caputo L.; Habtemariam S.; Lentini G. Molecular Simplification of Natural Products: Synthesis, Antibacterial Activity, and Molecular Docking Studies of Berberine Open Models. Biomedicines 2021, 9 (5), 452. 10.3390/biomedicines9050452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub I.; A Sieber S. β-Lactams as Selective Chemical Probes for the in Vivo Labeling of Bacterial Enzymes Involved in Cell Wall Biosynthesis, Antibiotic Resistance, and Virulence. J. Am. Chem. Soc. 2008, 130 (40), 13400–13409. 10.1021/ja803349j. [DOI] [PubMed] [Google Scholar]
- Li Q.; Pellegrino J.; Lee D. J.; Tran A. A.; Chaires H. A.; Wang R.; Park J. E.; Ji K.; Chow D.; Zhang N.; Brilot A. F.; Biel J. T.; van Zundert G.; Borrelli K.; Shinabarger D.; Wolfe C.; Murray B.; Jacobson M. P.; Mühle E.; Chesneau O.; Fraser J. S.; Seiple I. B. Synthetic Group A Streptogramin Antibiotics That Overcome Vat Resistance. Nature 2020, 586 (7827), 145–150. 10.1038/s41586-020-2761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitcheltree M. J.; Pisipati A.; Syroegin E. A.; Silvestre K. J.; Klepacki D.; Mason J. D.; Terwilliger D. W.; Testolin G.; Pote A. R.; Wu K. J. Y.; Ladley R. P.; Chatman K.; Mankin A. S.; Polikanov Y. S.; Myers A. G. A Synthetic Antibiotic Class Overcoming Bacterial Multidrug Resistance. Nature 2021, 599 (7885), 507–512. 10.1038/s41586-021-04045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson E. E. Natural Products as Chemical Probes. ACS Chem. Biol. 2010, 5 (7), 639–653. 10.1021/cb100105c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M. D.; Burkart M. D.; Leonard M. S.; Portonovo P.; Liang B.; Ding X.; Joullié M. M.; Gulledge B. M.; Aggen J. B.; Chamberlin A. R.; Sandler J.; Fenical W.; Cui J.; Gharpure S. J.; Polosukhin A.; Zhang H.-R.; Evans P. A.; Richardson A. D.; Harper M. K.; Ireland C. M.; Vong B. G.; Brady T. P.; Theodorakis E. A.; La Clair J. J. A Central Strategy for Converting Natural Products into Fluorescent Probes. ChemBioChem 2006, 7 (3), 409–416. 10.1002/cbic.200500466. [DOI] [PubMed] [Google Scholar]
- Chen X.; Wang Y.; Ma N.; Tian J.; Shao Y.; Zhu B.; Wong Y. K.; Liang Z.; Zou C.; Wang J. Target Identification of Natural Medicine with Chemical Proteomics Approach: Probe Synthesis, Target Fishing and Protein Identification. Signal Transduction Targeted Ther. 2020, 5 (1), 72 10.1038/s41392-020-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abouelhassan Y.; Garrison A. T.; Yang H.; Chávez-Riveros A.; Burch G. M.; Huigens R. W. Recent Progress in Natural-Product-Inspired Programs Aimed To Address Antibiotic Resistance and Tolerance. J. Med. Chem. 2019, 62 (17), 7618–7642. 10.1021/acs.jmedchem.9b00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison K. C.; Hergenrother P. J. Natural Products as Starting Points for the Synthesis of Complex and Diverse Compounds. Nat. Prod. Rep. 2014, 31 (1), 6–14. 10.1039/C3NP70063A. [DOI] [PubMed] [Google Scholar]
- Donner J.; Reck M.; Bergmann S.; Kirschning A.; Müller R.; Wagner-Döbler I. The Biofilm Inhibitor Carolacton Inhibits Planktonic Growth of Virulent Pneumococci via a Conserved Target. Sci. Rep. 2016, 6 (1), 29677 10.1038/srep29677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M.; Rutz K.; Kunze B.; Tomasch J.; Surapaneni S. K.; Schulz S.; Wagner-Döbler I. The Biofilm Inhibitor Carolacton Disturbs Membrane Integrity and Cell Division of Streptococcus Mutans through the Serine/Threonine Protein Kinase PknB. J. Bacteriol. 2011, 193 (20), 5692. 10.1128/JB.05424-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M.; Wagner-Döbler I. Carolacton Treatment Causes Delocalization of the Cell Division Proteins PknB and DivIVa in Streptococcus Mutans in Vivo. Front. Microbiol. 2016, 7, 684 10.3389/fmicb.2016.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze B.; Reck M.; Dötsch A.; Lemme A.; Schummer D.; Irschik H.; Steinmetz H.; Wagner-Döbler I. Damage of Streptococcus Mutans Biofilms by Carolacton, a Secondary Metabolite from the Myxobacterium Sorangium Cellulosum. BMC Microbiol. 2010, 10 (1), 199 10.1186/1471-2180-10-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T.; Kirschning A. Total Synthesis of Carolacton, a Highly Potent Biofilm Inhibitor. Angew. Chem., Int. Ed. 2012, 51 (4), 1063–1066. 10.1002/anie.201106762. [DOI] [PubMed] [Google Scholar]
- Hallside M. S.; Brzozowski R. S.; Wuest W. M.; Phillips A. J. A Concise Synthesis of Carolacton. Org. Lett. 2014, 16 (4), 1148–1151. 10.1021/ol500004k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Wang W.; Wang Y.; Zeng A.-P. Two-Dimensional Gel-Based Proteomic of the Caries Causative Bacterium Streptococcus Mutans UA159 and Insight into the Inhibitory Effect of Carolacton. Proteomics 2013, 13 (23–24), 3470–3477. 10.1002/pmic.201300077. [DOI] [PubMed] [Google Scholar]
- Fu C.; Sikandar A.; Donner J.; Zaburannyi N.; Herrmann J.; Reck M.; Wagner-Döbler I.; Koehnke J.; Müller R. The Natural Product Carolacton Inhibits Folate-Dependent C1Metabolism by Targeting FolD/MTHFD. Nat. Commun. 2017, 8 (1), 1529 10.1038/s41467-017-01671-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque C.; Stipp R. N.; Wang B.; Smith D. J.; Höfling J. F.; Kuramitsu H. K.; Duncan M. J.; Mattos-Graner R. O. Downregulation of GbpB, a Component of the VicRK Regulon, Affects Biofilm Formation and Cell Surface Characteristics of Streptococcus Mutans. Infect. Immun. 2011, 79 (2), 786–796. 10.1128/IAI.00725-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattos-Graner R. O.; Porter K. A.; Smith D. J.; Hosogi Y.; Duncan M. J. Functional Analysis of Glucan Binding Protein B from Streptococcus Mutans. J. Bacteriol. 2006, 188 (11), 3813–3825. 10.1128/JB.01845-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keohane C. E.; Steele A. D.; Fetzer C.; Khowsathit J.; Van Tyne D.; Moynié L.; Gilmore M. S.; Karanicolas J.; Sieber S. A.; Wuest W. M. Promysalin Elicits Species-Selective Inhibition of Pseudomonas Aeruginosa by Targeting Succinate Dehydrogenase. J. Am. Chem. Soc. 2018, 140 (5), 1774–1782. 10.1021/jacs.7b11212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W.; Cross A. R.; Crowe-McAuliffe C.; Weigert-Munoz A.; Csatary E. E.; Solinski A. E.; Krysiak J.; Goldberg J. B.; Wilson D. N.; Medina E.; Wuest W. M.; Sieber S. A. The Natural Product Elegaphenone Potentiates Antibiotic Effects against Pseudomonas Aeruginosa. Angew. Chem., Int. Ed. 2019, 58 (25), 8581–8584. 10.1002/anie.201903472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le P.; Kunold E.; Macsics R.; Rox K.; Jennings M. C.; Ugur I.; Reinecke M.; Chaves-Moreno D.; Hackl M. W.; Fetzer C.; Mandl F. A. M.; Lehmann J.; Korotkov V. S.; Hacker S. M.; Kuster B.; Antes I.; Pieper D. H.; Rohde M.; Wuest W. M.; Medina E.; Sieber S. A. Repurposing Human Kinase Inhibitors to Create an Antibiotic Active against Drug-Resistant Staphylococcus Aureus, Persisters and Biofilms. Nat. Chem. 2020, 12 (2), 145–158. 10.1038/s41557-019-0378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J.; Hein M. Y.; Luber C. A.; Paron I.; Nagaraj N.; Mann M. Accurate Proteome-Wide Label-Free Quantification by Delayed Normalization and Maximal Peptide Ratio Extraction, Termed MaxLFQ. Mol. Cell. Proteomics 2014, 13 (9), 2513–2526. 10.1074/mcp.M113.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartual S. G.; Straume D.; Stamsås G. A.; Muñoz I. G.; Alfonso C.; Martínez-Ripoll M.; Håvarstein L. S.; Hermoso J. A. Structural Basis of PcsB-Mediated Cell Separation in Streptococcus Pneumoniae. Nat. Commun. 2014, 5, 3842 10.1038/ncomms4842. [DOI] [PubMed] [Google Scholar]
- Rued B. E.; Alcorlo M.; Edmonds K. A.; Martínez-Caballero S.; Straume D.; Fu Y.; Bruce K. E.; Wu H.; Håvarstein L. S.; Hermoso J. A.; Winkler M. E.; Giedroc D. P. Structure of the Large Extracellular Loop of FtsX and Its Interaction with the Essential Peptidoglycan Hydrolase PcsB in Streptococcus Pneumoniae. mBio 2019, 10 (1), e02622–18. 10.1128/mBio.02622-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham L.-T.; Barendt S. M.; Kopecky K. E.; Winkler M. E. Essential PcsB Putative Peptidoglycan Hydrolase Interacts with the Essential FtsXSpn Cell Division Protein in Streptococcus Pneumoniae D39. Proc. Natl. Acad. Sci. U.S.A. 2011, 108 (45), E1061–E1069. 10.1073/pnas.1108323108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham L.-T.; Jensen K. R.; Bruce K. E.; Winkler M. E. Involvement of FtsE ATPase and FtsX Extracellular Loops 1 and 2 in FtsEX-PcsB Complex Function in Cell Division of Streptococcus Pneumoniae D39. mBio 2013, 4 (4), e00431-13 10.1128/mBio.00431-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs N. S.; Bruce K. E.; Naskar S.; Winkler M. E.; Roper D. I. The Pneumococcal Divisome: Dynamic Control of Streptococcus Pneumoniae Cell Division. Front. Microbiol. 2021, 12, 737396 10.3389/fmicb.2021.737396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quivey R. G. Jr.; Grayhack E. J.; Faustoferri R. C.; Hubbard C. J.; Baldeck J. D.; Wolf A. S.; MacGilvray M. E.; Rosalen P. L.; Scott-Anne K.; Santiago B.; Gopal S.; Payne J.; Marquis R. E. Functional Profiling in Streptococcus Mutans: Construction and Examination of a Genomic Collection of Gene Deletion Mutants. Mol. Oral Microbiol. 2015, 30 (6), 474–495. 10.1111/omi.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M.; Fives-Taylor P.; Wu H. The Utility of Affinity-Tags for Detection of a Streptococcal Protein from a Variety of Streptococcal Species. J. Microbiol. Methods 2008, 72 (3), 249–256. 10.1016/j.mimet.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senadheera M. D.; Guggenheim B.; Spatafora G. A.; Huang Y.-C. C.; Choi J.; Hung D. C. I.; Treglown J. S.; Goodman S. D.; Ellen R. P.; Cvitkovitch D. G. A VicRK Signal Transduction System in Streptococcus Mutans Affects gtfBCD, gbpB, and Ftf Expression, Biofilm Formation, and Genetic Competence Development. J. Bacteriol. 2005, 187 (12), 4064–4076. 10.1128/JB.187.12.4064-4076.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.